Abstract
Background:
Dahuang Mudan Decoction is commonly used in China for the treatment of acute pancreatitis. Nevertheless, the therapeutic efficacy of the drug remains a subject of debate, and its active ingredients and potential therapeutic targets remain to be determined. The present study used a network pharmacological approach to investigate the active ingredients and possible targets of the drug, and illustrated the clinical effectiveness of Dahuang Mudan Decoction in the treatment of acute pancreatitis by meta-analysis.
Methods:
The present study investigated the active ingredients of the constituent herbs of Dahuang Mudan Decoction using the TCMID database. In order to further identify molecular targets, Swiss Target Prediction, OMIM and Genecards databases was be used. The present study used metascape database for gene ontology function enrichment analysis and Kyoto Genome Encyclopedia pathway enrichment analysis. A gene interaction network diagram was established for predicting the main targets and mechanism of action to Dahuang Mudan Decoction for acute pancreatitis. To further illustrate the validity of the gene targets and the clinical efficacy of the drug, 13 relevant studies were included for meta-analysis and analyzed using the Cochrane Collaboration’s Review Manager 5.4 software.
Result:
After a thorough screening process, the present study identified three main components of Dahuang Mudan Decoction: kaempferol, quercetin and eupatin. These three major components have the potential to target 5 important proteins: AKT1, TNF-a, IL-6, TP53, HIF1A. In addition, pathway analyses by the Kyoto Genome Encyclopedia showed that Dahuang Mudan Decoction is active through the Pathways in cancer, AGE-RAGE signaling pathway in diabetic complications, PI3K-Akt signaling pathway, etc signaling pathway to act on acute pancreatitis. The results of meta-analysis showed that compared with the control group, the experimental group had superior performance in terms of overall treatment efficacy, reduction of hospital stays and inflammatory factor levels after treatment.
Conclusion:
In summary, network pharmacological studies have shown that Dahuang Mudan Decoction affects acute pancreatitis through different components, targets, and mechanisms. In addition, the meta-analysis study strongly supported the effectiveness of Dahuang Mudan Decoction in the treatment of acute pancreatitis.
Keywords: acute pancreatitis, Dahuang Mudan Decoction, evidence-based medicine, meta-analysis, network pharmacology
1. Introduction
Acute pancreatitis develops due to the abnormal activation of pancreatic enzymes in the pancreas, leading to digestive effects in the gland itself and surrounding tissues and organs. It is characterized by a localized inflammatory response in the pancreas as the primary feature, and in severe cases, it leads to organ dysfunction and clinical manifestations of acute abdomen.[1] In most mild cases, clinical remission is efficient with active treatment such as appropriate fluid resuscitation, pain and nausea control, etc. When acute pancreatitis develops into severe pancreatitis or progresses into chronic pancreatitis, it seriously jeopardizes the life and health of the patient. According to the relevant research department, the incidence of this disease is ranked as the second most common digestive disease, second only to appendicitis, and the global incidence of acute pancreatitis is increasing year by year.[2,3]
Dahuang Mudan Decoction is from “The Essentials of the Golden Chamber” by Zhang Zhongjing of the Eastern Han Dynasty. The formula includes Dahuang, which clears fire and expels blood stasis, acts as a laxative, and detoxifies. Mudan Pi cools blood and clears heat, promotes blood circulation, and dissipates blood stasis. The combination is effective for diarrhea caused by damp-heat stagnation in the intestines and bowels. Mangxiao disperses solid masses, aiding Dahuang in clearing solid heat. Taoren can activate blood and resolve stasis. Donggua Ren can induce diuresis to drain dampness, drains pus and eliminates carbuncles. The whole formula helps clear the intestines, eliminate carbuncles, and thus stops the pain.[4]
Dahuang Mudan Decoction is commonly used in clinical practice to treat abdominal symptoms, inflammatory aggregation, and patient prognosis caused by acute pancreatitis.[5] A large number of clinical and experimental studies have proven that Dahuang Mudan Decoction has good anti-inflammatory, decongestive, immune-regulating, and blood circulation-improving effects. However, the molecular mechanism of Dahuang Mudan Decoction in the treatment of acute pancreatitis is still unclear, which limits its promotion and development.[6]
Network pharmacology is a valuable tool for analyzing the complex relationships between drug components, protein targets, diseases and genes. This method has been widely used in the analysis of Chinese medicinal preparations. The present study used network pharmacology to select the active ingredients of Dahuang Mudan Decoction, identify its targets, and analyze the genes involved in the treatment of acute pancreatitis. The aim of the study was to investigate the potential links between the active ingredients of Dahuang Mudan Decoction and the targets of acute pancreatitis using network pharmacology. Meanwhile, we conducted a meta-analysis from the perspective of evidence-based medicine to validate the efficacy of Dahuang Mudan Decoction in the treatment of acute pancreatitis.[7,8]
2. Materials and methods
2.1. Network pharmacology analysis
2.1.1. Screening the active compounds
Drug potential active ingredient collection and target prediction: Dahuang Mudan Decoction contains 5 traditional Chinese medicines (Dahang, Mudan Pi, Taoren, Donggua Ren, and Mangxiao). Through the TCMSP (https://old.tcmsp-e.com/tcmsp.php) platform, oral bioavailability (OB) ≥ 0.3 and drug-like properties index (DL) ≥ 0.18 were used as the boundaries to screen the main active ingredients and targets of Dahuang Mudan Decoction. Based on the active ingredients and target genes of the TCMs screened from TCMSP, and supplemented accordingly by Swiss Target Prediction database (https://swisstargetprediction.ch/). At the same time, all the target genes queried in the database were organized and de-weighted.
2.1.2. Establishment of a database between disease and targets
The target genes involved in the acute pancreatitis process were obtained from 2 databases: Gene Cards (https://www.genecards.org/), which contains a variety of biological information such as genomic, transcriptomic, proteomic, and genetic information. OMIM (https://www.omim.org/), Online catalogue of human genes and genetic diseases.
2.1.3. Construction of the PPI network
The functional relationship between the ingredients in Dahuang Mudan Decoction and the targets involved in acute pancreatitis were visualized as a network using Cytoscape (version 3.7.1; https://www.cytoscape.org/). The nodes in the network represented the active constituents and the targets, and the edges represented the interactions between the active constituents and the targets.
Further, the interaction between these target proteins was shown as a network using an open-source online database, STRING (https://string-db.org/), which integrates a large number of known or predicted protein-protein interaction information in various organisms. Specifically, the ingredients in Dahuang Mudan Decoction and the targets involved in alopecia were uploaded to the STRING database (screened with confidence score > 0.4), and the study species were limited to“Homo sapiens.” Then the protein interaction relationships were downloaded as a TSV format, which could be visualized as the protein-protein interaction (PPI) network with Cytoscape. In the PPI network, the nodes represented proteins, and the edges represented the interactions.
2.1.4. Bioinformatic annotation
The gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were applied for the pathway enrichment analysis and the functional annotation analysis (https://metascape.org/gp/index.html). GO enrichment analysis included three categories: cellular component, biological process, and molecular function. Finally, the analytic results were represented with bar graphs, which were performed with an online bioinformatics tool (http://www.bioinformatics.com.cn/). In this study, P < .05 was considered statistically significant.
2.2. Meta-analysis
This study has been registered on PROSPERO. The registration number is CRD42024508770. Ethical approval is unnecessary because this is a literature-based study.
2.2.1. Databases and search strategies
This study searched PubMed, Web of Science, Cochrane library, Wan Fang, VIP, and CNKI databases and the China Clinical Research Registry for clinical randomized controlled studies of previous applications of Dahuang Mudan Decoction for acute pancreatitis. Search terms included “Dahuang Mudan Decoction,” “Dahuang Mudanpitang,” “Dahuang Mudan Decoction Plus Reduction,” “Pancreatitis,” “AP,” “Severe Pancreatitis,” “Randomised Controlled Trial,” “Clinical Controlled Trial,” “Placebo,” “Clinical Trial,” “Controlled Trial,” and so on. Search formula: (“Dahuang Mudan Decoction” OR “Dahuang Mudanpi tang” OR “Dahuang Mudan Decoction Plus Reduction”) AND (“Pancreatitis” OR “acute pancreatitis” OR “Severe Pancreatitis”)
2.2.2. Inclusion and exclusion criteria
Literatures: All papers were researched to the efficacy and safety of Dahuang Mudan Decoction in the treatment of acute pancreatitis with no language as well as time constraints. Participants: Patients with acute pancreatitis; denial of severe cardiac, pulmonary, hepatic, or renal insufficiency; allergy to Dahuang Mudan Decoction herbs and other herbal preparations; and no use of antibiotics or other medications for pancreatitis in the 2 weeks prior to treatment, and no comorbid serious infections. Intervention: There is no restriction on the mode of administration of Dahuang Mudan Decoction for acute pancreatitis, including oral intake and retention enema. There is no restriction on the therapeutic dose and duration of treatment. The control group includes conventional western medicine treatment or blood purification for acute pancreatitis, and the experimental group includes the addition of Dahuang Mudan Decoction or Dahuang Mudan Decoction plus reduction to the treatment plan of the control group. In addition, other symptom supportive treatments were not limited in both groups. Exclusion criteria: Case reports; animal studies; basic research; personal experience and review articles; incomplete data; duplication of literature; baseline conditions not assessed, etc.
2.2.3. Primary outcome indicators and secondary indicators
Primary outcome indicators include overall treatment efficacy, secondary outcome indicators include length of hospital stay, improved levels of inflammatory factors.
2.2.4. Risk of bias assessment
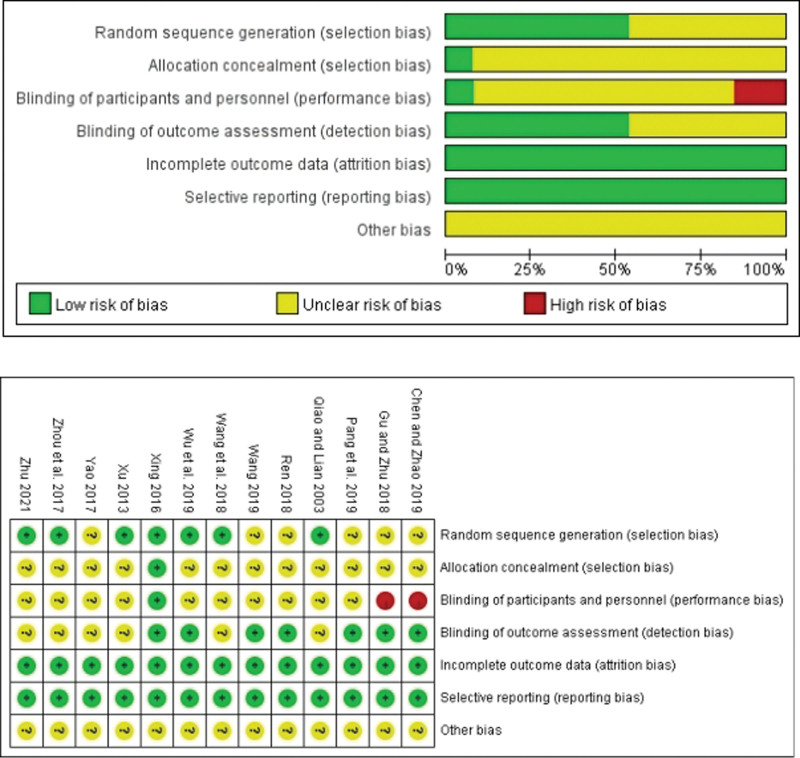
Two authors independently assessed the risk of bias as described in the Cochrane Handbook for the systematic review of interventions. We categorized potential bias as high, low or unclear. The following items were assessed: Random sequence generation, Allocation concealment, Blinding of participants and personnel, Blinding of outcome assessment, Incomplete outcome data, Selective reporting, other biases.
2.2.5. Research selection and statistic collection
After excluding duplicate publications, both authors first screened articles based on title and abstract. Full-text versions of papers that meet the inclusion criteria are then retained, and data on patient characteristics, treatment details and clinical outcomes are extracted independently. Disagreements, if any, will be resolved by a third author.
2.2.6. Statistical analysis and data synthesis
Review Manager 5.4 software provided by Cochrance Collaboration Network was used for meta-analysis. The counting data were expressed by Risk ratio (RR) and 95% confidence interval (95% CI). The continuous variable data were expressed by weighted mean difference (WMD) and 95% CI, and the test level was P < .05. I2 was used to analyze the heterogeneity of the included studies. If I2 ≤ 50%, P > .05, there was no heterogeneity, and fixed effect model was used. If I2 ≥ 50%, P < .05, If I2 ≥ 50%, P < .05, heterogeneity was considered and random effect model was used. Standard deviations and potential errors in labeling for certain indicators were calculated using methods provided by Cochrane.[9] We further analyze the source of heterogeneity, if the heterogeneity is too obvious, descriptive analysis is used. Funnel plot was used to test potential publication bias.
3. Results
3.1. Screening of the active ingredients
According to the TCMSP database search, 44 active ingredients of traditional Chinese medicines were obtained with OB ≥ 0.30 and DL ≥ 0.18 as the critical values. The TCMSP search data and the active ingredient targets queried in Swiss Target Prediction database were combined and de-emphasized after standardized annotation in Uniprot database, eventually a total of 628 targets were obtained.
3.2. Acute pancreatitis target search and intersection target acquisition
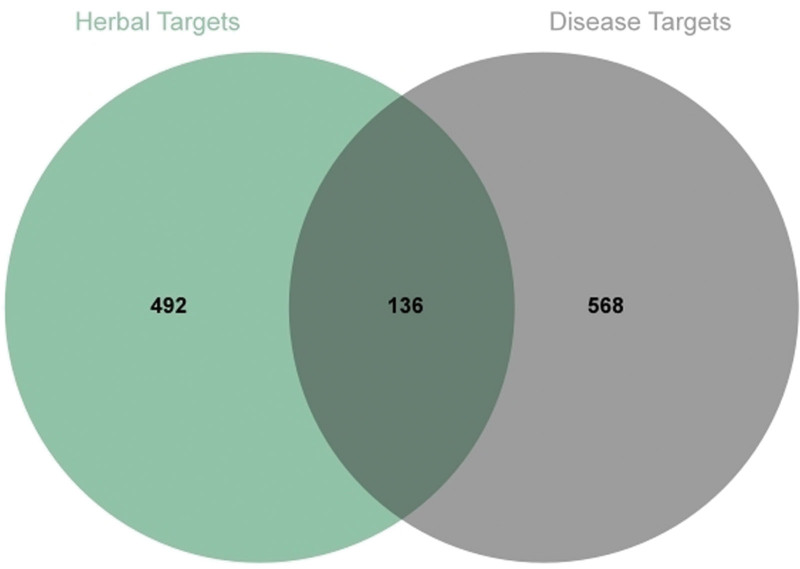
704 potential therapeutic targets were collected from OMIM, DrugBank, and GeneCards databases, which were intersected with the targets of traditional Chinese medicines, and 136 intersected targets were obtained by plotting the Venn diagram (Fig. 1).
Figure 1.
Venn diagram for screening of Chinese her ingredient targets and disease targets.
3.3. TCM active ingredient-target network construction
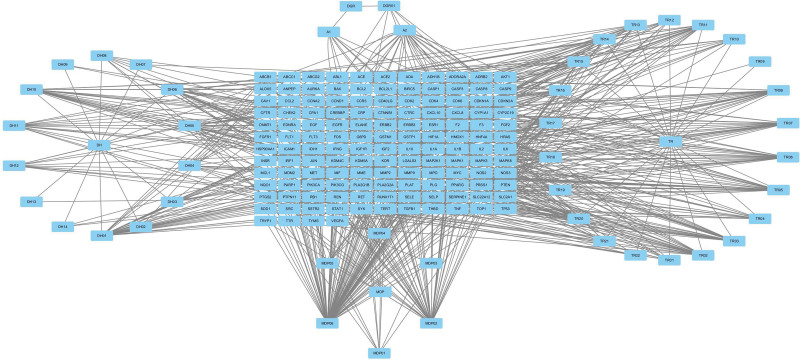
Using Cytoscape 3.7.2 software, we constructed a complex network diagram linking the components of traditional Chinese medicines with the intersection targets. Detailed compounds have been listed in order (Table 1). Statistically, the active ingredients with higher degrees include kaempferol, quercetin, and eupatin, which can be hypothesized to be key compounds primarily involved in the treatment of acute pancreatitis (Fig. 2).
Table 1.
Chemical composition of Dahuang Mudan Decoction; DH01-DH14 represent the 14 active ingredients of Dahuang; MDP01-MDP06 represent the 6 active ingredients of Mudan Pi; TR01-TR22 represent the 22 active ingredients of Taoren; A1 and A2 represent the ingredients common to Dahuang Mudan Decoction.
| Number | Ingredient name | Degree | Number | Ingredient name | Degree |
|---|---|---|---|---|---|
| DH01 | Eupatin | 30 | TR03 | 2,3-didehydro Gallic acid 77 | 20 |
| DH02 | Mutatochrome | 2 | TR04 | Gallic acid119 | 13 |
| DH03 | Physciondiglucoside | 7 | TR05 | Gallic acid 120 | 24 |
| DH04 | Procyanidin B-5,3’-O-gallate | 14 | TR06 | Gallic acid 121-isolactone | 20 |
| DH05 | rhein | 19 | TR07 | Gallic acid 122 | 19 |
| DH06 | Sennoside E_qt | 23 | TR08 | Gallic acid 122-isolactone | 20 |
| DH07 | Torachrysone-8-O-beta-D-(6’-oxayl)-glucoside | 2 | TR09 | gibberellin 17 | 2 |
| DH08 | Toralactone | 9 | TR10 | 4a-formyl-7alpha-hydroxy-1-methyl-8-methylidene-4aalpha,4bbeta-gibbane-1alpha,10beta-dicarboxylic acid | 9 |
| DH09 | Emodin-1-O-beta-D-glucopyranoside | 3 | TR11 | Gallic acid 30 | 21 |
| DH10 | Sennoside D_qt | 23 | TR12 | Gibberellin A44 | 11 |
| DH11 | palmidin A | 14 | TR13 | Gallic acid 54 | 15 |
| DH12 | aloe-emodin | 10 | TR14 | Gallic acid 60 | 13 |
| DH13 | gallic acid-3-O-(6’-O-galloyl)-glucoside | 2 | TR15 | Gallic acid 63 | 17 |
| DH14 | (-)-catechin | 4 | TR16 | gibberellin 7 | 18 |
| MDP01 | Mairin | 9 | TR17 | Gallic acid 77 | 15 |
| MDP02 | kaempferol | 47 | TR18 | Gallic acid 87 | 16 |
| MDP03 | (+)-catechin | 3 | TR19 | 3-O-p-coumaroylquinic acid | 7 |
| MDP04 | benzoyl paeoniflorin | 16 | TR20 | Populoside_qt | 20 |
| MDP05 | 5-[[5-(4-methoxyphenyl)-2-furyl]methylene]barbituric acid | 9 | TR21 | hederagenin | 14 |
| MDP06 | quercetin | 85 | TR22 | campesterol | 4 |
| TR01 | Sitosterol alpha1 | 10 | DGR01 | Stigmasterol | 7 |
| TR02 | 2,3-didehydro GA70 | 21 | A2 | beta-sitosterol | 28 |
| A1 | sitosterol | 10 |
Figure 2.
Construction of the compounds-target visual network of acute pancreatitis.
3.4. PPI network construction
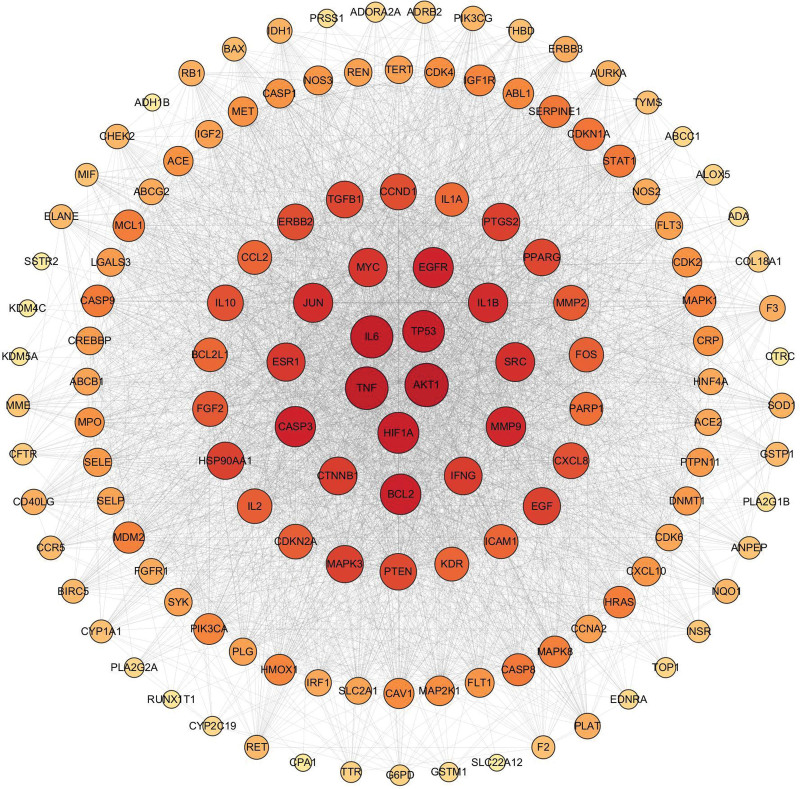
The intersecting targets were formed into a PPI network by STRING11.0 software (Fig. 3). Among them, AKT1, TNF, IL-6, TP53, HIF1A, BCL2, CASP3, EGFR, MMP9, IL1B has a more advanced degree ordering, so they are considered to be important targets on the protein interactions system (Table 2).
Figure 3.
A diagram of the interactions between the active ingredient targets, where darker colors indicate that the targets have more complex interactions and may play a greater role in the treatment.
Table 2.
The top 10 key targets of the degree value.
| Gene name | Protein name | Degree |
|---|---|---|
| AKT1 | AKT serine/threonine kinase 1 | 114 |
| TNF | Tumor Necrosis Factor | 111 |
| IL6 | Interleukin 6 | 109 |
| TP53 | Tumor protein p53 | 107 |
| HIF1A | Hypoxia-inducible factor 1-alpha | 104 |
| BCL2 | B-cell lymphoma-2 | 102 |
| CASP3 | Caspase 3 | 101 |
| EGFR | Epidermal growth factor receptor | 100 |
| MMP9 | Matrix metallopeptidase 9 | 98 |
| IL1B | Interleukin-1 beta | 97 |
3.5. Analysis of the biological functions and pathways of key target genes
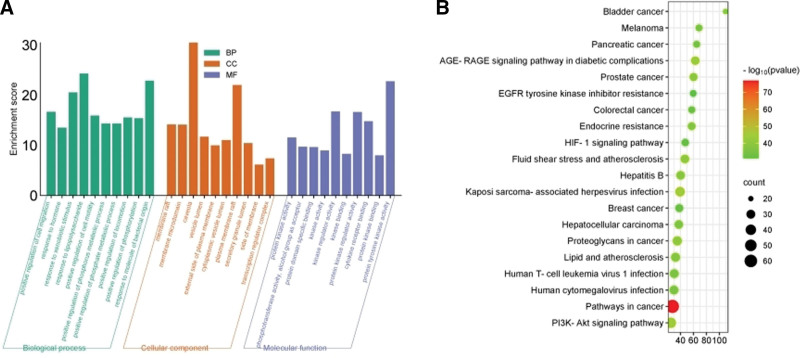
GO function enrichment analysis method was performed, yielding 1909 biological processes, 89 cellular components, and 172 molecular functions. Biological processes mainly include positive regulation of cell migration, response to hormones, and response to xenobiotic stimulus, among others. Cellular components mainly include membrane rafts, membrane microdomains and, and caveolae. Molecular functions mainly involve protein kinase activity, phosphotransferase activity, alcohol group as an acceptor, and protein domain specific binding etc (Fig. 4A). KEGG pathway enrichment analysis was conducted on the screened target proteins, resulting in a total of 195 enrichment outcomes. KEGG enrichment analysis of the key protein modules was performed to draw bubble diagrams of the top 20 pathways with the relevant pathway P values, in which the intersecting targets mainly acted on Pathways in cancer, Kaposi sarcoma-associated herpesvirus infection, PI3K-Akt signaling pathway and AGE-RAGE signaling pathway in diabetic complications, Fluid shear stress and atherosclerosis Proteoglycans in cancer (Fig. 4B).
Figure 4.
(A) GO enrichment analysis of cross genes between Dahuang Mudan Decoction and acute pancreatitis; (B) KEGG enrichment analysis of cross genes between Dahuang Mudan Decoction and acute pancreatitis. BP = biological process, CC = cell composition, GO = Gene ontology, KEGG = Kyoto encyclopedia of genes and genomes, MF = molecular function.
3.6. Incorporation of literature statistics and basic information
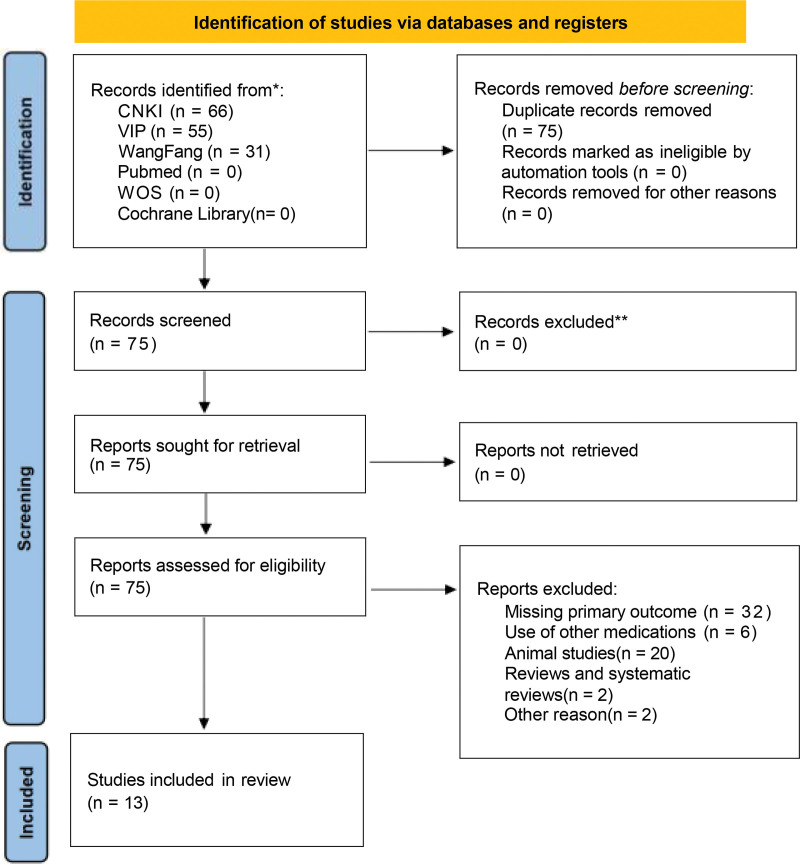
As depicted in the flow chart of the meta-analysis, we conducted a comprehensive search strategy across 7 databases and the Chinese Clinical Research Registry. Initially, 152 relevant papers were identified. After excluding 2 reviews, 20 animal studies, 6 papers that did not align with the research objectives or diseases, and 32 papers lacking primary outcome data, a total of 13 randomized controlled trials were included in this study[10–22] (Fig. 5). Baseline characteristics between the treatment and control groups were comparable across the trials. Eleven studies assessed overall treatment effectiveness, 8 studies evaluated improvement in inflammatory factors, and 4 studies measured hospitalization duration. Risk assessments for study inclusion were completed (Fig. 6). Basic information about the included studies was also summarized (Table 3).
Figure 5.
Study flow diagram for patient inclusion, screening, and final cohort selected for analysis.
Figure 6.
Cochrane collaboration risk of bias summary: evaluation of bias risk items for each included study.
Table 3.
Basic information about the included studies.
| Author/year | Number of case | Sex (Men/Women) | Age | EG | CG | Outcome indicator | |||
|---|---|---|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | ||||
| Chen and Zhao 2019[10] | 90 | 90 | 43/47 | 52/38 | 52.5 ± 6.9 | 52.5 ± 2.1 | A + C | D | a + b |
| Gu and Zhu 2018[11] | 29 | 29 | 18/11 | 21/8 | 54.27 ± 12.31 | 55.14 ± 10.72 | A + F | F | b |
| Pang, et al 2019[12] | 63 | 63 | 27/36 | 30/33 | 55.10 ± 3.10 | 55.30 ± 2.48 | A + D | D | d |
| Qiao and Lian 2003[13] | 35 | 23 | 14/21 | 14/11 | 48.5 ± 7.5 | 46.5 ± 9.5 | A + D | D | b |
| Ren 2018[14] | 40 | 40 | 29/11 | 28/12 | 54.34 ± 12.42 | 54.27 ± 12.38 | B + D | D | a |
| Wang, et al 2018[15] | 43 | 43 | 23/20 | 26/17 | 52.85 ± 11.40 | 50.27 ± 12.85 | A + F + D | F + D | a + c + d |
| Wang 2019[16] | 37 | 37 | 20/17 | 21/16 | 43.12 ± 1.12 | 43.15 ± 1.14 | B + D | D | a + c + d |
| Wu, et al 2019[17] | 29 | 29 | 18/11 | 17/12 | 50.7 ± 13.9 | 50.6 ± 13.8 | B + D | D | a |
| Xing 2016[18] | 50 | 50 | 26/24 | 31/19 | 36.38 ± 14.05 | 37.65 ± 13.14 | A + D | D | a + c + d |
| Xu 2013[19] | 30 | 30 | 18/12 | 19/11 | 22.10 ± 11.12 | 21.15 ± 12.35 | A + D | D | a |
| Yao 2017[20] | 40 | 40 | 21/19 | 20/20 | 36.4 ± 14.1 | 37.2 ± 14.0 | A + F | F | a + b |
| Zhou, et al 2017[21] | 65 | 65 | 39/26 | 40/25 | 48.65 ± 9.64 | 48.97 ± 8.67 | B + D | D | a + c |
| Zhu 2021[22] | 33 | 33 | 19/14 | 18/15 | 48.29 ± 5.64 | 48.91 ± 5.69 | A + D | D | a + c |
A: Chinese medicine retention enema; B: Oral or nasal administration of Chinese medicines; C: Somatostatin; D: Conventional treatment with western-style medicine; F: blood purification therapy; a: Overall response rate; b: Length of hospital stays; c: Serum TNF-a levels; d: Serum IL-6 levels.
CG = control group, EG = experimental group.
3.7. Overall response rate
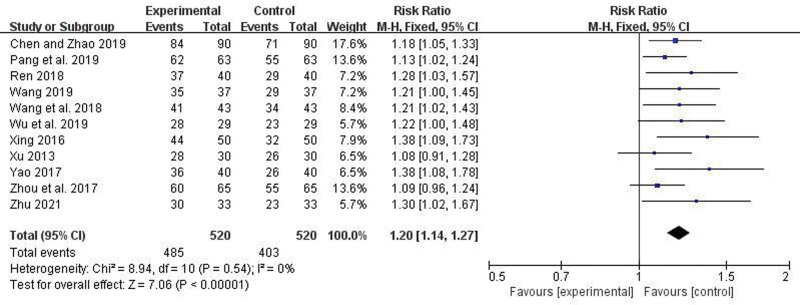
Eleven articles reported the overall response rate. There was no heterogeneity among the studies (I2 = 0%, P = .54), so a fixed-effect model was used. Meta-analysis indicated a statistically significant higher overall response rate in the trial group compared to the control group (RR = 1.20, 95% CI = 1.14–1.27, P < .00001) (Fig. 7).
Figure 7.
Forest plot comparing overall response rates between the 2 groups.
3.8. Length of hospital stays and level of improvement in inflammatory factors
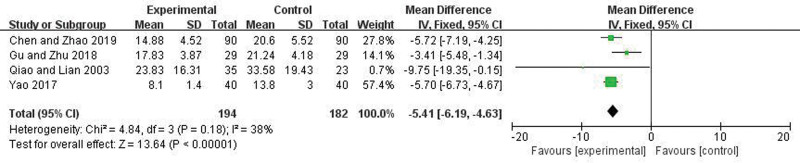
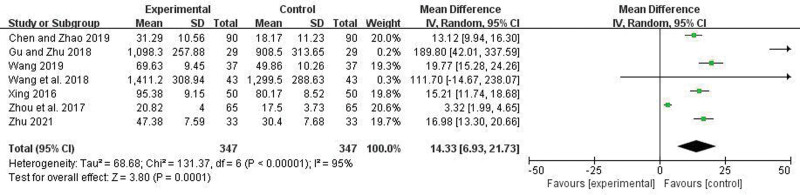
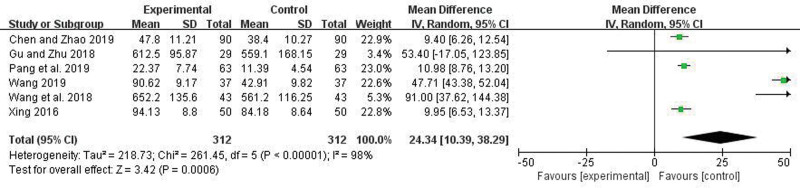
Four articles reported the Length of hospital stays. There was no heterogeneity among the studies (I2 = 38%, P = .18), so a fixed-effect model was applied. Meta-analysis showed that the hospitalization duration in the trial group was significantly less than in the control group, and the difference was statistically significant (OR = −5.41, 95% CI = −6.19 to −4.63, P < .00001) (Fig. 8). Seven articles reported the degree of improvement in TNF-a. There were heterogeneity among the studies (I2 = 95%, P < .00001), random effect model was used. Meta-analysis showed that the improved level of TNF-a in the trial group was more significant compared to the control group, and the difference was statistically significant (WMD = 14.33, 95% CI = 6.93–21.73, P = .0001) (Fig. 9). Six articles reported the degree of improvement in IL-6. There were heterogeneity among the studies (I2 = 98%, P < .00001), random effect model was used. Meta-analysis showed that the improved level of IL-6 in the trial group was more significant compared to the control group, and the difference was statistically significant (WMD = 24.34, 95% CI = 10.39–38.29, P = .0006) (Fig. 10).
Figure 8.
Forest plot comparing Length of hospitalization between the 2 groups.
Figure 9.
Forest plot comparing TNF-a between the 2 groups.
Figure 10.
Forest plot comparing IL-6 between the 2 groups.
3.9. Publication bias analysis
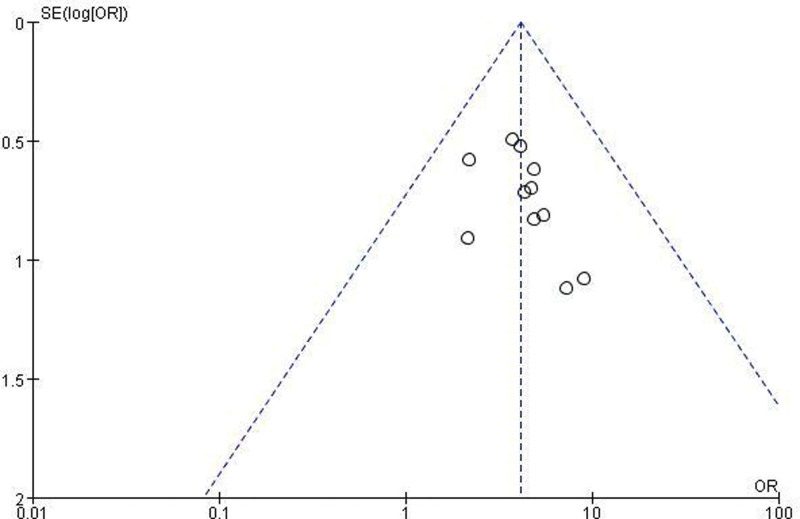
A funnel plot analysis was conducted to assess publication bias in the overall response rate. The plot exhibited good symmetry with scattered points evenly distributed within the funnel, indicating minimal influence of publication bias on the meta-analysis results (Fig. 11).
Figure 11.
Funnel plot of all the included studies for assessing publication bias.
4. Discussion
As a common emergency in the emergency department, the effectiveness and timeliness of treatment for acute pancreatitis significantly influence patient prognosis. In particular, the risks associated with severe pancreatitis are currently receiving significant attention. There is a growing interest in the use of traditional herbal medicines and extracorporeal therapies. Studies have shown that Chinese herbal medicines have several advantages in the treatment of acute pancreatitis.[23] Pancreatic injury is closely related to disturbances in intestinal function. A study on Chinese herbal recipe containing Dahuang showed that the use of Chinese herbs resulted in better recovery of intestinal function in patients with severe acute pancreatitis, as well as greater relief of their level of abdominal pain.[24] Chinese herbal medicine restores the intestinal mucosal barrier and modulates protein kinase C-mediated calcium release from colonic smooth muscle in the intestines to regulate peristalsis and simultaneously clear intestinal toxins.[25] Studies have shown that herbal enemas are also effective in the treatment of patients with acute pancreatitis.[26] The treatment of acute pancreatitis with retained enemas of Dahuang Mudan Decoction is centered on activating blood, resolving stasis, clearing heat, and draining dampness, which is useful in the improvement of the disease.
In this study, preliminary screening of network pharmacology showed that the active ingredients of Dahuang Mudan Decoction for the treatment of acute pancreatitis were mainly kaempferol, quercetin and eupatin. Experimental studies showed that kaempferol could inhibit the activation of NF-kB signaling pathway to reduce the production of inflammatory factors associated with pancreatitis, attenuate the inflammatory response, and improve the prognosis.[27] Meanwhile, kaempferol also inhibited the proliferation of human pancreatic cancer cells (PANC-1) and the protein expression levels of NF-kB and vascular endothelial growth factor (VEGF) in a dose-dependent manner, thereby inhibiting apoptosis and invasion of pancreatic cancer cells.[28] Quercetin is a class of flavonoids with positive effects on the prevention of inflammation. It can regulate proteins involved in various oncogenic and signaling pathways. Additionally, it can inhibit tumor necrosis factor-a (TNF-a) and the p38/MAPK signaling pathway through the up-regulation of miR-216b, thus having a protective effect on acute pancreatitis.[29] Moreover, quercetin can inhibit the migration activity of pancreatic cancer cells induced by epidermal growth factor.[30] Eupatin also has significant anti-inflammatory and neuroprotective effects.[31]
The main feature of pancreatitis is that pancreatic enzymes cause pancreatic auto-digestion, which leads to autophagy and apoptosis of pancreatic acinar cells. These effects may trigger a severe inflammatory response and release inflammatory factors. Inflammatory cells and pancreatic acinar cells then release a variety of inflammatory mediators, including pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-a).[32]The increase in the level of these factors leads to an increase in vascular permeability, activation of leukocytes, and destruction of tissues. The release of inflammatory mediators is the key factor in the pathogenesis of pancreatitis, and it can aggravate the condition, leading to the involvement of organs outside the pancreas.[33]
Relevant studies have provided strong evidence for clinical disease diagnosis through the screening or modeling of target genes, indicating the importance of target genes in disease development.[34] The top 10 targets according to network pharmacology were AKT1, TNF-a, IL-6, TP53, HIF1A, BCL2, CASP3, EGFR, MMP9, IL1B. Among them, IL-6 and TNF-a are important regulators in the inflammatory response.[35] On the one hand, IL-6 can regulate the inflammatory response by phosphorylating STAT3 at specific sites through the MAPKAPK-2 signal transduction pathway, allowing it to enter the nucleus and bind to chromosomal DNA.[36] On the other hand, TNF-a is an important pro-inflammatory factor. A rapid rise in its level will lead to the aggravation of pancreatitis, and inhibition of it will cause apoptosis of pancreatic acinar cells, resulting in a significant reduction of pancreatitis.[37]
Aggregations of different cells may have different immune landscapes or KEGG expression.[38] KEGG enrichment analysis of the key protein module of the relevant pathways ranked in the top 20 by P value was visualized using a bubble diagram, in which the intersection of the targets are mainly in the AGE-RAGE signaling pathway in diabetic complications, the PI3K-Akt signaling pathway, etc.
Diabetic complications of the AGE-RAGE signaling pathway can activate NF-kB to cause the expression and release of a large number of adhesion molecules, growth factors, and pro-inflammatory factors. This can lead to inflammation and oxidative stress. Therefore, the main components of traditional Chinese medicine act on this signaling to a certain extent, influencing the development of acute pancreatitis.[39] The PI3K-Akt signaling pathway can regulate apoptosis, oxidative stress, autophagy, and more. Wang et al[40] found that the activation of PI3K signaling pathway could be inhibited by propargylglycine in severe acute pancreatitis (SAP) rats. This inhibition reduced the activity of NF-kB, which led to the inhibition of apoptotic genes, a reduction in the release of inflammatory factors, and alleviation of the clinical symptoms of acute pancreatitis. Laura et al[41] showed that PI3K/Akt signaling pathway can activate NF-kB to induce oxidative stress in alveolar cells, and at the same time mediate the downstream pathway leading to inflammatory reactions in the pancreas. Another study showed that rhubarbin contained in the traditional Chinese medicine Dahuang can inhibit autophagy-related genes and reduce the severity of acute pancreatitis in rats.[42] All of the above can illustrate the important role of the main signaling pathways in acute pancreatitis, and the results of network pharmacology show that they can be influenced by the main active ingredients of traditional Chinese medicine.
The results of the meta-analysis showed that the treatment group had a significant advantage in terms of overall efficacy, level of improvement in inflammatory factors, and shorter hospital stays compared with the control group. The effect of Dahuang Mudan Decoction on various of inflammatory factors can be further supported.
However, there were some shortcomings in this study, including the lack of mention of blinding and allocation concealment in the 13 included studies. In addition, the methodological quality of the included studies was average, and gray literature such as unpublished studies was not included. The overall sample size for the systematic evaluation was small, and assessments of adverse effects and safety were lacking. The limitations of the Network Pharmacology TCMIP platform database may have resulted in an incomplete collection of active ingredients of drugs. Therefore, future studies need to expand the sample size and optimize the clinical trial specifications to better leverage the advantages of traditional Chinese medicine in clinical practice. Finally, further verification of the specific mechanisms behind the results of this study is necessary through in vivo and in vitro experiments.
5. Conclusion
In summary, network pharmacological studies have shown that Dahuang Mudan Decoction affects acute pancreatitis through multiple components, targets and mechanisms. In addition, evidence-based medicine provides strong support for the effectiveness of Dahuang Mudan Decoction in the treatment of acute pancreatitis.
Author contributions
Conceptualization: Jinhan Chen, Mengjie Jiang, Mingxian Chen.
Data curation: Jinhan Chen, Mengjie Jiang, Yuou Ying, Yuan Ji, Yuying Chi, Linghui Tao, Fuping Wu.
Investigation: Yuan Ji, Yuying Chi, Linghui Tao, Fuping Wu.
Methodology: Jinhan Chen, Mengjie Jiang, Yuou Ying, Mingxian Chen.
Supervision: Jinhan Chen.
Writing – original draft: Jinhan Chen, Mengjie Jiang, Mingxian Chen.
Writing – review & editing: Jinhan Chen, Mingxian Chen.
Abbreviations:
- GO
- gene ontology
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- PPI
- protein-protein interaction
This work was funded by State Administration of Traditional Chinese Medicine Science and Technology Department-Zhejiang Provincial Administration of Traditional Chinese Medicine Co-construction of Key Laboratory of Research on Prevention and Treatment for depression syndrome (no. GZY-ZJ-SY-2402); State Administration of Traditional Chinese Medicine National Clinical Outstanding Talents Training Program (Chinese medicine Education Letter [2022] no. 1); Zhejiang Province 551 Health Talent Training Project (Zhejiang Provincial Health Commission Office [2021] no. 40).
The study did not involve clinical research and therefore did not require ethics committee review.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
How to cite this article: Chen J, Jiang M, Ying Y, Ji Y, Chi Y, Tao L, Wu F, Chen M. Network pharmacological mechanism analysis and evidence-based medical validation of Dahuang Mudan Decoction in the treatment of acute pancreatitis. Medicine 2024;103:37(e39679).
Contributor Information
Jinhan Chen, Email: chenmingxian2005@126.com.
Mengjie Jiang, Email: 2507383043@qq.com.
Yuou Ying, Email: yingyuou22@163.com.
Yuan Ji, Email: jiyuan0117@126.com.
Yuying Chi, Email: 1625659576@qq.com.
Linghui Tao, Email: 316881605@qq.com.
Fuping Wu, Email: wufuping0213@163.com.
References
- [1].Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pan creatitis. Lancet. 2020;396:726–34. [DOI] [PubMed] [Google Scholar]
- [2].Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162:122–34. [DOI] [PubMed] [Google Scholar]
- [3].Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59:128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. [DOI] [PubMed] [Google Scholar]
- [5].Zhuo Y, Cui L, Li C, et al. Progress in the study of the mechanism of action of traditional Chinese medicine compound formula for the treatment of acute pancreatitis. Chin J Surg Integr Tradit West Med. 2019;25:394–8. [Google Scholar]
- [6].Yang D, Sun Y, Wang Y, et al. Research progress on mechanism of Dahuang Mudan decoction in diagnosis and treatment of acute pancreatitis. J Pract Tradit Chin Intern Med. 2023;37:9–11. [Google Scholar]
- [7].Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25:1110–1. [DOI] [PubMed] [Google Scholar]
- [8].Huang SJ, Mu F, Li F, et al. Systematic elucidation of the potential mechanism of Erzhi Pill against drug-induced liver injury via network pharmacology approach. Evid Based Complement Alternat Med. 2020;2020:6219432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. 2019;9:143–76. [Google Scholar]
- [10].Chen L, Zhao J. Clinical efficacy of growth inhibitor combined with Dahuang Mudan decoction retention enema in the treatment of acute pancreatitis. Mod Pract Med. 2019;31:1638–40. [Google Scholar]
- [11].Gu X, Zhu Y. Dahuang Mudan decoction retention enema combined with blood purification for the treatment of severe acute pancreatitis and the clinical effect on intestinal mucosal barrier function and inflammatory factors. Mod J Integr Tradit Chin West Med. 2018;27:3513–6. [Google Scholar]
- [12].Pang K, Liu J, Li X. Effect of enema with Dahuang Mudan decoction on inflammatory response and microcirculation in patients with severe acute pancreatitis. Guizhou Med J. 2019;43:605–7. [Google Scholar]
- [13].Qiao H, Lian Y. Clinical observation on the treatment of acute pancreatitis by enema with Dahuang Mudan decoction. J Emerg Tradit Chin Med. 2003;04:326. [Google Scholar]
- [14].Ren Y. Clinical observation on modified Dahuang Mudan decoction combined with western medicine in the treatment of acute pancreatitis. Guangming J Chin Med. 2018;33:1178–80. [Google Scholar]
- [15].Wang R, Cao Z, Meng J. Clinical efficacy of Dahuang Mudan decoction retention rnema combined with blood purification in the treatment of severe acute pancreatitis and its effect on the barrier function of intestinal mucosa and inflammatory factors in patients. J Emerg Tradit Chin Med. 2018;27:1618–20. [Google Scholar]
- [16].Wang X. Comprehensive treatment of acute pancreatitis in 37 cases by Dahuang Mudan decoction. Guangming J Chin Med. 2019;34:575–7. [Google Scholar]
- [17].Wu W, Lin D, He J. Analysis of the clinical effect of combined Chinese and Western medicine in the treatment of pancreatitis. Shenzhen J Integr Tradit Chin West Med. 2019;29:19–20. [Google Scholar]
- [18].Xing Y. Analysis of the clinical effect of Dahuang Mudan decoction retention enema in the treatment of acute pancreatitis. J Emerg Tradit Chin Med. 2016;25:1634–1635 + 1644. [Google Scholar]
- [19].Xu Z. Clinical observation of Dahuang Mudan decoction clyster in the treatment of acute pancreatitis. Mod Diagn Treat. 2013;24:1973–5. [Google Scholar]
- [20].Yao R. Application effect of Dahuang Mudan decoction retention enema combined with blood purification in the treatment of severe acute pancreatitis. Mod Diagn Treat. 2017;28:4530–1. [Google Scholar]
- [21].Zhou J, Gao C, Tang X. Effect on inflammation and stress response of acute pancreatitis by Dahuang Mudan decoction. Inf Tradit Chin Med. 2017;34:62–6. [Google Scholar]
- [22].Zhu Y. Clinical observation on Dahuang Mudan decoction in the treatment of severe acute pancreatitis. Chin Med Mod Distance Educ China. 2021;19:81–3. [Google Scholar]
- [23].Lin J, Han C, Dai N, Bi S, Du D, Xia Q. Effectiveness of Chengqi-series decoctions in treating severe acute pancreatitis: a systematic review and meta-analysis. Phytomedicine. 2023;113:154727. [DOI] [PubMed] [Google Scholar]
- [24].Zhang CL, Lin ZQ, Luo RJ, et al. Chai-Qin-Cheng-Qi decoction and carbachol improve intestinal motility by regulating protein kinase C-mediated Ca release in colonic smooth muscle cells in rats with acute necrotising pancreatitis. Evid Based Complement Alternat Med. 2017;2017:5864945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patel BK, Patel KH, Bhatia M, Iyer SG, Madhavan K, Moochhala SM. Gut microbiome in acute pancreatitis: a review based on current literature. World J Gastroenterol. 2021;27:5019–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang S, Zhou T, Long K, et al. Enema of traditional Chinese medicine for patients with severe acute pancreatitis. J Vis Exp. 2023;191:e64831. [DOI] [PubMed] [Google Scholar]
- [27].Yang C, Yue Z, Lianlian H, et al. Interention effect mechanism kaempferol on caerulein-induced acute pancreatitis in mice. Shandong Med. 2021;65:41–4. [Google Scholar]
- [28].Zhuo C, Shengnan L, Keijian W, et al. Effects of different doses of kaempferol on proliferation and apoptosis of pancreatic cancer PANC-1 cells. Shandong Med. 2018;58:27–30. [Google Scholar]
- [29].Sheng B, Zhao L, Zang X, et al. Quercetin inhibits caerulein-induced AP through regulating miR-216b by targeting MAP2K6 and NEAT1. Inflammopharmacology. 2021;29:549–59. [DOI] [PubMed] [Google Scholar]
- [30].Lee J, Han SI, Yun JH, Kim JH. Quercetin 3-O-glucoside suppresses epidermal growth factor-induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumour Biol. 2015;36:9385–93. [DOI] [PubMed] [Google Scholar]
- [31].Hattori T, Fujitsuka N, Kurogi A, Shindo S. Effect of Onpi-to (TJ-8117) on TGF-beta 1 in rats with 5/6 nephrectomized chronic renal failure. Nihon Jinzo Gakkai Shi. 1996;38:475–83. [PubMed] [Google Scholar]
- [32].Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–38. [DOI] [PubMed] [Google Scholar]
- [33].Kim MJ, Kim DU, Choi JW, et al. Silymarin attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2020;49:89–95. [DOI] [PubMed] [Google Scholar]
- [34].Yu H, Ji X, Ouyang Y. Unfolded protein response pathways in stroke patients: a comprehensive landscape assessed through machine learning algorithms and experimental verification. J Transl Med. 2023;21:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Severgnini M, Takahashi S, Rozo LM, et al. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1282–92. [DOI] [PubMed] [Google Scholar]
- [36].Malleo G, Mazzon E, Genovese T, et al. Etanercept attenuates the development of cerulein-induced acute pancreatitis in mice: a comparison with TNF-alpha genetic deletion. Shock. 2007;27:542–51. [DOI] [PubMed] [Google Scholar]
- [37].Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130–40. [DOI] [PubMed] [Google Scholar]
- [38].Yu H, Si G, Si F. Mendelian randomization validates the immune landscape mediated by aggrephagy in esophageal squamous cell carcinoma patients from the perspectives of multi-omics. J Cancer. 2024;15:1940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang X, Liu J, Yang Y, Zhang X. An update on the potential role of advanced glycation end products in glycolipid metabolism. Life Sci. 2020;245:117344. [DOI] [PubMed] [Google Scholar]
- [40].Wang G, Han B, Zhou H, et al. Inhibition of hydrogen sulfide synthesis provides protection for severe AP rats via apoptosis pathway. Apoptosis. 2013;18:28–42. [DOI] [PubMed] [Google Scholar]
- [41].Gaman L, Dragos D, Vlad A, et al. Phytoceuticals in acute pancreatitis: targeting the balance between apoptosis and necrosis. Evid Based Complement Alternat Med. 2018;2018:5264592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu X, Li C, Song H, et al. Emodin attenuates autophagy response to protect the pancreas from acute pancreatitis failure. Pancreas. 2018;47:892–7. [DOI] [PubMed] [Google Scholar]