Abstract
The Veterans Health Administration (VHA) listed the infliximab (IFX) biosimilar, IFX-dyyb (Inflectra), on the Veterans Affairs National Formulary (VANF) in May 2017. In September 2018, biosimilar IFX-abda (Renflexis) became the VANF IFX product. The recommended formulary changes from one IFX biosimilar to another provided a unique opportunity to study IFX utilization patterns in IFX-naïve Veterans with Inflammatory Bowel Disease (IBD). This study aimed to describe IFX and healthcare utilization during the 365 days after initiation with IFX reference product (RP) or biosimilars IFX-dyyb and IFX-adba. This descriptive study was performed using the VHA Corporate Data Warehouse. All Veterans initiated on IFX-RP (Remicade) or biosimilars IFX-dyyb and IFX-adba between September 1, 2016 and December 30, 2019 were included and followed for 365 days. Veterans enrolled in the VHA for at least 365 days with no evidence of IFX before their index date were considered IFX-naïve. Continuous data on IFX use, laboratory measurements, and healthcare utilization were reported with means, 95% confidence interval (CI), medians, and interquartile ranges. Frequency, proportions, and 95% CIs were presented for categorical variables. Statistical tests included ANOVA and Kruskal–Wallis for continuous outcomes, Poisson regression for count-based outcomes (i.e., healthcare utilization visits), and Chi-square for dichotomous outcomes. The study identified 1763 IFX-naïve patients with IBD, and 785, 441, and 537 was indexed to RP, IFX-dyyb, and IFX-adba, respectively. Statistical differences were observed in IFX utilization measures related to dosing, adherence, and persistence. The proportion of days covered (PDC) during the 365-day follow-up period varied among the IFX groups: IFX-RP at 66%, IFX-dyyb at 60%, and IFX-abda at 69% (P value < .001). Persistence with the index IFX product during the 365-day follow-up period also varied: IFX-RP at 43%, IFX-dyyb at 32%, and IFX-abda at 51% (P value < .001). Healthcare utilization and laboratory findings were similar among the IFX groups. IFX utilization and laboratory patterns were clinically similar among the IFX biosimilars and RP groups, suggesting that providers did not modify their practice with biosimilars. Statistically significant differences in IFX utilization patterns are explained by formulary dynamics when the VANF product switched from IFX-dyyb to IFX-abda.
Keywords: Crohn’s disease (CD), descriptive epidemiology, inflammatory bowel disease (IBD), real-world evidence, ulcerative colitis (UC)
1. Introduction
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), defines a spectrum of chronic immune-mediated inflammatory gastrointestinal disorders that cause impaired quality of life and often require hospitalization and surgery.[1–5] Biological therapies, such as anti-tumor necrosis factor alpha monoclonal antibodies, have revolutionized the management of IBD.[6] Infliximab (IFX), the first tumor necrosis factor inhibitor (TNFi)-approved in the United States (US) for IBD, has been proven to induce mucosal healing, prolong periods of remission, and improve quality of life, leading to a reduction in hospitalizations and surgical procedures.[7] IFX, nevertheless, is expensive, and its costs can result in access inequalities[8,9] and a profound impact on pharmacy budgets.[10] The introduction of IFX biosimilars has been anticipated to generate competition and result in substantial cost savings for the Veterans Health Administration (VHA)[11] and other healthcare systems.[12]
Biosimilar IFX-dyyb (Inflectra) received Food and Drug Administration approval in April 2016 and IFX-abda (Renflexis) in April 2017 for all their indications of the originator product following an expedited review process. These biosimilars received approval for UC and CD through extrapolation. Extrapolation allows biosimilars to be approved for all reference product (RP) indications without clinical trials in each indicated disease. For example, IFX-dyyb approval for IBD was extrapolated based on data from clinical trials in ankylosing spondylitis and rheumatoid arthritis.[13,14]
Physicians who treat IBD patients were initially cautious about using biosimilars due to concerns with their approval process that extrapolated efficacy and safety data.[15,16] Nevertheless, a growing collection of literature from clinical trials[17–19] and real-world observations[20–22] continues to provide reassuring data that biosimilars are effective, safe, and comparable to reference biologics in treatment-naïve patients. Most studies, however, have focused on IFX-dyyb rather than IFX-abda.[23]
In May 2017, the VHA Pharmacy Benefits Management (PBM) recommended IFX-dyyb as the preferred product for new initiations of IFX. In September 2018, IFX-abda was awarded a national contract and became the VHA PBM preferred IFX product. IFX-abda remains the preferred product by the VHA in place of IFX-RP, Remicade, for IFX initiations. Biosimilar adoption in the VHA appears to be outpacing academic institutions;[11] nevertheless, few reports have examined the real-world utilization of IFX biosimilars in the VHA. This study sought to describe real-world experiences with biosimilar IFX among Veteran patients with IBD. Specifically, IFX utilization patterns, healthcare utilization, and laboratory results were used to describe biosimilar IFX in IBD patients previously naïve to IFX products during the initial 365 days.
2. Methods
2.1. Population, data sources, and study design
This cohort study described the use of IFX-RP and IFX biosimilars in US Veterans diagnosed with IBD who received their first IFX exposure between September 1, 2016 and December 30, 2019 while receiving care in the VHA. All US Veterans aged ≥18 on the day of their first IFX dispensing who had a clinic visit at least 365 days before their index IFX exposure were eligible for this study of IFX-naïve IBD patients. The National VHA setting included all VHA facilities that provided IFX infusions during the study period, except for 5 stations removed from the analysis since they contained medication labeling errors that prevented differentiating IFX-RP from IFX-biosimilars.
The VHA Corporate Data Warehouse (CDW) provided the clinical and administrative data for care delivered by VHA providers from 145 VHA Medical Centers. The CDW data domains used included patient (e.g., demographics), inpatient and outpatient encounters, inpatient and outpatient pharmacy dispensing, vital signs, and laboratory and chemistry domains.[24] We used the Compensation and Pension Records Interchange (CAPRI) system, which provides National “read-only” access to Veteran electronic health records and medical notes to inform algorithm development and confirm the correct classification of clinical concepts.
Trends in IFX product selection, IFX use, healthcare utilization, and laboratory measurements pertinent to IFX were examined during a follow-up period of 365 days. The key features of the historical cohort design are described in Appendix A, Supplemental Digital Content, http://links.lww.com/MD/N457 of the online supplement. The IFX lookback period included all relevant pharmacy data after the VHA enforcement of Centers for Medicare and Medicaid Services coding standards (2005) to reduce the likelihood of misclassifying IFX- naïve status. The study eligibility window was September 1, 2016 and December 30, 2019, allowing for a 1-year follow-up for every patient. The baseline (BL) and follow-up periods were relative to the patients’ first IFX infusion index date. The naïve user assessment window and BL period encompassed 365 days before the IFX index date. IFX utilization, healthcare utilization (including hospitalizations, emergency, and specialty care), and laboratory measurements were described during a follow-up period of 365 days.
This study was approved by the University of Utah Institutional Review Board and the Salt Lake City VHA Research Service.
2.2. Measurement
2.2.1. IFX exposure classification
A previously validated algorithm that harmonized VHA pharmacy data in the CDW was used to identify the dispensing of IFX reference products or biosimilars (IFX-RP, IFX-dyyb, or IFX-abda).[25,26] Patients were classified as IFX-naïve when the date of their first observed IFX infusion from the CDW occurred during the eligibility period.[27] Patients were assigned to one of the 3 exposure groups (IFX-RP, IFX-dyyb, and IFX-abda) based on their index IFX product.
2.2.2. IBD diagnosis
A rule-based algorithm was used to assign all Veteran patients indexed on IFX to a treatment indication. The algorithm prioritized proximity to the IFX index date and IBD diagnoses (UC, CD). Appendix B, Supplemental Digital Content, http://links.lww.com/MD/N457 of the online supplement provides the International Classification of Disease (ICD)-9-Clinical Modification (CM) and ICD-10-CM codes. More complex coding algorithms were considered, such as those requiring 2 or more IBD diagnoses[20] during the BL period or IBD medications,[21] which did not alter the relative indicators of IBD classification. These indicators included visits to a gastroenterologist, measures of fecal inflammation, and the use of IBD medications during the BL period.
2.2.3. IFX exposure utilization outcomes
IFX utilization was assessed during a follow-up period of 365 days. We previously described the data workflows for extracting, classifying, and standardizing dispensing information for IFX.[28] In brief, the data workflows were designed to include records associated with treatment received by removing duplicates, errors, and mislabeled dispensing events. The workflow also standardizes dispensing information for medications administered in the hospital and infusion suites with information in outpatient pharmacy dispensing records, such as the total quantity dispensed in milligrams (mg) and the intended duration of treatment (i.e., days’ supply). Since the Food and Drug Administration-approved maintenance dosing schedule of IFX for UC and CD is 56 days, we assigned a 56-day supply to indicate they had treatment coverage for 56 days, which is important when estimating adherence and persistence measures.
IFX utilization measures included the average initial dose, maximum dose, average dose, average weight-based dose, average interval between dispensing events, average cumulative dose, average interval between the last dispensing of IFX and the end of the follow-up period (i.e., extant interval), and the proportion of days covered (PDC). The PDC was calculated by dividing the IFX treatment days by days in the follow-up period (365 days) and multiplying by 100.[29,30] Since the maintenance dosing schedule is every 56 days, we assumed 56 days of coverage or fewer (based on actual intervals) after each dispensing/administration event. For example, if a patient had their second infusion 86 days after their first infusion, then they had a 20-day gap in treatment. On day 132 the numerator in the PDC calculation is (56 + 56 = 112) and the denominator is (56 + 56 + 20 = 132) resulting in 84.8% of days during the 132 day interval with IFX coverage. If the patient had their second infusion 54 days after their first then the numerator and denominator on day 110 are 110, with a PDC equal to 100 percent. Patients who had their last dispensing for their index IFX product more than 86 days (56 + 30) before the end of the follow-up period were considered non-persistent with their index IFX treatment. The above measures were based on the index IFX product. Additionally, the IFX product switching from the index IFX was recorded and described. We also measured adherence to any IFX product during follow-up.
2.2.4. Healthcare utilization outcomes
Healthcare Utilization measures included counts of gastroenterology outpatient visits (VHA stop codes 301, 321), emergency care (EC) visits (urgent care clinic stop code 131, and emergency department [ED] stop code 130), and inpatient admissions to acute medical and surgical wards, which included Intensive Care Unit, medical, and surgical bed section from the inpatient discharge diagnosis table. Discharges from rehabilitation, mental health, and long-term care were excluded.
The Healthcare Cost and Utilization Project Clinical Classification Software Revised,[31] which classifies ICD-10-CM codes into clinically meaningful categories, was used to describe the top 5 principal discharge diagnoses for each treatment group. The goal was to describe patterns in the documented reasons for hospital admissions across index IFX products to explore differences that may represent safety or effectiveness concerns.
2.2.5. IFX-related laboratory results
Laboratory measures of liver function (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), fecal inflammation (fecal calprotectin [FC] and fecal lactoferrin), general inflammation C-reactive protein (CRP) was assessed at BL and during the follow-up period. IFX trough and antibodies to IFX were also assessed if measured clinically as part of the routine standard of care.[32–34]
When more than one laboratory test was available during the study period, the test result closest to the index date (during the BL period) represented the patient’s BL measurement, and the test closest to the end of the study period provided the follow-up measurement. IFX concentration levels were considered troughs when collected within 7 days before the IFX dispensing event. The list of logical observation identifier names and codes used for each laboratory concept is provided in the online supplement’s Appendix C, Supplemental Digital Content, http://links.lww.com/MD/N457.
Attempts were made to standardize aggregated laboratory results for each concept. Mathematical conversions were applied to standardized measured units when more than 1 unit was reported within a laboratory concept. Additional rules were applied to standardize string values containing alphanumeric characters or values that delimit a range, such as < 5 mcg/g, as informed by the published Laboratory Rosetta Stone (LabRS).[35] Laboratory values that referred to an addendum or comments were chart-reviewed for FC, lactoferrin, IFX concentrations, and antibodies for IFX (ATI) to extract and standardize the reported values.
FC values exceeding 150 mg/g were considered an indicator of active inflammation.[36,37] Lactoferrin was recorded as a dichotomous value in the CDW and treated as evidence of inflammation or no evidence of inflammation. CRP levels exceeding 5mg/dL were considered an indicator of general inflammation.[36,37]
IFX concentrations were reported as mean detected values, whereas ATIs were reported as undetected or detected due to assay dependence in ATI measurement; no thresholds were established.[32,33,38]
2.2.6. Baseline demographics, comorbidity, and baseline medications
The CDW patient domain provided demographic characteristics such as age, gender, race, and ethnicity. Race was obtained from self-reported races when available. The most frequent race was chosen when patients listed more than 1 race category (categories did not include mixed races).[39] To maintain Veterans Affairs race categories, Veterans with more than 1 race at an equal frequency had their race chosen at random. Patient health factors were used to classify smoking status into current, former, and never smokers.[40,41] Body mass index was calculated by dividing weight in kilograms (kg) by height in meters (m) squared (kg/m2) using the median of each person’s recorded height measurement during the 365 day BL period—heights <55 inches or larger than 82 inches were not included. The Deyo-Charlson Comorbidity Index[42] adapted to ICD-10 coding was used to generate a composite comorbidity score to compare differences among index IFX groups.[43]
The use of IBD-related medications was described for each IFX treatment group. Corticosteroids, 5-aminosalicylates, non-TNFi biologics, TNFi biologics, Janus kinase (JAK) inhibitors, and oral small molecules were categorized by therapeutic classification. Appendix D, Supplemental Digital Content, http://links.lww.com/MD/N457 of the online supplement provides the list of generic products queried for each category.
2.3. Secondary analysis
A secondary analysis was conducted for the IBD study population’s IFX utilization, healthcare utilization, and IBD-laboratory measured by infliximab product at the follow-up period of 183 days (available in the online supplement (Appendices E–G, Supplemental Digital Content, http://links.lww.com/MD/N457, http://links.lww.com/MD/N457, http://links.lww.com/MD/N457).
2.4. Statistical approach
This study assessed IFX utilization, healthcare utilization, and IBD-related laboratory measures for index IFX-RP and biosimilar groups. Continuous data were presented as mean, standard deviation, and 95% confidence interval (CI). Medians and interquartile ranges (IQR) were also computed to describe the central tendencies of group measures more accurately. Categorical data were presented as frequency, percentages, and binomial exact 95% CIs. This study aimed to describe observed IFX treatment and utilization patterns to assess whether clinically meaningful differences indicated potential variation in perceived or actual treatment effectiveness or safety. Statistical tests included ANOVA and Kruskal Wallis for continuous outcomes, Poisson regression for count-based outcomes (i.e., healthcare utilization visits), and Chi-square for dichotomous outcomes. Missing data were reported instead of imputed. Data processing was conducted using Microsoft Server Management Studio (SQL) 17.4, and descriptive statistics were computed using Statistical Analysis System (SAS) 9.4.
3. Results
3.1. Study population and index IFX groups
After applying classification criteria, 3394 Veteran patients were identified with IBD during the eligibility period between September 1, 2016 and December 30, 2019. We classified 1763 IFX users as naïve and 1631 experienced IFX users (i.e., they entered the eligibility period with IFX or had a prior exposure before restarting IFX during the eligibility period). This study describes IFX measures only for the 1763 IFX- naïve users classified as having IBD.
Patients were indexed into treatment groups based on the first IFX product dispensed during the eligibility period. We found 785 patients started on IFX-RP, 441 on IFX-dyyb, and 537 started on IFX-abda. Table 1 provides BL demographics by IFX groups. Differences in missing ethnicity were observed among IFX groups with 14.5% (95% CI: 11.5–17.5) in IFX-abda, 12.2% (95% CI: 9.2–15.3) in IFX-dyyb, and 9.9% (95% CI: 7.8–12) in the IFX-RP group (P value = .04). Differences were also observed in the proportion of former smokers with 41.7% (95% CI: 37.5–45.9) in IFX-abda, 32.9% (95% CI: 28.5–37.3) in IFX-dyyb, and 31.7% (95% CI: 28.5–35) in the IFX-RP group (P value < .001). Refer to Table 1 for a detailed description of patient demographics, and BL medication use for the distributions did not reach statistical significance.
Table 1.
Baseline demographics IFX-naïve IBD population.
| IBD naïve (n = 1763) | IFX-RP (n = 785) | IFX-dyyb (n = 441) | IFX-abda (n = 537) | P values | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Age at index (yr) | 51.6 ± 16.9 | 50.4–52.8 | 51.1 ± 16.6 | 49.5, 52.6 | 53.4 ± 16.5 | 52–54.7 | .072 |
| Gender | N | Col % (95% CI) | N | Col % (95% CI) | n | Col % (95% CI) | |
| Male | 697 | 88.8 (88.6, 91) | 392 | 88.9 (86, 91.8) | 468 | 87.2 (84.3, 90) | .601 |
| Race | N | Col % (95% CI) | N | Col % (95% CI) | n | Col % (95% CI) | |
| White | 586 | 74.6 (71.6, 77.7) | 311 | 70.5 (66.3, 74.8) | 390 | 72.6 (68.9, 76.4) | .287 |
| Black | 102 | 13 (10.6, 15.4) | 78 | 17.7 (14.1, 21.3) | 75 | 14 (11, 16.9) | .075 |
| Others | 29 | 3.7 (2.4, 5) | 9 | 2 (0.7, 3.4) | 17 | 3.2 (1.7, 4.7) | .278 |
| Missing | 68 | 8.7 (7.8, 12) | 43 | 9.8 (7, 12.5) | 55 | 10.2 (7.7, 12.8) | .603 |
| Ethnicity | N | Col % (95% CI) | N | Col % (95% CI) | n | Col % (95% CI) | |
| Hispanic | 57 | 7.3 (5.5, 9.1) | 29 | 6.6 (4.3, 8.9) | 34 | 6.3 (4.3, 8.4) | .785 |
| Non-Hispanic | 650 | 82.8 (80.2, 85.4) | 358 | 81.2 (77.5, 84.8) | 425 | 79.1 (75.7, 82.6) | .245 |
| Missing | 78 | 9.9 (7.8, 12) | 54 | 12.2 (9.2, 15.3) | 78 | 14.5 (11.5, 17.5) | .040 |
| BMI at index | 28.6 ± 5.4 | 28.2–28.9 | 28.7 ± 5.7 | 28.2–29.3 | 28.9 ± 5.6 | 28.4–29.4 | .522 |
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
|---|---|---|---|---|---|---|---|
| DCCI | 0.7 ± 1.3 | 0 (0–1) | 0.7 ± 1.3 | 0 (0–1) | 0.8 ± 1.4 | 0 (0–1) | .186 |
| Smoking | N | Col % (95% CI) | N | Col % (95% CI) | N | Col % (95% CI) | |
| Current | 204 | 26 (22.9, 29.1) | 102 | 23.1 (19.2, 27.1) | 111 | 20.7 (17.3, 24.1) | .079 |
| Former | 249 | 31.7 (28.5, 35) | 145 | 32.9 (28.5, 37.3) | 224 | 41.7 (37.5, 45.9) | .000 |
| Never | 330 | 42 (38.6, 45.5) | 192 | 43.5 (38.9, 48.2) | 202 | 37.6 (33.5, 41.7) | .131 |
| Missing | 2 | 0.3 (0.03, 0.9) | 2 | 0.5 (0.1, 1.6) | 0 | 0 | .325 |
| IBD | N | Col % (95% CI) | N | Col % (95% CI) | n | Col % (95% CI) | |
| Ulcerative colitis | 351 | 44.7 (41.2, 48.2) | 200 | 45.4 (40.7, 50) | 226 | 42.1 (37.9, 46.3) | .526 |
| Crohn’s disease | 434 | 55.3 (51.8, 58.8) | 241 | 54.6 (50, 59.3) | 311 | 57.9 (53.7, 62.1) | .526 |
| Previous medication | N | Col % (95% CI) | N | Col % (95% CI) | n | Col % (95% CI) | |
| Corticosteroid | 280 | 35.7 (32.3, 39) | 177 | 40.1 (35.6, 44.7) | 185 | 34.5 (30.4, 38.5) | .156 |
| 5-ASA | 437 | 55.7 (52.2, 59.1) | 258 | 58.5 (53.9, 63.1) | 300 | 55.9 (51.7, 60.1) | .599 |
| Non-TNFi biologics | 71 | 9.0 (7, 11.1) | 29 | 6.6 (4.3, 8.9) | 37 | 6.9 (4.8, 9) | .198 |
| Non-IFX TNFi | 278 | 35.4 (32.1, 38.8) | 174 | 39.5 (34.9, 44) | 193 | 35.9 (31.9, 40) | .345 |
| JAK inhibitor | 22 | 2.8 (1.7, 4) | 22 | 5 (3, 7) | 16 | 3 (1.5, 4.4) | .104 |
| OSM | 313 | 39.9 (36.5, 43.3) | 182 | 41.3 (36.7, 45.9) | 209 | 38.9 (34.8, 43) | .756 |
Note: Continuous variables were evaluated using ANOVA, and categorical or dichotomous outcomes were evaluated using Chi-square.
5-ASA = 5-aminosalicylates, BMI = body mass index, DCCI = Deyo–Charlson comorbidity index, IBD = inflammatory bowel disease, JAK = Janus kinase inhibitors, OSM = oral small molecule (other than JAK inhibitors), TNFi = tumor necrosis factor inhibitor.
3.2. Adoption
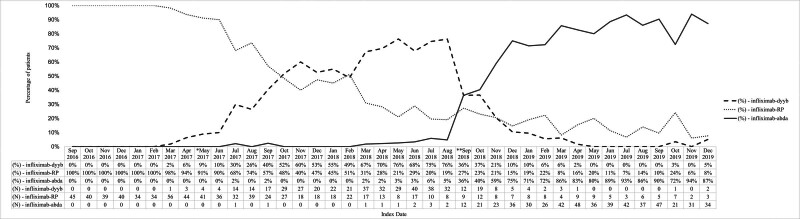
IFX-dyyb was listed as the Veterans Affairs National Formulary (VANF) product in May 2017, and IFX-abda gained VANF approval and became the preferred IFX product in September 2018. Figure 1 describes IFX-biosimilar initiation patterns within the context of changing VANF. The uptake of the biosimilar IFX within the VHA was markedly slower for IFX-dyyb than for IFX-abda. It took IFX-dyyb 183 days after VANF designation to be chosen for at least half of the new IFX infusion cases and 335 days to become the predominant product across the VHA. In contrast, IFX-abda achieved dominance within 90 days of its designation status as the preferred VANF product.
Figure 1.
IFX product initiation in IFX-naïve veterans with IBD diagnoses in relation to Veterans Affairs National Formulary (VANF). IFX-RP = Remicade, IFX-dyyb = Inflectra, IFX-abda = Renflexis. *May 2017: IFX-dyyb added as Veterans Affairs National Formulary (VANF) IFX product. **September 2018: IFX-abda added as Veterans Affairs National Formulary (VANF) IFX product.
3.3. IFX utilization
Dosing measures: differences in IFX product dosing were observed among IFX groups. Specifically, differences were observed in average accumulative dose (P value = .003) and max dose (P value = .003).
Measures of persistence and adherence: differences were observed in all measures used to infer persistence and adherence during 365 day follow-up that includes an average number of dispensing/administration (P value < .001) and the average interval between dispensing/administration (P value < .001), and the interval between the last dispensing and end of follow-up (P value < .001). As a result, significant differences in PDCs were observed among index treatments: IFX-abda (72%, 95% CI: 69–75), IFX-dyyb (60%, 95% CI: 57–63), and IFX-RP (66%, 95% CI: 63–68) (P value < .001). Similarly, significant differences in persistence on index treatment were observed among IFX-abda (51%, 95% CI: 46–55), IFX-dyyb (32%, 95% CI: 28–36), and IFX-RP (43%, 95% CI: 39–46) (P value < .001). Differences in persistence at the broader IFX class level were also observed among IFX-abda (56.2%, 95% CI: 51.9–60.5), IFX-dyyb (62.9%, 95% CI: 57.2–66.5), and IFX-RP (62.9%, 95% CI: 59.4–66.4; P value < .001). See Table 2.
Table 2.
IFX utilization measures and adherence during the 365-days follow-up period.
| IBD naïve (n = 1763) | IFX-RP | IFX-dyyb | IFX-abda | P value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Number of patients | 785 | 441 | 537 | ||||
| Starting dose (mg) | 495.28 ± 178.83 | 482.75, 507.81 | 505.23 ± 189.15 | 487.53, 522.93 | 503.18 ± 175.15 | 488.33, 518.03 | .585 |
| Max dose (mg) | 576.31 ± 242.12 | 559.34, 593.27 | 613.33 ± 271.82 | 587.89, 638.77 | 622.12 ± 277.28 | 598.61, 645.62 | .003 |
| Average weight based dose (mg/kg) | 5.84 ± 1.63 | 5.73, 5.96 | 6.04 ± 1.78 | 5.87, 6.2 | 6.01 ± 1.84 | 5.85, 6.16 | .097 |
| Average dose (mg) | 519.57 ± 175.83 | 507.25, 531.89 | 540.73 ± 200.1 | 522, 559.45 | 538.49 ± 187.43 | 522.61, 554.38 | .08 |
| Average accumulative dose | 3231.52 ± 2409.54 | 3062.7, 3400.34 | 3204.06 ± 2426.53 | 2976.97, 3431.16 | 3640.99 ± 2279.75 | 3447.73, 3834.24 | .003 |
| Average number of dispensing | 6.1 ± 3.54 | 5.85, 6.35 | 5.71 ± 3.21 | 5.41, 6.01 | 6.6 ± 2.91 | 6.35, 6.85 | <.001 |
| Average interval days (pt > 1 dispensing) | 41.15 ± 21.46 | 39.57, 42.73 | 37.22 ± 12.6 | 35.98, 38.47 | 40.34 ± 11.58 | 39.32, 41.36 | <.001 |
| Extant interval (interval between last dispensing and end of follow-up) | 156.94 ± 131.87 | 147.7, 166.18 | 181.24 ± 129.78 | 169.1, 193.39 | 134.13 ± 123.8 | 123.64, 144.63 | <.001 |
| PDC | 66 ± 33 | 63, 68 | 60 ± 33 | 57, 63 | 72 ± 31 | 69, 75 | <.001 |
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | ||
| Persistent on index product | 341 (43%) | 39%, 46% | 142 (32%) | 28%, 36% | 275 (51%) | 46%, 55% | <.001 |
| Persistent on any IFX product at 365 days | 494 (62.9%) | 59.4%, 66.4% | 273 (62.9%) | 57.2%, 66.5% | 302 (56.2%) | 51.9%, 60.5% | .041 |
| Switch to | N | Non-persistent (%) | n | Non-persistent (%) | n | Non-persistent (%) | |
|---|---|---|---|---|---|---|---|
| IFX-RP | 35 | 11.7 | 21 | 8 | |||
| IFX-dyyb | 128 | 28.8 | 11 | 4.2 | |||
| IFX-abda | 59 | 13.3 | 124 | 41.5 |
Note: Continuous variables were evaluated using ANOVA, and categorical or dichotomous outcomes were evaluated using Chi-square.
IBD = inflammatory bowel disease, IFX = infliximab, IFX-abda = Renflexis, IFX-dyyb = Inflectra, IFX-RP = Remicade.
3.4. Health care utilization
Differences in baseline and follow-up GI visits were observed among IFX-abda (3.88, 95% CI: 3.65–4.11), IFX-dyyb (3.74, 95% CI: 3.5–3.98), and IFX-RP (3.37, 95% CI: 3.19–3.49) (P value < .001). No statistical differences were observed in ED or Inpatient visits (Table 3). Table 3 also presents the 5 most prevalent categories of principal discharge diagnoses during baseline and follow-up periods. A statistical difference in baseline discharge diagnosis of gastrointestinal hemorrhage was observed among IFX groups (P value = .047). During the follow-up period, the predominant diagnosis for all treatment cohorts was regional enteritis along with ulcerative colitis (UC), followed closely by gastrointestinal hemorrhage, but no differences were observed during the follow-up period.
Table 3.
Health care utilization during the 365-days follow-up period.
| IFX-RP (n = 785) | IFX-dyyb (n = 441) | IFX-abda (n = 537) | P values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outpatient GI visits | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||||
| Baseline | Number of GI visits per patient | 3.37 (3.19–3.49) | 3.74 (3.5–3.98) | 3.88 (3.65–4.11) | <.001 | ||||
| N | % | N | % | N | % | ||||
| Patients with a GI visit | 711 | 90.6 | 412 | 93.4 | 507 | 94.4 | .023 | ||
| Follow-up | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||||
| Number of GI visits per patient | 4.38 (4.31–4.45) | 4.62 (4.53–4.71) | 4.04 (3.96–4.12) | <.001 | |||||
| N | % | N | % | N | % | ||||
| Patients with a GI visit | 714 | 91.0 | 409 | 92.7 | 492 | 91.6 | .556 | ||
| Emergency care (EC) visits | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||||
| Baseline | Number of EC visits per patient | 1.36 (1.14–1.59) | 1.39 (1.20–1.57) | 1.28 (1.1–1.44) | .268 | ||||
| N | % | N | % | N | % | ||||
| Patients with an EC visit | 385 | 49 | 238 | 54 | 281 | 52.3 | .214 | ||
| Follow-up | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||||
| Number of ED visits per patient | 1.22 (1.05–1.4) | 1.22 (1.04–1.4) | 1.12 (0.95–1.3) | .211 | |||||
| N | % | N | % | N | % | ||||
| Patients with an EC visit | 355 | 45.2 | 224 | 50.8 | 255 | 47.5 | .172 | ||
| Inpatient visits | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||||
| Baseline | Number of inpatient visits per patient | 0.46 (0.4–0.53) | 0.49 (0.41–0.58) | 0.45 (0.38–0.52) | .56 | ||||
| N | % | N | % | N | % | ||||
| Patients with an Inpatient visit | 219 | 27.9 | 139 | 31.5 | 162 | 30.2 | .377 | ||
| Follow-up | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||||
| Number of inpatient visits per patient | 0.57 (0.49–0.65) | 0.58 (0.47–0.68) | 0.54 (0.45–0.64) | .762 | |||||
| N | % | N | % | N | % | ||||
| Patients with any | 240 | 30.6 | 137 | 31.1 | 162 | 30.2 | .955 | ||
| Top 5 principal discharge diagnoses by frequency (by HCUP-CCSR category) | All treatment groups | ||||||||
| Baseline | N | % | N | % | N | % | N | % | |
| Total number of inpatient visits | 819 | 46.5 | 363 | 46.2 | 217 | 49.2 | 239 | 44.5 | .337 |
| Regional enteritis and ulcerative colitis (DIG011) | 349 | 19.8 | 148 | 18.9 | 93 | 21.1 | 108 | 20.1 | .626 |
| Gastrointestinal hemorrhage (DIG021) | 89 | 5 | 30 | 3.8 | 31 | 7.0 | 28 | 5.2 | .047 |
| Intestinal obstruction and ileus (DIG012) | 64 | 3.6 | 32 | 4.1 | 17 | 3.9 | 15 | 2.8 | .453 |
| Bacterial infections (INF003) | 53 | 3 | 21 | 2.7 | 14 | 3.2 | 18 | 3.4 | .757 |
| Peritonitis and intra-abdominal abscess (DIG016) | 47 | 3 | 26 | 2.7 | 11 | 3.2 | 10 | 3.4 | .757 |
| Follow-up | N | % | N | % | N | % | N | % | |
| Total number of inpatient visits | 992 | 56.3 | 447 | 56.9 | 254 | 57.6 | 291 | 54.2 | .496 |
| Regional enteritis and ulcerative colitis (DIG011) | 464 | 23.3 | 207 | 26.3 | 130 | 29.5 | 127 | 23.6 | .120 |
| Gastrointestinal hemorrhage (DIG021) | 103 | 5.8 | 47 | 6 | 24 | 5.4 | 32 | 6 | .918 |
| Intestinal obstruction and ileus (DIG012) | 91 | 5.2 | 43 | 5.5 | 20 | 4.5 | 28 | 5.2 | .772 |
| Bacterial infections (INF003) | 70 | 4 | 31 | 3.9 | 21 | 4.8 | 18 | 3.4 | .531 |
| Septicemia (INF002) | 33 | 1.9 | 17 | 2.2 | 7 | 1.6 | 9 | 1.7 | .713 |
Note: Categorical or dichotomous outcomes were evaluated using Chi-square and visit counts were evaluated using Poisson regression.
HCUP-CCSR = Healthcare Cost and Utilization Project Clinical Classifications Software Refined.
3.5. Laboratory findings
Table 4 presents IBD-related laboratory measures during the baseline and follow-up periods. No statistical differences were observed among the IFX groups.
Table 4.
IBD-related laboratory measures during baseline and the 365-days follow-up period.
| Lab Results | IFX-RP | IFX-dyyb | IFX-abda | P values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | Mean ± SD | Median (IQR) | t | Mean ± SD | Median (IQR) | t | Mean ± SD | Median (IQR) | ||
| Number of patients | n = 785 | n = 441 | n = 537 | |||||||
| Liver function | ||||||||||
| Baseline ALT (U/L) | 750 | 27.17 ± 17.45 | 23 (16–33) | 430 | 26.86 ± 17.87 | 23 (15–33) | 526 | 29.73 ± 28.55 | 23 (16–34) | .532 |
| Follow-up ALT (U/L) | 747 | 29.75 ± 21.66 | 24 (17–36) | 423 | 32.63 ± 29.91 | 25 (16–37) | 512 | 30.68 ± 24.87 | 24 (16–36) | .805 |
| Baseline AST (U/L) | 744 | 22.52 ± 11.89 | 20 (15–26) | 433 | 22.18 ± 13.38 | 19 (15–25) | 529 | 23.19 ± 15.18 | 20 (15–25) | .495 |
| Follow-up AST (U/L) | 746 | 25.42 ± 14.65 | 22 (17–28) | 426 | 28.76 ± 34.98 | 22 (17–29) | 513 | 25.78 ± 15.92 | 22 (17–29) | .696 |
| IFX troughs | ||||||||||
| Follow-up IFX (mcg/mL) | 171 | 6.72 ± 8.48 | 3.97 (1–8.6) | 127 | 6.75 ± 8.15 | 4.38 (1.24–8.6) | 146 | 6.97 ± 7.77 | 4.05 (1.2–10.2) | .648 |
| Antibodies for IFX (ATI) | t | Yes | Detected of measured, % | t | Yes | Detected of measured, % | t | Yes | Detected of measured, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| ATI Detected | 246 | 119 | 48.4 | 183 | 86 | 47 | 240 | 110 | 45.8 | .854 |
| Inflammation measures | ||||||||||
| Baseline fecal calprotectin > 150 (µg/g) | 144 | 104 | 72 | 103 | 79 | 77 | 153 | 119 | 77 | .510 |
| Follow-up fecal calprotectin > 150 (µg/g) | 121 | 71 | 59 | 104 | 69 | 66 | 157 | 92 | 59 | .389 |
| Baseline lactoferrin | 38 | 32 | 84 | 28 | 25 | 89 | 29 | 27 | 93 | .522 |
| Follow-up lactoferrin | 12 | 8 | 67 | 16 | 12 | 75 | 14 | 11 | 79 | .782 |
| Baseline CRP > 5 mg/dL | 326 | 54 | 16.6 | 195 | 31 | 15.9 | 281 | 31 | 11 | .125 |
| Follow-up CRP > 5 mg/dL | 321 | 20 | 6.2 | 170 | 14 | 8.2 | 254 | 16 | 6.3 | .664 |
Note: Kruskal–Wallis test was used to analyze continuous labs, and categorical or dichotomous outcomes were evaluated using Chi-square.
CRP = C-reactive protein, t = number of patients tested.
3.6. Secondary analysis
Secondary analyses that include study results during the follow-up period of 183 days are available in the Supplemental File. See Appendix E, Supplemental Digital Content, http://links.lww.com/MD/N457 for IFX utilization during the 183-day follow-up period, Appendix F, Supplemental Digital Content, http://links.lww.com/MD/N457 for health care utilization during the 183-day follow-up period, and Appendix G, Supplemental Digital Content, http://links.lww.com/MD/N457 for IBD-related laboratory measures during the 183-day follow-up period. Results for IFX use, healthcare utilization, and laboratory measures of interest were similar when comparing the 183- and 365-day study intervals.
4. Discussion
In this National VHA study designed to describe the VHAs experience with IFX-biosimilars in a population of IBD patients who were previously naïve to IFX, we found that IFX-biosimilar use was well-established in the VHA with a small proportion of patients identified as naïve to IFX being initiated on IFX-RP since IFX-abda gained VANF approval in September 2018. In contrast, it took approximately 6 months for the biosimilar IFX-dyyb to achieve predominance in the IBD population after the VANF assignment in May 2017. The rapid IFX-abda adoption may represent increased comfort due to experience with biosimilar IFX among gastroenterologists, accumulating evidence in support of biosimilars, and swift and systematic action by VHA PBM to deploy and enforce a VANF compliance initiative.
Consistent with randomized trials and observational studies that found similarities in the experiences of IFX biosimilars and IFX-RP.[18–21,44,45] Notable differences in measures associated with IFX product adherence and persistence were, nevertheless, observed. We found that 41.5% of patients who were nonpersistent with index IFX-dyyb switched to IFX-abda, and approximately 40% of patients who were not persistent with IFX-RP switched to IFX biosimilars. In particular, IFX-abda demonstrated seemingly greater adherence and persistence than IFX-RP and IFX-dyyb; however, this difference appeared to be a result of formulary pressure to switch patients to the current VANF product (IFX-abda). Only 12% of patients who were not persistent with IFX-abda switched to another IFX product, indicating the reduced persistence with index IFX-RP and IFX-abda was due to formulary pressure to switch patients to IFX-abda. The VHA recommended that all IBD patients on IFX-RP and IFX-dyyb should be evaluated for a possible switch to IFX-abda due to cost savings; nevertheless, the treating physician retained the final decision to switch.[21] Even though statistical differences were identified with IFX utilization measures, they were not deemed clinically meaningful since they could be explained by formulary dynamics within the Veterans Affairs. This pressure to switch patients to the VANF product would affect measures related to adherence and persistence for index IFX-RP and IFX-dyyb. In addition, a high proportion of patients indexed on IFX-RP or IFX-dyyb remained on an IFX-product at the end of the 365 day follow-up period, when we accounted for switching among IFX products.
The healthcare utilization measures for gastroenterology services by outpatient gastroenterologist visits, ED visits, and inpatient admissions did not indicate differences in surveillance patterns among IFX biosimilars and IFX-RP. The similarity in ED visits and inpatient admissions among IFX products was a crude measure of potential safety and effectiveness concerns. The top 2 reasons for inpatient admissions across IFX products were regional enteritis and UC and gastrointestinal hemorrhage. Intestinal obstruction and ileus were the third or fourth cause of admission among IFX products. Additional examination of admissions did not identify systematic differences in patient experience across IFX products.
Optimizing outcomes in IBD requires rapid and sustained control of inflammation and disease remission. The current target is mucosal healing; however, the gold standard, endoscopy with histological confirmation, is insufficient for therapeutic monitoring due to its high cost and invasiveness.[46,47] FC has received much attention as a noninvasive biomarker to inform therapeutic and treatment optimization; however, validated thresholds to indicate clinical outcomes are variable and not well-established. During the study period, there was little consensus among IBD experts on how FC should be used in IBD treatment monitoring strategies and the range of FC associated with mucosal healing.[48–51] However, recent AGA guidelines address this issue by recommending a biomarker-based treatment monitoring strategy that considers FC threshold > 150 mg/g along with symptom severity and additional markers of inflammation to inform treatment and the need for endoscopic assessment.[36,37]
We found that providers were more likely to assess FC in patients indexed on IFX biosimilars, which may be explained by historical changes in the use of these biomarkers to monitor inflammatory activity rather than increased surveillance due to uncertainty in biosimilar effectiveness since the marginal differences were resolved when conditioning on study year. See Appendix H, Supplemental Digital Content, http://links.lww.com/MD/N457 in the online supplement for a summary of the number of patients with fecal biomarker tests at each interval in the study window.
Over the past 5 years, there has been a significant shift in the interest in laboratory surrogates of treatment response. However, the role of FC as a noninvasive biomarker of gut inflammation in this context was unclear and not formalized during the study window.[34,48–55] Now that AGA guidelines formalized the use of inflammatory biomarkers for treatment modification they will likely be ordered more routinely. These data can then be used to optimize treatment modification within a treat-to-target framework.[56,57]
Therapeutic drug monitoring of serum drug levels and antidrug antibodies is a principal means of elucidating the reasons for anti-TNF treatment failure and maximizing long-term response to these agents.[58] The association between serum IFX and clinical response in IBD is well-established.[32,33] This study did not observe meaningful differences in the average IFX trough concentration in patients measured during the 365 day follow-up, indicating similar performance between IFX-biosimilar and IFX-RP.
Immunogenicity is a well-established phenomenon common to most biologics, including IFX. The formation of anti-drug antibodies is associated with loss of clinical response and adverse effects.[32,33] ATI is associated with decreased clinical effectiveness and increased infusion-related reactions.[58,59] We found no meaningful differences in the proportion of ATI tests with detected antibodies during follow-up. Since this was a real-world study and the authors did not have control over measurement protocols, our ability to detect ATIs was dependent on the treating provider’s decision to conduct laboratory measures of ATIs, which ranged from 27% to 41% of the index group for IFX-abda and IFX-dyyb, respectively. Since ATI measurement was not routine in all patients, the decision to test may be influenced by a perceived lack of primary or secondary treatment response. This is a limitation of real-world data and applies to all laboratory biomarkers of treatment response, including measures of inflammation.
CRP is a nonspecific marker of inflammation. Although erythrocyte sedimentation rate measurements were common in our sample, they are not favored since they lack sensitivity to IBD activity compared to CRP.[54] For this reason, we only reported CRP values > 5 as a marker of inflammation. IFX was implicated in inducing elevated liver enzymes in IBD patients.[60] AST and ALTs were measured for most of our study population; however, the average values during follow-up were similar across index IFX products indicating patients had similar responses with biosimilar IFX.
5. Conclusion
Previous VHA studies evaluated the effectiveness of switching from IFX-RP to biosimilar IFX (predominately IFX-dyyb)[20,21]; however, this study is unique because we followed IFX- naïve initiators with IBD to describe index IFX product utilization, healthcare utilization, and laboratory measures during a follow-up period of 365 days. We found that IFX utilization and laboratory measures of biosimilar IFX were similar to IFX-RP. Differences observed in IFX utilizations were recognized as reactions to formulary dynamics and not patient or provider behaviors. Providers were more likely to assess fecal measures of gut inflammation in patients indexed on IFX biosimilars, which is explained by historical changes in the use of biomarkers to monitor inflammatory activity rather than increased surveillance due to uncertainty in biosimilar effectiveness. This study highlights the VHA experience of placing biosimilar IFX on the national formulary and may inform future strategies for addressing biosimilars as they continue to become available for usage.
Author contributions
Conceptualization: Jessica Walsh, Jessica Johnson, Grant W. Cannon, David Wu, Brian C. Sauer.
Data curation: Shardool Patel, Derek Pinnell, Shaobo Pei, Jorge Rojas, Anitha Rathod.
Formal analysis: Shardool Patel, Jessica Walsh, Shaobo Pei, Wei Chen, Jorge Rojas, Anitha Rathod, Grant W. Cannon.
Funding acquisition: Jessica Walsh, Grant W. Cannon, Brian C. Sauer.
Investigation: Shardool Patel, Jessica Walsh, Derek Pinnell, Jessica Johnson, Andrew Gawron, Jeffrey R. Curtis, Joshua F. Baker, Grant W. Cannon, Brian C. Sauer.
Methodology: Shardool Patel, Jessica Walsh, Derek Pinnell, Jessica Johnson, Grant W. Cannon, Brian C. Sauer.
Project administration: Shardool Patel, Jessica Walsh, Grant W. Cannon, Brian C. Sauer.
Resources: Brian C. Sauer.
Supervision: Grant W. Cannon, Brian C. Sauer.
Validation: Wei Chen.
Visualization: Wei Chen, Miao Lai.
Writing – original draft: Shardool Patel, Jessica Walsh, Jessica Johnson, Grant W. Cannon, Miao Lai, Brian C. Sauer.
Writing – review & editing: Shardool Patel, Jessica Walsh, Jessica Johnson, Andrew Gawron, Jeffrey R. Curtis, Joshua F. Baker, Grant W. Cannon, David Wu, Miao Lai, Brian C. Sauer.
Supplementary Material
Abbreviations:
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- ATI
- antibodies-to-infliximab
- BL
- baseline
- CD
- Crohn’s disease
- CDW
- Corporate Data Warehouse
- CI
- confidence interval
- CMS
- Centers for Medicare and Medicaid Services
- CRP
- C-reactive protein
- ED
- emergency department
- FC
- fecal calprotectin
- GI
- gastroenterology
- IBD
- inflammatory bowel disease
- ICD
- International Classification of Diseases
- ICU
- Intensive Care Unit
- IFX
- infliximab
- IQR
- interquartile range
- PBM
- Pharmacy Benefits Management
- PDC
- proportion of days covered
- RP
- reference product
- TNFi
- tumor necrosis factor inhibitor
- UC
- ulcerative colitis
- US
- United States
- VANF
- Veterans Affairs National Formulary
- VHA
- Veterans Health Administration
Work supported by an Investigator Initiated Research Project with Merck Research & Organon.
Merck & Co., Inc. & Organon were involved in reviewing the research plan and manuscript. Jessica Walsh and Brian C. Sauer received funding from Merck & Co., Inc. to study the use of infliximab in the Veterans Health Administration. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Salt Lake City Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. Derek Pinnell and Miao Lai were supported by the Veterans Affairs Advanced Fellowship Program with the Office of Academic Affiliations. Shardool Patel, Derek Pinnell, Shaobo Pei, Wei Chen, Jorge Rojas, Anitha Rathod, Jessica Johnson, Andrew Gawron, Grant W. Cannon, and Miao Lai declare they have no relevant financial interests. Jessica Walsh reports receiving research funding from AbbVie, Pfizer, and Merck and consultancy agreements with UCB, Janssen, Lilly, and Novartis. Joshua F. Baker reports consultancy agreements with or receiving honoraria from Bristol-Myers Squibb, Pfizer, Cumberland Pharma, and CorEvitas. Jeffrey R. Curtis reports consultancy agreements with or receiving honoraria from ACR, AbbVie, Amgen, ArthritisPower, Aqtual, Bendcare, BMS, CorEvitas, FASTER, GSK, Janssen, Labcorp, Lilly, Myriad, Novartis, Revero, Pfizer, Sanofi, Scipher, Setpoint, TNacity Blue Ocean, and UCB. David Wu is a former employee of Merck & Co., Inc.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Patel S, Walsh J, Pinnell D, Pei S, Chen W, Rojas J, Rathod A, Johnson J, Gawron A, Curtis JR, Baker JF, Cannon GW, Wu D, Lai M, Sauer BC. Real-world experience with biosimilar infliximab-adba and infliximab-dyyb among infliximab-naïve patients with inflammatory bowel disease in the Veterans Health Administration. Medicine 2024;103:37(e39476).
Contributor Information
Jessica Walsh, Email: Jessica.Johnson@hsc.utah.edu.
Derek Pinnell, Email: Derek.Pinnell@utah.edu.
Shaobo Pei, Email: shaobo.pei@utah.edu.
Wei Chen, Email: Wei.Chen@utah.edu.
Jorge Rojas, Email: Jorge.Rojas@hsc.utah.edu.
Anitha Rathod, Email: anitha.rathod@utah.edu.
Jessica Johnson, Email: Jessica.Johnson@hsc.utah.edu.
Andrew Gawron, Email: andrew.gawron@hsc.utah.edu.
Jeffrey R. Curtis, Email: jrcurtis@uabmc.edu.
Joshua F. Baker, Email: Joshua.Baker@pennmedicine.upenn.edu.
Grant W. Cannon, Email: Grant.Cannon@hsc.utah.edu.
David Wu, Email: dwwmd@hotmail.com.
Miao Lai, Email: Miao.Lai@pharm.utah.edu.
Brian C. Sauer, Email: brian.sauer@utah.edu.
References
- [1].Solberg IC, Vatn MH, Høie O, et al.; IBSEN Study Group. Clinical course in Crohn’s disease: results of a norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–8. [DOI] [PubMed] [Google Scholar]
- [2].Solberg IC, Lygren I, Jahnsen J, et al.; IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431–40. [DOI] [PubMed] [Google Scholar]
- [3].Monstad I, Hovde O, Solberg IC, Moum BA. Clinical course and prognosis in ulcerative colitis: results from population-based and observational studies. Ann Gastroenterol. 2014;27:95–104. [PMC free article] [PubMed] [Google Scholar]
- [4].Hovde O, Kempski-Monstad I, Småstuen MC, et al. Mortality and causes of death in Crohn’s disease: results from 20 years of follow-up in the IBSEN study. Gut. 2014;63:771–5. [DOI] [PubMed] [Google Scholar]
- [5].Høivik ML, Bernklev T, Solberg IC, et al.; IBSEN Study Group. Patients with Crohn’s disease experience reduced general health and vitality in the chronic stage: ten-year results from the IBSEN study. J Crohns Colitis. 2012;6:441–53. [DOI] [PubMed] [Google Scholar]
- [6].Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12:537–45. [DOI] [PubMed] [Google Scholar]
- [7].Annese V, Duricova D, Gower-Rousseau C, Jess T, Langholz E. Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the epidemiology committee of ECCO. J Crohns Colitis. 2016;10:216–25. [DOI] [PubMed] [Google Scholar]
- [8].Hlavaty T, Letkovsky J. Biosimilars in the therapy of inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2014;26:581–7. [DOI] [PubMed] [Google Scholar]
- [9].Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities? Front Pharmacol. 2019;10:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cannon GW, DuVall SL, Haroldsen CL, et al. Clinical outcomes and biologic costs of switching between tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. Adv Ther. 2016;33:1347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baker JF, Leonard CE, Re VL, Weisman MH, George MD, Kay J. Biosimilar uptake in academic and veterans health administration settings: influence of institutional incentives. Arthritis Rheumatol. 2020;72:1067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Talathi S, Baig KRKK. Biosimilars in inflammatory bowel disease. J Dig Dis. 2020;21:610–20. [DOI] [PubMed] [Google Scholar]
- [13].Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Solitano V, D’Amico F, Fiorino G, Peyrin-Biroulet L, Danese S. Biosimilar switching in inflammatory bowel disease: from evidence to clinical practice. Expert Rev Clin Immunol. 2020;16:1019–28. [DOI] [PubMed] [Google Scholar]
- [16].Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease—an update. J Crohns Colitis. 2017;11:26–34. [DOI] [PubMed] [Google Scholar]
- [17].Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393:1699–707. [DOI] [PubMed] [Google Scholar]
- [18].Jørgensen KK, Olsen IC, Goll GL, et al.; NOR-SWITCH study group. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389:2304–16. [DOI] [PubMed] [Google Scholar]
- [19].Jørgensen KK, Goll GL, Sexton J, et al. Efficacy and safety of CT-P13 in inflammatory bowel disease after switching from originator infliximab: exploratory analyses from the NOR-SWITCH main and extension trials. BioDrugs. 2020;34:681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ho SL, Niu F, Pola S, Velayos FS, Ning X, Hui RL. Effectiveness of switching from reference product infliximab to infliximab-Dyyb in patients with inflammatory bowel disease in an integrated healthcare system in the United States: a retrospective, propensity score-matched, non-inferiority cohort study. BioDrugs. 2020;34:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khan N, Patel D, Pernes T, et al. The efficacy and safety of switching from originator infliximab to single or double switch biosimilar among a nationwide cohort of inflammatory bowel disease patients. Crohns Colitis 360. 2021;3:otab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Smith JT, Velayos FS, Niu F, et al. Retrospective cohort study comparing infliximab-dyyb and infliximab in biologic-naive patients with inflammatory bowel disease in the United States. Crohns Colitis 360. 2021;3:otab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bhat S, Limdi JK, Cross RK, Farraye FA. Does similarity breed contempt? A review of the use of biosimilars in inflammatory bowel disease. Dig Dis Sci. 2021;66:2513–32. [DOI] [PubMed] [Google Scholar]
- [24].Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the veterans health administration. Health Aff (Millwood). 2014;33:1203–11. [DOI] [PubMed] [Google Scholar]
- [25].Sauer B, Teng C, Burningham Z, Cannon G. Errata to NLP study of infusion notes to identify outpatient infusions in the VA. Pharmacoepidemiol Drug Saf. 2015;24:1225–6. [DOI] [PubMed] [Google Scholar]
- [26].Nelson SD, Lu CC, Teng CC, et al. The use of natural language processing of infusion notes to identify outpatient infusions. Pharmacoepidemiol Drug Saf. 2014;24:86–92. [DOI] [PubMed] [Google Scholar]
- [27].Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22:1–6. [DOI] [PubMed] [Google Scholar]
- [28].Sauer BC, Teng CC, He T, Leng J, Lu CC. Effectiveness and costs of biologics in veterans with rheumatoid arthritis. Am J Pharm Benefits. 2015;7:280. [Google Scholar]
- [29].Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2016;40:1280–8. [DOI] [PubMed] [Google Scholar]
- [30].Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–61. [DOI] [PubMed] [Google Scholar]
- [31].HCUP. Clinical Classifications Software Refined (CCSR)for ICD-10-CM Diagnoses. Healthcare Cost and Utilization Project (HCUP). Clinical Classifications Software Refined (CCSR)for ICD-10-CM Diagnoses. Healthcare Cost and Utilization Project (HCUP). Published March 1, 2021. Available at: https://hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp. Accessed July 26, 2021. [Google Scholar]
- [32].Melmed GY, Irving PM, Jones J, et al. Appropriateness of testing for anti–tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin Gastroenterol Hepatol. 2016;14:1302–9. [DOI] [PubMed] [Google Scholar]
- [33].Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1655–68.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patel V, Seif S, Barrett T. 2523 Noninvasive biomarkers for inflammatory bowel disease: drawbacks and potential. J Clin Transl Sci. 2018;2(Suppl 1):22. [Google Scholar]
- [35].Hauser RG, Quine DB, Ryder A. LabRS: a Rosetta stone for retrospective standardization of clinical laboratory test results. J Am Med Inform Assoc. 2017;25:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ananthakrishnan AN, Adler J, Chachu KA, et al.; AGA Clinical Guidelines Committee. AGA clinical practice guideline on the role of biomarkers for the management of Crohn’s disease. Gastroenterology. 2023;165:1367–99. [DOI] [PubMed] [Google Scholar]
- [37].Singh S, Ananthakrishnan AN, Nguyen NH, et al.; AGA Clinical Guidelines Committee. AGA clinical practice guideline on the role of biomarkers for the management of ulcerative colitis. Gastroenterology. 2023;164:344–72. [DOI] [PubMed] [Google Scholar]
- [38].Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–34. [DOI] [PubMed] [Google Scholar]
- [39].Gonsoulin M. Using SQL to “Sort Out” Race in CDW: A Method for Cleaning Multiple Values of Race. In: The Researchers Notebook. VA Information Resource Center; 2016. [Google Scholar]
- [40].McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Golden SE, Hooker ER, Shull S, et al. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J. 2019;26:1507–15. [DOI] [PubMed] [Google Scholar]
- [42].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [43].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- [44].Fiorino G, Manetti N, Armuzzi A, et al.; PROSIT-BIO Cohort. The PROSIT-BIO Cohort. Inflamm Bowel Dis. 2017;23:233–43. [DOI] [PubMed] [Google Scholar]
- [45].Milassin A, Fábián A, Molnár T. Switching from infliximab to biosimilar in inflammatory bowel disease: overview of the literature and perspective. Therap Adv Gastroenterol. 2019;12:1756284819842748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Iacucci M, Ghosh S. Looking beyond symptom relief: evolution of mucosal healing in inflammatory bowel disease. Therap Adv Gastroenterol. 2011;4:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Abreu MT, Sandborn WJ; IOIBD Defining Endpoints and Biomarkers in Inflammatory Bowel Disease Writing Group. Defining endpoints and biomarkers in inflammatory bowel disease: moving the needle through clinical trial design. Gastroenterology. 2020;159:2013–8.e7. [DOI] [PubMed] [Google Scholar]
- [48].Jusué V, Chaparro M, Gisbert JP. Accuracy of fecal calprotectin for the prediction of endoscopic activity in patients with inflammatory bowel disease. Dig Liver Dis. 2018;50:353–9. [DOI] [PubMed] [Google Scholar]
- [49].Reinisch W, Panaccione R, Bossuyt P, et al. Association of biomarker cutoffs and endoscopic outcomes in Crohn’s disease: a post hoc analysis from the CALM study. Inflamm Bowel Dis. 2020;26:1562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Musci JOD, Cornish JS, Däbritz J. Utility of surrogate markers for the prediction of relapses in inflammatory bowel diseases. J Gastroenterol. 2016;51:531–47. [DOI] [PubMed] [Google Scholar]
- [51].Dai C, Cao Q, Jiang M. Accuracy of consecutive fecal calprotectin measurements to predict relapse in inflammatory bowel disease patients. J Clin Gastroenterol. 2019;53:314. [DOI] [PubMed] [Google Scholar]
- [52].Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kato J, Yoshida T, Hiraoka S. Prediction of treatment outcome and relapse in inflammatory bowel disease. Expert Rev Clin Immunol. 2019;15:667–77. [DOI] [PubMed] [Google Scholar]
- [54].Lichtenstein GR, McGovern DPB. Using markers in IBD to predict disease and treatment outcomes: rationale and a review of current status. Am J Gastroenterol Suppl. 2016;3:17–26. [Google Scholar]
- [55].Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta-analysis. J Gastrointestin Liver Dis. 2018;27:299–306. [DOI] [PubMed] [Google Scholar]
- [56].Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1042–50.e2. [DOI] [PubMed] [Google Scholar]
- [57].Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- [58].Casteele NV, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8. [DOI] [PubMed] [Google Scholar]
- [60].Parisi I, O’Beirne J, Rossi RE, et al. Elevated liver enzymes in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2016;28:786–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.