Abstract
A combination of antimicrobial and endotoxin-neutralizing activities is desired in order to prevent progression from infection to sepsis due to the release of lipopolysaccharide from dying gram-negative bacteria. Lipopolyamines have emerged as a new type of endotoxin-neutralizing compound, but their antimicrobial activity has not been investigated. We synthesized a series of 10 oleoylamines differing in the polyamino head group, particularly in the number and separation between nitrogen atoms and the position of the oleoyl moiety. Compounds showed activity against both gram-negative and gram-positive bacteria in the micromolar range. Compounds were able to provide penetration of ethidium bromide into bacteria, indicating effects on the bacterial membrane. Oleoylamines neutralized endotoxin in Limulus amoebocyte lysate assays and by neutralization of tumor necrosis factor alpha release in human blood. Comparison of biological activities of compounds identified structural properties responsible for antimicrobial activity, and quantitative structure-property relationship analysis provided a quantitative model for prediction of activity of oleoylamines.
Sepsis, the leading cause of mortality in intensive care units, is triggered by components of bacterial cells, most notably endotoxin (lipopolysaccharide [LPS]), from gram-negative bacteria. LPS can be released from dying bacteria either through the action of the body's natural defense system or due to the administration of antibiotics (10, 21). Upon cellular recognition of LPS, inflammatory mediators are produced which may lead to serious physiological damage ranging from hypotension or disseminated vascular coagulation to multiple organ failure (3, 29). Antimicrobial agents still represent the most important treatment to prevent sepsis, but they are insufficient, since there is currently no effective endotoxin-neutralizing treatment which would sequester LPS and prevent excessive response by the innate immune system. Lipopolyamines have recently shown promising results for neutralization of endotoxin (2, 5-7, 14). Lipopolyamines are polycationic amphiphilic molecules originally used as DNA transfection agents. DOSPER [1,3-dioleoyloxy-2-(6-carboxyspermyl)propylamide] binds and neutralizes LPS with an affinity comparable to polymyxin B, a polycationic peptide antibiotic of microbial origin, which is one of the most potent LPS neutralizers, but with significant toxicity (4, 8, 27). DOSPER is noncytotoxic to mammalian cells and has shown protective effects against LPS-induced lethality in murine models (7, 25). No antimicrobial activity has been reported for lipopolyamines that were able to neutralize LPS (2).
Lipopolyamines are therefore of considerable interest due to their low toxicity, low immunogenicity, ease of chemical synthesis, and defined molecular structure. In the present study, we synthesized a group of novel lipopolyamines which contain a polar nitrogen-containing head group with an attached hydrophobic oleoyl chain. Based on the presence of proline in several proline-rich antimicrobial peptides (26), we prepared three compounds which incorporate this residue. Prepared oleoylamines were tested for their antibacterial, membrane-permeabilizing, endotoxin-neutralizing, and hemolytic activities. Compounds were active against Escherichia coli and Staphylococcus aureus and were able to neutralize endotoxin as well. Their biological activity was correlated to their structure in order to obtain descriptors for the subsequent improved generations of compounds.
MATERIALS AND METHODS
Materials.
All chemicals were of the highest quality commercially available and obtained from Sigma-Aldrich Co. N,N-Bis[2-(trifluoroacetamido)ethyl]amine, N-[4-(trifluoroacetamido)butyl]-N-[3-(trifluoroacetamido)propyl]amine, and (2,2,2-trifluoroacetylamino)acetyl chloride were prepared according to the literature (1). Laboratory glassware used in the chromogenic Limulus amoebocyte lysate (LAL) assay was thoroughly cleaned and baked dry for 4 h at 180°C to render it free of contaminating LPS. Pipette tips from their original packing were wrapped in aluminum foil piece by piece and autoclaved for 45 min at 131°C and 1.2 × 105 Pa. For endotoxin neutralization assays, smooth LPS from E. coli O55:B5 (Sigma Aldrich) was used.
Synthesis of oleoylamines. General methods.
The 1H (300 MHz, internal Me4Si) and 13C (75 MHz) nuclear magnetic resonance (NMR) spectra were recorded in CDCl3. In all cases, 1H NMR, 13C NMR, mass spectrometry (MS), and infrared spectra were consistent with assigned structures.
(i) Oleic acid bis(2-aminoethyl)amide (compound 1).
Oleoyl chloride (2.4 ml, 6.12 mmol) was added to a cold (0°C) solution of N,N-bis[2-(trifluoroacetamido)ethyl]amine (1.50 g, 5.1 mmol), Et3N (0.85 ml, 6.12 mmol), and DMAP (4-dimethylaminopyridine, catalytic amount [cat.]) in dry CH2Cl2 (30 ml) and stirred at room temperature (RT) overnight. The reaction mixture was washed with 1 M HCl, 5% aqueous NaHCO3, and H2O and dried (Na2SO4). The solvent was evaporated, and the crude product was purified by chromatography on silica gel, eluting with CH2Cl2/EtOAc/Et3N (30:10:0.25), furnishing a light yellowish oil (1.61 g, 56%). This N-trifluoroacetylated (N-TFA) protected product was dissolved in MeOH (50 ml) and cooled to 0°C, and NaBH4 (0.58 g, 15.5 mmol) was added. The mixture was stirred at RT overnight and then concentrated. The residue was partitioned between H2O (20 ml) and CH2Cl2 (20 ml). The organic layer was dried (Na2SO4) and concentrated. The residue was dissolved in MeOH (10 ml), and 36% HCl was added to precipitate the product as its HCl salt. It was then suspended in H2O and basified to pH 10 with 1 M NaOH, and the product was extracted with CH2Cl2, dried (Na2SO4), and concentrated to obtain an oil (0.79 g, 75%). For 1H NMR, δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.28 [m, 20H, (CH2)6, (CH2)4], 1.63 (m, 2H, COCH2CH2), 2.01 (m, 4H, CH2CH = CHCH2), 2.37 (m, 2H, COCH2), 2.86 and 2.87 (2t, 4H, 2× CH2NH2; J1 = 6.8 Hz, J2 = 6.9 Hz), 3.39 (m, 4H, CH2NCH2), and 5.35 (m, 2H, CH = CH) (underlining denotes protons appearing in the 1H NMR signal). For MS (FAB [fast atom bombardment]), m/z was 368.3 (M+ + 1).
(ii) Oleic acid (4-aminobutyl)-(3-aminopropyl)amide (compound 2).
The compound was prepared in the same manner as compound 1, starting from N-[4-(trifluoroacetamido)butyl]-N-[3-(trifluoroacetamido)propyl]amine. For 1H NMR, δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.28 [m, 20H, (CH2)6, (CH2)4], 1.38 to 1.77 [m, 8H, COCH2CH2, NCH2CH2, NCH2(CH2)2CH2NH2], 2.01 (m, 4H, CH2CH = CHCH2), 2.30 (m, 2H, COCH2), 2.62 to 2.78 (m, 4H, 2× CH2NH2), 3.20 to 3.46 (m, 4H, CH2NCH2), and 5.34 (m, 2H, CH = CH). For MS (FAB), m/z was 410.4 (M+ + 1).
(iii) Oleic acid bis(2-DEAE)amide (compound 3).
Oleoyl chloride (1.5 ml, 4.5 mmol) was added to a cold (0°C) solution of tetraethylenediethylenetriamine (1.0 ml, 3.9 mmol), Et3N (0.7 ml, 5.0 mmol), and DMAP (cat.) in dry CH2Cl2 (25 ml) and stirred at RT overnight. The concentrated residue was partitioned between 0.5 M H2SO4 (20 ml) and ether (20 ml). The aqueous layer was washed again with ether (20 ml) and basified to pH 10 with 2 M NaOH. The product was extracted with CH2Cl2 (2 × 20 ml), dried (Na2SO4), and concentrated to obtain a slightly yellowish oil (1.51 g, 81%). For 1H NMR, δ values were 0.88 (t, 3H, CH3; J = 7.3 Hz), 1.03 (t, 12H, 4× NCH2CH3; J = 7.2 Hz), 1.29 [m, 20H, (CH2)6, (CH2)4], 1.64 (m, 2H, COCH2CH2), 2.00 (m, 4H, CH2CH = CHCH2), 2.31 (t, 2H, COCH2; J = 7.6 Hz), 2.56 [m, 12H, 2× (CH3CH2)2NCH2], 3.38 (m, 4H, 2× NHCH2), and 5.34 (m, 2H, CH = CH). For MS (FAB), m/z was 480.5 (M+ + 1).
(iv) N1-Oleoyldiethylenetriamine (compound 4).
Diethylenetriamine (300 mg, 2.9 mmol) and benzophenoneimine (1.2 ml, 7.2 mmol) in CH2Cl2 (20 ml) were stirred at RT overnight. The mixture was concentrated and washed with petroleum ether (2 × 15 ml). The residue was concentrated and suspended in dioxane (10 ml), and 2 M HCl (10 ml) was added. The mixture was stirred at RT for 3 h, partially concentrated, diluted with H2O (10 ml), and washed with ether. The aqueous phase was basified to pH 10 with 2 M NaOH and extracted with CH2Cl2. The concentrated residue was dissolved in MeOH (10 ml), and 36% HCl was added to obtain a precipitate. This precipitate was filtered and then dissolved in H2O and basified to pH 10 with 1 M NaOH, and the product was extracted with CH2Cl2 (2 × 15 ml). The combined extracts were dried (Na2SO4) and concentrated to obtain an oil which solidified partially upon standing (0.10 g, 36%). For 1H NMR, δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.28 [m, 20H, (CH2)6, (CH2)4], 1.69 (m, 2H, COCH2CH2), 2.00 (m, 4H, CH2CH = CHCH2), 2.16 (t, 2H, COCH2; J = 7.8 Hz), 2.68 (m, 2H, NH2CH2), 2.79 (m, 4H, CH2NHCH2), 3.65 (m, 2H, CH2NHCO), 5.34 (m, 2H, CH = CH), and 6.19 (br s, 1H, NHCO). For MS (FAB), m/z was 368.4 (M+ + 1).
(v) Oleic acid bis[2-(2-aminoethylamino)ethyl]amide (compound 5).
(2,2,2-Trifluoroacetylamino)ethylbromide (0.93 mg, 4.3 mmol) was added to a solution of compound 1 (75 mg, 0.21 mmol) and N, N-diisopropylethylamine (110 μl, 0.63 mmol) in toluene, and the mixture was refluxed for 5 h. The cold mixture was filtered and concentrated and redissolved in MeOH (5 ml), and NaBH4 was added (47 mg, 1.25 mmol). After being stirred at RT overnight, the concentrated residue was partitioned between H2O (10 ml) and CH2Cl2 (10 ml). The organic layer was dried (Na2SO4) and concentrated. The residue was dissolved in MeOH (5 ml), and HCl gas was bubbled in followed by ether to precipitate the product as its HCl salt (75 mg, 60%). For analyses, the product was suspended in H2O, basified to pH 10 with 1 M NaOH, extracted with CH2Cl2, dried (Na2SO4), and concentrated to obtain an oil. For 1H NMR, δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.28 [m, 20H, (CH2)6, (CH2)4], 1.62 (m, 2H, COCH2CH2), 2.01 (m, 4H, CH2CH = CHCH2), 2.18 (m, 2H, COCH2), 2.43 to 2.93 [m, 12H, 2× CH2NH(CH2)2NH2], 3.33 (m, 4H, CH2NCH2), and 5.34 (m, 2H, CH = CH). For MS (FAB), m/z was 454.4 (M+ + 1).
(vi) Oleic acid [4-(2-aminoethylamino)butyl]-[3-(2-aminoethylamino)propyl]amide (compound 6).
The compound was prepared in the same manner as that for compound 4, starting from compound 2. For 1H NMR, δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.02 to 1.85 [m, 28H, (CH2)6, (CH2)4, NCH2(CH2)2CH2NH2, NCH2CH2, COCH2CH2], 1.92 (m, 4H, CH2CH = CHCH2), 2.22 (m, 2H, COCH2), 2.28 to 2.83 [m, 12H, 2× CH2NH(CH2)2NH2], 3.09 to 3.32 (m, 4H, CH2NCH2), and 5.33 (m, 2H, CH = CH). For MS (FAB), m/z was 368.4 (M+ + 1).
(vii) N,N-Bis[2-(glycinamido)ethyl]oleoylamide (compound 7).
(2,2,2-Trifluoroacetylamino)acetyl chloride (1.13 g, 5.85 mmol) in CH2Cl2 was added to a cold (0°C) solution of compound 1 (0.90 g, 2.45 mmol), Et3N (1.0 ml, 7.53 mmol), and DMAP (cat.) in CH2Cl2 (25 ml) and stirred at RT overnight. The reaction mixture was washed with 1 M HCl, 5% aqueous NaHCO3, and H2O. The organic layer was concentrated, and the crude extract was purified by chromatography on silica gel, eluting with CH2Cl2/EtOAc/Et3N (40:10:0.25), to obtain a light yellowish oil (1.43 g). This N-TFA protected product was dissolved in MeOH (30 ml) and cooled to 0°C, and NaBH4 (0.50 g, 13.2 mmol) was added. The mixture was stirred at RT overnight and concentrated. The residue was partitioned between H2O (30 ml) and CH2Cl2 (30 ml), and the organic layer was dried (Na2SO4). After concentration, the residue was dissolved in MeOH (25 ml), and 36% HCl was added to precipitate the product as its HCl salt. This was suspended in H2O and basified to pH 10 with 1 M NaOH, and the product was extracted with CH2Cl2, dried (Na2SO4), and concentrated to obtain an oil which solidified partially upon standing (0.75 g, 64%). For 1H NMR, δ values were 0.86 (t, 3H, CH3; J = 6.6 Hz), 1.25 [m, 20H, (CH2)6, (CH2)4], 1.48 (m, 2H, COCH2CH2), 1.98 (m, 4H, CH2CH = CHCH2), 2.31 (m, 2H, COCH2), 2.85 to 3.64 [m, 12H, N(CH2CH2)2, 2× COCH2NH2], and 5.33 (m, 2H, CH = CH). For MS (FAB), m/z was 482.4 (M+ + 1).
(viii) (N-Oleoyl-l-prolyl)-[N,N-bis(2-aminoethyl)]amide (compound 8).
Oleoyl chloride (1.5 ml, 4.5 mmol) was added to a cold (0°C) suspension of l-proline (1.00 g, 8.7 mmol), Et3N (1.4 ml, 10.5 mmol), and DMAP (cat.) in CH2Cl2 (40 ml) and stirred at RT overnight. The reaction mixture was washed with 1 M HCl and saturated aqueous NaCl solution and dried (Na2SO4). The concentrated residue was purified by chromatography on silica gel, eluting with ether/hexane/AcOH (10:10:0.2) to obtain N-oleoyl-l-proline as a colorless oil (2.21 g). Oxalyl chloride was added to a cold (0°C) solution of N-oleoyl-l-proline and DMAP (cat.) in CH2Cl2 (25 ml) (0.56 ml, 6.4 mmol) and stirred at RT for 3 h. This solution was added to a cold (0°C) solution of N,N-bis[2-(trifluoroacetamido)ethyl]amine (1.80 g, 6.1 mmol) and Et3N (1.7 ml, 12 mmol) in CH2Cl2 (25 ml) and stirred at RT overnight. The mixture was washed with 5% aqueous NaHCO3 and saturated aqueous NaCl solution, dried (Na2SO4), and concentrated to obtain a light yellowish oil (1.80 g). This N-TFA protected compound was suspended in MeOH (20 ml) and cooled to 0°C, and NaBH4 (1.32 g, 35 mmol) was added. The mixture was stirred at RT overnight and concentrated. The residue was partitioned between H2O (50 ml) and CH2Cl2 (50 ml), and the organic layer was dried (Na2SO4). The concentrated residue was dissolved in MeOH (30 ml), and HCl gas was bubbled in to precipitate the product as its HCl salt. It was then suspended in H2O and basified to pH 10 with 1 M NaOH, and the product was extracted with CH2Cl2, dried (Na2SO4), and concentrated to obtain an oil (0.73 g, 35%). For 1H NMR (CDCl3), δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.28 [m, 20H, (CH2)6, (CH2)4], 1.62 (m, 2H, COCH2CH2), 1.86 to 2.35 [m, 10H, CH2CH = CHCH2, COCH2, CH(CO)(CH2)2], 2.79 to 3.15 (m, 4H, 2× CH2NH2), 3.39 to 3.75 (m, 6H, CH2NHCH2, NCH2), 4.88 (m, 1H, CH), and 5.34 (m, 2H, CH = CH). For MS (FAB), m/z was 465.4 (M+ + 1).
(ix) (N-Oleoyl-l-prolyl)-N,N-bis[2-(diethylamino)ethyl]amide (compound 9).
Acid chloride of N-oleoyl-l-proline (0.77 g, 1.94 mmol) in CH2Cl2 (5 ml) was added to a cold (0°C) solution of tetraethylenediethylenetriamine (0.5 ml, 1.95 mmol), Et3N (0.8 ml, 5.8 mmol), and DMAP (cat.) in CH2Cl2 (25 ml). The mixture was stirred at RT overnight and washed with 5% aqueous NaHCO3 and saturated aqueous NaCl solution and dried (Na2SO4). After concentration, a light yellowish oil (0.86 g, 77%) was obtained. For 1H NMR (CDCl3), δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.05 [m, 12H, 2× N(CH2CH3)2], 1.28 [m, 20H, (CH2)6, (CH2)4], 1.63 (m, 2H, COCH2CH2), 1.84 to 2.35 and 2.51 to 2.92 [2m, 22H, CH2CH = CHCH2, COCH2, NCH(CH2)2, 2× CH2N(CH2CH3)2], 3.28 to 3.76 (m, 6H, CH2NCH2, NCH2), 4.74 (m, 1H, CH), and 5.34 (m, 2H, CH = CH). For MS (FAB), m/z was 577.5 (M+ + 1).
(x) (Nα-Oleoyl-l-lysyl-l-prolyl)-N,N-bis[2-(diethylamino)ethyl]amide (compound 10).
Oleoyl chloride (2.3 ml, 5.95 mmol) was added to a cold (0°C) solution of Nɛ-(trifluoroacetyl)-l-lysyl-l-proline (2.0 g, 5.89 mmol), Et3N (1.67 ml, 12.1 mmol), and DMAP (cat.) in dry CH2Cl2 (50 ml). The mixture was stirred at RT overnight and washed with 1 M HCl, 5% aqueous NaHCO3, and H2O and dried (Na2SO4). The solvent was evaporated, and the crude extract was purified by chromatography on silica gel, eluting with CH2Cl2/EtOAc/Et3N (30:10:0.25) to obtain a light yellowish oil (1.99 g). Oxalyl chloride (0.30 ml, 3.46 mmol) was added to a cold (0°C) solution of [Nα-oleoyl-Nɛ-(trifluoroacetyl)-l-lysyl]-l-proline and DMAP (cat.) in CH2Cl2 (30 ml). The solution was stirred at RT for 3 h. This solution was added to a cold (0°C) solution of tetraethylenediethylenetriamine (0.85 ml, 3.33 mmol) and Et3N (1.3 ml, 9.89 mmol) in CH2Cl2 (25 ml) and stirred at RT overnight. The mixture was washed with 5% aqueous NaHCO3 and saturated aqueous NaCl solution, dried (Na2SO4), and concentrated to obtain a light yellowish oil (1.53 g). This N-TFA protected product was suspended in MeOH (20 ml) and cooled to 0°C, and NaBH4 (0.43 g, 11.5 mmol) was added. The mixture was stirred at RT overnight and concentrated. The residue was partitioned between H2O (40 ml) and CH2Cl2 (40 ml), and the organic layer was dried (Na2SO4). The concentrated residue was dissolved in MeOH (30 ml), and HCl gas was bubbled in, precipitating the product as its HCl salt. This salt was suspended in H2O and basified to pH 10 with 1 M NaOH, and the product was extracted with CH2Cl2, dried (Na2SO4), and concentrated to obtain an oil (0.93 g, 22%). For 1H NMR (CDCl3), δ values were 0.88 (t, 3H, CH3; J = 6.6 Hz), 1.02 [m, 12H, 2× N(CH2CH3)2], 1.16 to 1.69 [m, 26H, (CH2)6, (CH2)4, (CH2)2CH2NH2, COCH2CH2], 1.87 to 2.25 and 2.44 to 2.89 [2m, 26H, CH2CH = CHCH2, COCH2, 2× CH2N(CH2CH3)2, CHCH2, CH2CH2, CH2NH2], 3.13 to 3.87 (m, 6H, CH2NCH2, NCH2), 4.64 to 4.84 (m, 1H, 2× CH), and 5.34 (m, 2H, CH = CH). For MS (FAB), m/z was 705.6 (M+ + 1).
Bacterial strains and growth conditions.
Escherichia coli (NCTC 8007, serotype O111 K58 H2) was provided by Ignacio Moriyon, University of Navarra, Pamplona, Spain, and Escherichia coli strain DC2 (CGSC 7139) was obtained from the E. coli Genetic Stock center (Yale University, New Haven, CT). Staphylococcus aureus subsp. aureus Rosenbach (clinical isolate) (ATCC 25923) was obtained from the American Type Culture Collection (Manassas, VA). Bacterial cultures were stored at −80°C and grown on Luria-Bertani (LB) medium at 37°C. Antimicrobial activity was determined using a standard broth microdilution assay in LB medium (23).
Determination of permeabilizing activity.
To assess the damage to the bacterial cell integrity, we have used ethidium bromide, which intercalates into the DNA inside bacteria and cannot cross the cell membrane (9, 22). Bacteria were grown to the logarithmic phase (optical density at 600 nm of 0.6), centrifuged at 3,500 rpm at room temperature for 10 min, and washed twice with water. Ethidium bromide (30 μM) in water was added into wells of a 96-well microtiter plate containing 100 μl of cell suspension. Various concentrations of oleoylamines were added, and the time course of the increase in fluorescence emission as a result of ethidium bromide binding to DNA inside bacterial cells was measured with a Perkin-Elmer LS-50 luminescence fluorimeter. Emission spectra at 595 nm were recorded at 25°C with excitation at 545 nm. Slit widths were set to 2.5 nm. The concentration where 50% maximal fluorescence intensity increase occurred (50% bacterial cell-permeabilizing concentration) was determined from the sigmoidal fit of at least five data points at different oleoylamine concentrations.
Neutralization of proinflammatory cytokine-stimulating activity of LPS.
Whole human blood was obtained from a healthy volunteer and anticoagulated with 0.105 M sodium citrate VacutainerTM (Becton Dickinson). Citrated whole blood was diluted 1:1 in RPMI 1640 medium. Five microliters of oleoylamines at final concentrations of 1 μM and LPS at the final concentration of 10 ng/ml were added to 100 μl of diluted blood and incubated at 37°C for 4 h. After the incubation, samples were centrifuged for 5 min at 400 × g at room temperature, and supernatants were collected for assay. Secreted tumor necrosis factor alpha (TNF-α) was measured using an OptEIA ELISA kit from BD Biosciences.
Determination of endotoxin-neutralizing activity.
A quantitative chromogenic version of the LAL assay (QCL-1000; Biowhittaker, Walkersville, MD) was used for in vitro LPS neutralization assay. A constant concentration of LPS (0.5 endotoxin units) was incubated with various concentrations of oleoylamines at 37°C for 10 min in 96-well endotoxin-free microtiter plates. A total of 50 μl of this mixture was added to the equal volumes of the LAL reagent and endotoxin-free water, and the mixture was incubated for an additional 10 min at 37°C, after which 100 μl of the chromogenic substrate solution was added to each well. The reaction was terminated after 6 min by the addition of acetic acid to 20%, and the absorbance at 405 nm was read with a microplate reader (3550-UV; Bio-Rad, Hercules, CA). The remaining biologically active LPS in the reaction mixtures was quantified from standard curves, which were linear in the range from 0.1 to 0.5 endotoxin units. The concentration where 50% inhibition of chromogenic LAL response to LPS occurred (endotoxin-neutralizing activity [ENC50]) was determined.
Determination of hemolytic activity.
Heparin (4 μl at 5,000 IU/ml) was added to 100 μl of fresh peripheral blood from a healthy volunteer and centrifuged at 2,000 rpm for 10 min at room temperature. We washed the pellet of red blood cells with phosphate-buffered saline (PBS) and prepared a 2% (vol/vol) suspension of erythrocytes in PBS. Fifty microliters of oleoylamines in the concentration range from 10−6 to 10−2 M in PBS and 50 μl of erythrocyte suspension were incubated at 37°C for 1 h. For a positive control, we used 2% (vol/vol) Triton X-100 in PBS, which caused 100% hemolysis. After the incubation, samples were centrifuged for 5 min at 2,200 rpm at room temperature, and absorbance at 405 nm was measured (13). The concentration of each oleoylamine at which 50% hemolysis compared to positive control occurred was defined as the effective hemolytic concentration.
Quantitative structure-activity relationships (QSAR).
Molecular structures of synthesized oleoylamines were constructed and briefly geometry optimized by the HyperChem, version 6.01, program (Hypercube, Gainesville, FL). Molecular descriptors (constitutional, geometric, electrostatic, and topological) of all the compounds were calculated based on structures of compounds using the program CODESSA (Comprehensive Descriptors for Structural and Statistical Analysis), version 2.20 (Semichem, Kansas City, MO). The log partition coefficient (log P) was calculated using Hyperchem and imported as an additional descriptor into CODESSA, along with the number of hydrogen bond donors and hydrogen bond acceptors and distances between nitrogen atoms (shortest, longest, average, distance between nitrogen atoms, and bordering attached oleoyl moiety). Altogether, 96 descriptors were used for each compound. Correlations between biological activity data (MICs for E. coli and S. aureus, E. coli permeabilization, endotoxin neutralization, and hemolytic activity) transformed into the log of their reciprocal value and descriptors were evaluated by CODESSA, using heuristic and multilinear regression, where we have limited the maximal number of descriptors to two. The cross-validated r2 [r2(cv)] value was calculated using the leave-one-out approach as implemented in CODESSA. The net charge of the compounds at pH 7 was calculated using the SPARC on-line calculator, version 2003 (http://ibmlc2.chem.uga.edu/sparc/index.cfm).
RESULTS
Design and chemical synthesis of oleoylamines.
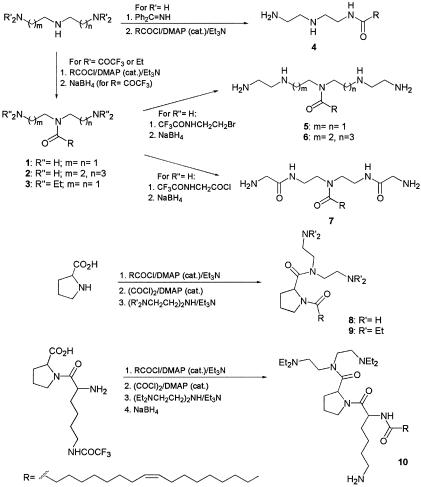
During the preparation of peptide-lipid conjugates (17), we have observed in control experiments that oleoylamine had antimicrobial and endotoxin-neutralizing activities, while saturated alkylamines containing 6 to 18 carbon units were not active. This prompted us to prepare a series of oleoylamines with variations in their head groups, particularly the number of nitrogen atoms, their separation, and the position of the oleoyl group attachment to the head group aimed at maximizing the interaction with components of bacterial cell membrane components and particularly with LPS. Synthesis of designed oleoylamines 1 to 10 is described in Materials and Methods and is schematically represented in Fig. 1.
FIG. 1.
Scheme of the chemical synthesis of oleoylamines.
Antibacterial activity.
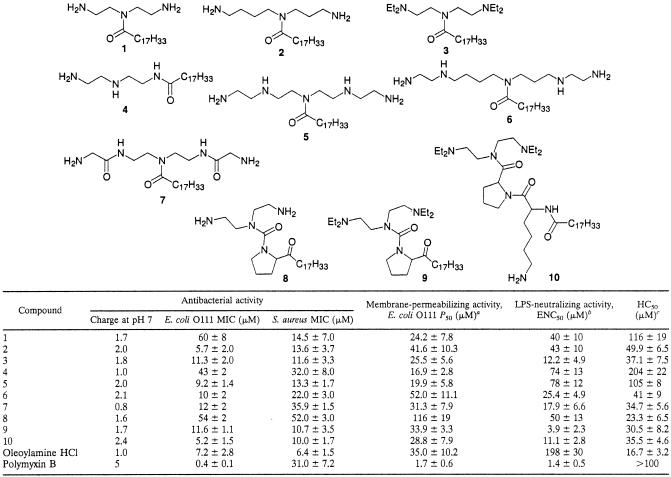
MICs obtained by the broth dilution method for the tested compounds were in the range between 5 and 60 μM for E. coli. MICs for S. aureus were in a similar range, without of any systematically lower or higher values. The lowest MICs were observed for compounds 2, 5, 10, and 6 (for E. coli) and 10, 9, 3, and 2 (for S. aureus) (Table 1). Nondividing bacteria incubated in the phosphate buffer were sensitive at significantly lower concentrations of oleoylamines, which was determined from the count of surviving bacteria (CFU). E. coli strain DC2 had the same susceptibility to oleoylamines as serotype O111.
TABLE 1.
Biological activities of synthesized oleoylamines
P50, 50% bacterial cell-permeabilizing concentration.
As determined by LAL assay.
HC50, concentration required for 50% hemolysis.
Bacterial cell-permeabilizing activity.
Compounds proposed to be taken up by the self-promoted cell uptake pathway, such as polymyxin B, destabilize the bacterial outer membrane of gram-negative bacteria (12) and increase their permeability (24, 30). DNA binding compound, which does not penetrate intact bacterial cells, was used as the probe for permeability studies (5). Oleoylamines demonstrated cell-permeabilizing activity in the concentration range between 17 and 100 μM. Half-maximal response occurred at the lowest concentration for compounds 4, 5, and 1 (Table 1), but there was weak correlation with antimicrobial activity. Oleoylamine also had significant permeabilizing activity as well as antimicrobial activity.
Endotoxin-neutralizing activity.
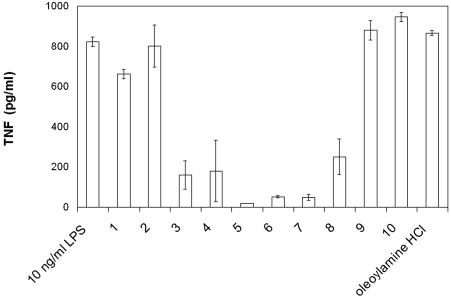
The most potent neutralizers of LPS in the LAL assay were oleoylamines 9, 10, and 3, with ENC50 between 4 and 12 μM. Compound 9 neutralized LPS at 3.9 μM, which is close to the concentration of polymyxin B under the same experimental conditions (1.4 μM). Inhibition of biological activity of LPS was also determined in whole human blood. Compounds inhibited TNF-α release from LPS-stimulated human blood at concentrations lower than those in the LAL test (Fig. 2), with the most potent activity with compounds 5 to 7 and slightly lower activity with compounds 3 and 4, while polyamine and compounds 1, 2, 9, and 10 showed almost no inhibition, which is surprising, particularly for compound 9, which was quite potent in LAL assay. Cytokine release inhibition was observed at concentrations well bellow their cytotoxic activity, which therefore cannot be accountable for decreased cytokine release.
FIG. 2.
Neutralization of LPS-induced TNF-α release from human blood by 1 μM oleoylamines and 10 ng/ml LPS.
Hemolytic activity.
The majority of synthesized oleoylamines with the exception of compound 8 showed low hemolytic activity at their respective MIC concentrations. Compounds with the lowest hemolytic activity were the ones with the smallest head group (compounds 4 and 1) and compound 5, while oleoylamine had strong hemolytic activity. Hemolytic activity of compound 4 occurred at a higher concentration than that in the case of polymyxin B (28).
QSAR analysis.
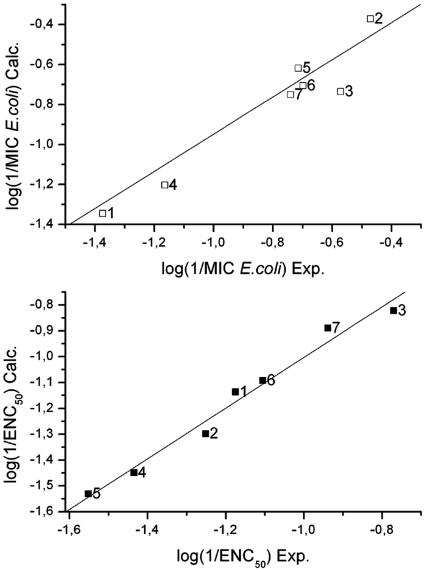
Activities of oleoylamines were correlated with a range of different constitutional, geometrical, topological, and electrostatic descriptors based on their structure. We found that log P was not useful as a descriptor of activity; therefore, the activities were not simply due to hydrophobicity. The best descriptors for correlations between experimental and calculated properties were selected by CODESSA. In order to maintain the high data-to-descriptor ratio and to avoid overfitting, we limited the number of descriptors to two. Reliability of each model was cross-validated using the leave-one-out approach. Despite the low number of descriptors, good correlation was obtained, particularly for antimicrobial activity [log(1/MICE. coli) and log(1/MICS. aureus)] and log(1/ENC) with r2 values between 0.7 and 0.9. Omitting the proline-containing compounds (compounds 8 to 10) from the analysis and restricting the data set to a family of closely related compounds (compounds 1 to 7) improved the r2 correlation to 0.93 (cross-validated r2 of 0.78) and F value to 26.5 for MIC (E. coli), while for endotoxin neutralization, the r2 increased to 0.98 [r2(cv) = 0.92] and the F value increased to 88.6 (Table 2). The most significant descriptors were the smallest distance between nitrogen atoms (NNmin) and geometrical indices ZX shadow and YZ shadow, which reflect the shape of the molecule projected onto the planes oriented with respect to its moments of inertia and topological descriptors such as the Balaban index (for a detailed definition, see reference 1a), the Kier index, and average information content, which in our set of compounds primarily reflect the branching of the molecules.
TABLE 2.
QSAR analysis, best correlations, and used descriptors of the regression analysis
| Antimicrobial activity | r2 | F value | r2 (cv) | Descriptors |
|---|---|---|---|---|
| All compounds | ||||
| Log(1/MICE. coli) | 0.70 | 8.23 | 0.28 | Kier flexibility, NNmin |
| Log(1/MICS. aureus) | 0.69 | 7.84 | 0.33 | Avg structural information content (order 2), relative no. of rings |
| Log(1/ENC50) | 0.72 | 8.81 | 0.36 | Avg information content (order 1), no. of O atoms |
| Only compounds 1-7 | ||||
| Log(1/MICE. coli) | 0.93 | 26.5 | 0.78 | NNmin, ZX shadow |
| Log(1/MICS. aureus) | 0.96 | 50.0 | 0.92 | Avg information content (order 2), ZX shadow/ZX rectangle |
| Log(1/ENC50) | 0.98 | 88.6 | 0.92 | YZ shadow/YZ rectangle, Balaban index |
DISCUSSION
Neutralization of endotoxin by lipopolyamines has been reported for DOSPER (7) and for the terminally modified spermidine and spermine derivatives (2), while antimicrobial activity and their mechanisms of action received little attention up to now. Free polyamines have low antibacterial activity (20), and the lipopolyamine DOSPER showed no activity against E. coli at concentrations up to 75 μg/ml (7). Oleoylamines reported here are active against gram-negative and gram-positive bacteria, while the lipopeptide polymyxin B has very low activity against gram-positive bacteria. In our set of compounds, there is no simple correlation between antimicrobial activity and the number of nitrogen atoms, since one of the most potent compounds (compound 2) contained only three nitrogen atoms. Increased separation between the nitrogen atoms (six bonds between the terminal nitrogen atoms in compound 1 compared to nine bonds in compound 2), however, improves the activity, particularly against E. coli and to a lower extent against S. aureus. The activity of amphiphilic compounds is probably targeted against the inner bacterial membrane containing a large fraction of anionic lipids, which is also indicated by bacterial cell permeabilization. The presence of cardiolipin, a lipid with two negative-charge centers at the defined distance in gram-negative as well as in gram-positive bacteria (15), may contribute to the activity of oleoylamines against E. coli and S. aureus since our compounds are designed against the amphiphiles with two anionic-charge centers.
All synthesized compounds had a net positive charge and hydrophobic moiety, which seem to be the necessary structural features for the ability to bind and neutralize LPS (5). Correlation between the separation of nitrogen atoms in polyamines and binding to LPS has already been noted previously, (8) but no upper limit has been established. Nine bonds separating nitrogen atoms, which correspond to the maximal distance of 11 Å in compound 2, seem to be the threshold distance in our series of compounds, since extending this distance to 12 and 15 bonds (corresponding to 13 and 17 Å) in compounds 5 and 6, respectively, did not additionally improve the MICs. The threshold distance matches the distance between the anionic phosphate groups of lipid A (5, 6). Terminally acylated spermine derivatives were previously shown to sequester LPS. The position of oleoyl substitution in the center of oleoylamines was favored in comparison to the terminal modification as seen from the comparison between compounds 2 and 4, with the latter being worse in all respects, including activity against S. aureus, except in membrane-permeabilizing and hemolytic activities. The best compounds had slightly better antimicrobial activity than oleoylamine, which, however, was much worse in its ability to neutralize LPS and was strongly hemolytic. Differences between simple (LAL) and complex (blood) endotoxin binding assays indicate the effect of other interactions besides LPS-oleoylamine interactions, such as adsorption to blood proteins or lipids, turnover of the compounds, or the state of aggregation, which may affect their bioactivity. Compounds 4 and 5, which had low activity in the LAL assay, performed well in the blood assay, although there is generally a good correlation between the both assays. It is interesting that those two compounds also had the lowest hemolytic activity. Concentrations used in the blood assay were well below the hemolytic concentration of all compounds; however, the release of even small amounts of hemoglobin from the erythrocytes present in the assay might have affected the result, since it has been shown that hemoglobin enhances the LPS-induced production of TNF-α (12). At the stage of development of bioactive compounds, the use of less complex assay systems (such as the LAL assay) with a low number of clearly defined variables is preferred to sustain the progressive rational improvement, while more complex but also biologically more relevant assays may be included in parallel or at later stages.
In the context of acylated lactoferrin fragments (17, 18), the oleoyl modification was not as effective as the lauryl group. Increased neutralization of endotoxin by compounds containing more than three nitrogen atoms in comparison to oleoylamine is probably due to the tighter interaction with the lipid A moiety involving both phosphate groups of lipid A. From the ability to permeabilize bacterial cells, we deduce that compounds act on the bacterial inner membrane, which contains a high fraction of acidic phospholipids (16). However, in all cases, MICs were lower than concentrations needed to permeabilize bacteria. Proline-containing compound 10 had good endotoxin-neutralizing activity in the LAL assay as well as a low MIC. In comparison to compound 9, it contains an additional arginine moiety, which improved the MIC for E. coli but didn't improve the activity against S. aureus. In almost all respects, compounds 9 and 10 were better than compound 8, from which they differ in the diethyl modification of the terminal amino groups. The higher activity of tertiary amines also applies for linear polyamines, as seen from the comparison between compounds 1 and 3. QSAR analysis showed good correlations for a series of closely related compounds. Although the physical interpretation and design of improved compounds based on the model using topological and geometrical descriptors such as the Balaban index (1a) are not as straightforward as, e.g., separation between cationic groups or direct comparison of properties and structures, as discussed above, the QSAR analysis allows quantitative prediction of the properties of novel compounds using equations such as that shown in the legend of Fig. 3 before the actual synthesis is attempted. Our results provide directions for the future improvement of antibacterial and endotoxin-neutralizing activities of lipopolyamines, particularly concerning the structure-activity relationships of the polyamine backbone, which is probably to a large extent uncoupled from a type of acyl chain modification. QSAR predictions of biological properties of compounds, designed by the implementation of rationalized differences between compounds described above, quantitatively support the possible future progress in the design of more effective compounds. However, the best endotoxin neutralizers do not necessarily also have the best antimicrobial activity, as previously shown in many peptides (11). Oleoylamines represent, from the point of chemical synthesis and pharmacological properties, an alternative to cationic peptides and probably share the same targets in bacterial cells with them.
FIG. 3.
Correlation between experimental (Exp.) and calculated (Calc.) properties based on two descriptors. Top panel, MICs (E. coli) of compounds 1 to 7; bottom panel, ENCs for compounds 1 to 7. Equations for the line representing the best correlations shown in the top and bottom panels are as follows: log(1/MICE. coli) = 4.66 + 0.29 · NNmin + 0.0225 · ZX shadow and log(1/ENC50) = −8.0 + 6.4 · YZ shadow + 6.18 · Balaban index, respectively.
Acknowledgments
This research was supported by the Ministry of Higher Education, Science, and Technology of Slovenia and in part by the European project ANEPID, within the 5th Framework program.
We thank Ignacio Moriyon, University of Navarra, Pamplona, Spain, for providing us E. coli strain O111 K58 H2. We thank Janja Lukač for her technical help.
REFERENCES
- 1.Asseline, U., M. Chassignol, J. Draus, M. Durand, and J. C. Maurizot. 2003. Synthesis and properties of oligo-2′-deoxyribonucleotides containing internucleotidic phosphoramidate linkages modified with pendant groups ending with either two amino or two hydroxyl functions. Bioorg. Med. Chem. 11:3499-3511. [DOI] [PubMed]
- 1a.Balaban, A. T. 1982. Highly discriminating distance based topological index. Chem. Phys. Lett. 89:399-404. [Google Scholar]
- 2.Blagbrough, I. S., A. J. Geall, and S. A. David. 2000. Lipopolyamines incorporating the tetraamine spermine, bound to an alkyl chain, sequester bacterial lipopolysaccharide. Bioorg. Med. Chem. Lett. 10:1959-1962. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A., J. H. Turner, and C. M. Kunin. 1974. Prevention of the generalized Shwartzman reaction and endotoxin lethality by polymyxin B localized in tissues. Infect. Immun. 10:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David, S. A. 2001. Towards a rational development of anti-endotoxin agents: novel approaches to sequestration of bacterial endotoxins with small molecules. J. Mol. Recognit. 14:370-387. [DOI] [PubMed] [Google Scholar]
- 6.David, S. A., B. Bechtel, C. Annaiah, V. I. Mathan, and P. Balaram. 1994. Interaction of cationic amphiphilic drugs with lipid A: implications for development of endotoxin antagonists. Biochim. Biophys. Acta 1212:167-175. [DOI] [PubMed] [Google Scholar]
- 7.David, S. A., R. Silverstein, C. R. Amura, T. Kielian, and D. C. Morrison. 1999. Lipopolyamines: novel antiendotoxin compounds that reduce mortality in experimental sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 43:912-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duwe, A. K., C. A. Rupar, G. B. Horsman, and S. I. Vas. 1986. In vitro cytotoxicity and antibiotic activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 30:340-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govorunov, I. G., N. V. Kosarev, I. Evtodienko, and E. O. Puchkov. 1982. [Permeability of the membrane of the Escherichia coli envelope for ethidium bromide.] Mikrobiologiia 51:731-734. (In Russian.) [PubMed] [Google Scholar]
- 10.Holzheimer, R. G. 2001. Antibiotic induced endotoxin release and clinical sepsis: a review. J. Chemother. 13:159-172. [DOI] [PubMed] [Google Scholar]
- 11.Jerala, R., and M. Porro. 2004. Endotoxin neutralizing peptides. Curr. Top. Med. Chem. 4:1173-1184. [DOI] [PubMed] [Google Scholar]
- 12.Jurgens, G., M. Muller, M. H. Koch, and K. Brandenburg. 2001. Interaction of hemoglobin with enterobacterial lipopolysaccharide and lipid A. Physicochemical characterization and biological activity. Eur. J. Biochem. 268:4233-4242. [DOI] [PubMed] [Google Scholar]
- 13.Kang, J. H., M. K. Lee, K. L. Kim, and K. S. Hahm. 1996. Structure-biological activity relationships of 11-residue highly basic peptide segment of bovine lactoferrin. Int. J. Pept. Protein Res. 48:357-363. [DOI] [PubMed] [Google Scholar]
- 14.Leon-Ponte, M., M. G. Kirchhof, T. Sun, T. Stephens, B. Singh, S. Sandhu, and J. Madrenas. 2005. Polycationic lipids inhibit the pro-inflammatory response to LPS. Immunol. Lett. 96:73-83. [DOI] [PubMed] [Google Scholar]
- 15.Lohner, K. 2001. The role of membrane lipid composition in cell targeting of antimicrobial peptides, p. 149-165. In K. Lohner (ed.), Development of novel antimicrobial agents: emerging strategies. Horizon Scientific Press, Norfolk, England.
- 16.Lohner, K., and E. J. Prenner. 1999. Differential scanning calorimetry and X-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim. Biophys. Acta 1462:141-156. [DOI] [PubMed] [Google Scholar]
- 17.Majerle, A., J. Kidrič, and R. Jerala. 2000. Production of stable isotope enriched antimicrobial peptides in Escherichia coli: an application to the production of a 15N-enriched fragment of lactoferrin. J. Biomol. NMR 18:145-151. [DOI] [PubMed] [Google Scholar]
- 18.Majerle, A., J. Kidrič, and R. Jerala. 2003. Enhancement of antibacterial and lipopolysaccharide binding activities of a human lactoferrin peptide fragment by the addition of acyl chain. J. Antimicrob. Chemother. 51:1159-1165. [DOI] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Moore, R. A., N. C. Bates, and R. E. Hancock. 1986. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 29:496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, D. C. 1998. Antibiotic-mediated release of endotoxin and the pathogenesis of gram-negative sepsis. Prog. Clin. Biol. Res. 397:199-207. [PubMed] [Google Scholar]
- 22.Mortimer, F. C., D. J. Mason, and V. A. Gant. 2000. Flow cytometric monitoring of antibiotic-induced injury in Escherichia coli using cell-impermeant fluorescent probes. Antimicrob. Agents Chemother. 44:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opal, S. M., J. E. Palardy, N. Parejo, and D. C. Morrison. 2001. Lipopolyamines as a therapeutic strategy in experimental Gram-negative bacterial sepsis. J. Endotoxin Res. 7:35-38. [PubMed] [Google Scholar]
- 26.Otvos, L., Jr. 2002. The short proline-rich antibacterial peptide family. Cell Mol. Life Sci. 59:1138-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rifkind, D. 1967. Prevention by polymyxin B of endotoxin lethality in mice. J. Bacteriol. 93:1463-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rustici, A., M. Velucchi, R. Faggioni, M. Sironi, P. Ghezzi, S. Quataert, B. Green, and M. Porro. 1993. Molecular mapping and detoxification of the lipid A binding site by synthetic peptides. Science 259:361-365. [DOI] [PubMed] [Google Scholar]
- 29.Ulevitch, R. J. 2000. Molecular mechanisms of innate immunity. Immunol. Res. 21:49-54. [DOI] [PubMed] [Google Scholar]
- 30.Vaara, M., and T. Vaara. 1983. Polycations as outer membrane-disorganizing agents. Antimicrob. Agents Chemother. 24:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]