Abstract
When bacteria assume the biofilm mode of growth, they can tolerate levels of antimicrobial agents 10 to 1,000 times higher than the MICs of genetically equivalent planktonic bacteria. The properties of biofilms that give rise to antibiotic resistance are only partially understood. Inhibition of antibiotic penetration into the biofilm may play a role, but this has not been proven directly. In this report, penetration of the glycopeptide antibiotic vancomycin into viable Staphylococcus aureus biofilms was analyzed by confocal scanning laser microscopy using a fluorescently labeled derivative of the drug. We found that while vancomycin bound to free-floating bacteria in water within 5 min, it took more than 1 h to bind to cells within the deepest layers of a biofilm. These results indicate that the antibiotic is transported through the depth of the biofilm but that the rate is significantly reduced with respect to its transport through flowing water. This suggests that, whereas planktonic bacteria were rapidly exposed to a full bolus of vancomycin, the bacteria in the deeper layers of the biofilm were exposed to a gradually increasing dose of the drug due to its reduced rate of penetration. This gradual exposure may allow the biofilm bacteria to undergo stress-induced metabolic or transcriptional changes that increase resistance to the antibiotic. We also investigated the role of poly-N-acetylglucosamine, an important component of the S. aureus biofilm matrix, and found that its production was not involved in the observed decrease in the rate of vancomycin penetration.
Staphylococcus aureus is an exceptionally adaptable organism and has repeatedly proven its ability to resist novel chemotherapeutic agents. Less than 20 years after the discovery of penicillin, the organism acquired the β-lactamase gene. In addition, methicillin resistance has been spreading rapidly in recent years (17), and the first fully vancomycin-resistant isolates have recently appeared (1, 3, 4). To complicate matters further, most strains of S. aureus are capable of assuming the biofilm mode of growth, and when bacteria are growing in a biofilm state, they are resistant to antibiotic levels 10- to 1,000-fold higher than genetically identical planktonic bacteria. Consequently, staphylococcal infections involving biofilm formation, which may include native valve endocarditis, chronic osteomyelitis, and medical device-related infections, can be extremely difficult to treat with antibiotics, are often chronic or relapsing, and frequently necessitate invasive procedures, such as removal of the infected tissue or device (5, 8).
The properties of biofilms that result in antibiotic resistance may include slow growth, phenotypic heterogeneity, the presence of persister cells, inactivation of antibiotics within the biofilm exopolysaccharide matrix, and limitations on antibiotic penetration imparted by the biofilm matrix (18). The relative contributions of these properties to resistance are not well understood. In particular, a role for the staphylococcal exopolysaccharide matrix, composed primarily of a polymer of β-1-6-linked N-acetylglucosamine (PNAG) in S. aureus and Staphylococcus epidermidis, as a diffusion barrier has not been conclusively established (14). A study with S. epidermidis suggests that PNAG restricts antibiotic penetration (13), and another found that preincubation of glycopeptides with staphylococcal polysaccharide “slime,” containing an unknown quantity of PNAG, reduced their efficacy (10). In contrast, others report free diffusion of certain antibiotics through staphylococcal biofilms (9, 22), and it has been argued that the diffusion coefficient of small molecules, such as antibiotics, through the biofilm exopolymeric matrix is roughly equivalent to the diffusion coefficient of water (19).
Most published studies of diffusion in biofilms focus on an endpoint after a number of hours and fail to address the rate with which antibiotics are transported. The rate of transport is important because mixing an antibiotic into a suspension of planktonic bacteria rapidly exposes all cells to the full antibiotic dose. If, however, the rate of antibiotic penetration through a biofilm is decreased with respect to the rate of transport through a liquid, such as media or blood, then the bacteria may be exposed to a gradually increasing dose of the antibiotic and may have time to mount a defensive response to the compound. In support of this idea, bacteria have been shown to increase transcription of stress-associated genes, such as heat shock protein homologues and cell wall synthesis genes within an hour of exposure to low doses of cell wall-active antibiotics (21). An additional problem with many previous studies is that they demonstrate that antibiotics move from one side of an intact biofilm to the other but do not prove that they actually reach their cellular target (22). Thus, antibiotics could traverse the biofilm through the exopolymeric matrix and water channels such that the bacteria are not actually exposed to an inhibitory dose.
The glycopeptide vancomycin exerts its bactericidal activity by binding to terminal d-Ala-d-Ala residues within the peptidoglycan wall, thereby blocking transpeptidation (16). Vancomycin is an important antistaphylococcal drug, but its efficacy against biofilms is particularly poor (15). We have taken advantage of the availability of a biologically active, fluorescently labeled derivative of vancomycin to analyze binding of the antibiotic to its target in the bacterial cell wall within a viable biofilm (7). Our results demonstrate that vancomycin binds rapidly to planktonic bacteria and binds quickly to the surface of the biofilm but that binding to cells within the deepest layers requires more than an hour of exposure to the antibiotic. Our findings suggest that this represents a decreased rate of penetration of vancomycin into the biofilm rather than an inability of the drug to bind to the bacteria. We also demonstrate that this effect is not dependent upon elaboration of the exopolysaccharide component of the biofilm matrix, PNAG.
MATERIALS AND METHODS
Staphylococcal strains and media.
S. aureus MN8 was originally isolated as the cause of a case of toxic shock syndrome by Patrick Schlievert, Minneapolis, MN. Strain MN8m is a spontaneous mutant of strain MN8 that exhibits greatly enhanced PNAG production (12). Strain NCTC 10833 (ATCC 25904) is a clumping factor-positive variant of a throat swab isolate and is commonly referred to as strain Newman in the literature. Partial deletion of the ica locus by insertional inactivation to produce strains 10833Δica::tet and MN8Δica::tet, which are PNAG negative, was performed by the method of Cramton et al. (6). The strains were grown at 37°C in tryptic soy broth containing 1% glucose (TSBG).
Antibiotic susceptibility testing.
For minimal bactericidal concentrations (MBCs) and time-kill curves of planktonic bacteria, overnight cultures were diluted to an optical density at 650 nm of 0.3 and then diluted 1:10 in fresh TSBG. For MBCs, 100-μl aliquots were placed in wells containing twofold dilutions of antibiotic (final concentrations of 0 to 20 μg/ml) for 24 h at 37°C. Viable cells were enumerated as follows; serial 10-fold dilutions of the contents of wells were made in duplicate, and 10 μl of each dilution was pipetted onto a tryptic soy agar (TSA) plate. The plate was tilted so that the 10-μl drop would run down the surface of the agar. Six drops could be plated simultaneously on a single TSA plate using a multichannel pipettor. Plates were incubated at 37°C overnight, and the viable bacteria were enumerated. For time-kill curves, a subinhibitory dose of antibiotic (1 μg/ml [0.58 nmol] BodipyFL-VAN [Molecular Probes, Eugene, OR] or 0.862 μg/ml [0.58 nmol] vancomycin), an inhibitory dose in the trough serum concentration range (15 μg/ml [8.7 μmol] BodipyFL-VAN or 12.9 μg vancomycin-HCl/ml [8.7 μmol]), or an inhibitory dose in the peak serum concentration range (45 μg/ml [26 nmol] BodipyFL-VAN or 38.6 μg/ml [26 nmol]) vancomycin was added, samples were taken every 1 to 2 h for 12 h, and viable cells were enumerated. For biofilms, overnight cultures were grown in TSBG and diluted 1:100 in fresh TSBG, and 200-μl aliquots were placed into individual wells of a flat-bottom, tissue culture-treated, 96-well microtiter plate (Corning, Acton, MA). The plates were incubated at 37°C for 24 h. Weak biofilm-producing strains were manipulated very carefully so that the bacteria were not dislodged from the wells, resulting in approximately equal numbers of bacteria present in the wells irrespective of the strain being tested. After 24 h, the media were removed, 200 μl TSBG containing dilutions of antibiotic was added (ranging from 0 to 2,500 μg/ml in 250-μg/ml increments), and the microtiter plates were incubated for 20 h at 37°C. The following day, the contents of the wells were gently sonicated (power level of 2) with the microtip of a sonic dismembrator to dislodge the biofilms and to break up aggregates, and the viable bacteria were enumerated. The number of bacteria remaining in each antibiotic dilution was compared to the number of bacteria per well before the addition of antibiotic, and the MBC was recorded as the concentration required to reduce the original bacterial count by 99.9%. All resistance determinations were performed in triplicate, and the statistical significance of the MBCs was obtained using an unpaired t test.
Flow cytometry.
Bacteria were grown planktonically in TSBG with shaking for 16 h. The planktonic bacteria were gently sonicated and diluted 1:10 in water, and the bacteria were incubated at 21°C in 2 μg BodipyFL-VAN/ml for 0 to 60 min. After incubation with the vancomycin derivative, bacteria were washed four times with H2O. Cell suspensions were subjected to flow cytometry using a fluorescein isothiocyanate (FITC) filter at the Children's Hospital FACS (fluorescence-activated cell sorting) Core Facility (Boston, MA). Each experiment was performed a minimum of three times with similar results.
CSLM.
Biofilms were grown overnight in TSBG in 35-mm collagen-coated glass coverslip-bottom dishes (MatTek Co., Ashland, MA). The following day, the media were removed, and the biofilm was washed once in H2O. All confocal images were obtained at the Harvard Center for Neurodegeneration and Repair, Boston, MA. Biofilms were observed using a 63× water immersion objective and a Zeiss 510 confocal microscope with 488-nm and 633-nm excitation wavelengths and 500- to 530-nm (green fluorescence representing BodipyFL-VAN and Syto 9) and >545-nm (light reflected by bacterial cells, which is depicted in red) emission filters. The biofilms were approximately 30 to 40 μm thick, and Z-slices were obtained every 2 microns. BodipyFL-VAN was added to the biofilm at a final concentration of 15 μg/ml (8.7 μmol) and Syto 9 at a final concentration of 3.5 μg/ml (8.7 μmol), and Z-stack images were obtained every 1 to 5 min for at least 1 h. Mixing by pipetting up and down was performed once per minute between scans for the duration of the time series. Side-view images of the biofilms were converted to Adobe Photoshop files. A region near the center of the biofilm was selected, a histogram of the region was obtained, and the mean intensity value of green pixels was divided by the mean intensity of the red pixels to obtain a semiquantitative measure of accumulation of the fluorophore in the biofilm. Ratios of green/red intensity values were obtained for the same region at different time points to obtain the rate of fluorophore transport. All confocal scanning laser microscopy (CSLM) experiments were performed three times with similar results, and standard deviations of green/red intensity ratios are shown.
Quantification of BodipyFL-VAN transport through transwells.
Transport of BodipyFL-VAN across polytetrafluoroethylene (PTFE), 12-well, 0.4-μm-pore-size transwell membranes (Corning, Acton, MA) was measured by adding 30 μg BodipyFL-VAN to 1.75 ml H2O in the lower chamber and measuring the fluorescence intensity of 10-μl samples taken from the 0.25 ml H2O in the upper chamber every 1 to 5 min. Light emitted in the 500- to 530-nm range was recorded in relative light units (RLU) using a 96-well plate format fluorimeter with 488-nm excitation. Continuous mixing was performed by adding a small magnetic stir bar to the lower chamber and stirring at approximately 100 rpm. For some of the experiments, the membrane was excised from the transwell, leaving the upper and lower chambers continuous.
RESULTS
Sensitivities of planktonic and biofilm bacteria to vancomycin.
We tested the MBCs of planktonic cells of S. aureus strains MN8m, MN8Δica::tet, 10833, and 10833Δica::tet to vancomycin and to BodipyFL-VAN. We found that for planktonic cultures, the activity of the fluorescent antibiotic was not significantly different from that of vancomycin (P > 0.05, t tests, for all pairwise comparisons of the two antibiotics against one strain), with MBCs ranging from 1.5 to 5 μg/ml (Fig. 1A). We noted that the MBCs of vancomycin and BodipyFL-VAN were significantly higher for the PNAG-negative strains MN8Δica::tet and 10833Δica::tet than for the PNAG-positive strains MN8m and 10833 (P < 0.05, one-way analysis of variance; P < 0.05 for MN8Δica::tet versus MN8m and P < 0.05 for 10833Δica::tet versus 10833, Tukey's multiple comparison tests for both vancomycin and BodipyFL-VAN) but did not pursue this finding further. Time-kill curves using a subinhibitory dose (0.58 nmol), an inhibitory dose in the trough serum concentration range (8.7 nmol), and an inhibitory dose of the antibiotics in the peak serum concentration range (26 nmol) demonstrated that the rate of killing of strain MN8Δica::tet by BodipyFL-VAN was at least as great as that of vancomycin (Fig. 1B).
FIG. 1.
Vancomycin MBCs for planktonic and biofilm cultures of S. aureus. A. The concentrations of vancomycin and BodipyFL-VAN required to effect a 99.9% kill (MBCs) of planktonically growing PNAG-negative strains MN8Δica::tet and 10833Δica::tet and the PNAG-positive strains MN8m and 10833 were not significantly different (P > 0.5) as determined by an unpaired t test. B. Time-kill curves for strain MN8Δica::tet using 1 μg/ml (solid black lines) BodipyFL-VAN (diamonds) or vancomycin (squares), 15 μg/ml (solid gray lines) BodipyFL-VAN (triangles) or vancomycin (crosses), or 45 μg/ml (dotted black lines) BodipyFL-VAN (crosses) or vancomycin (circles) demonstrate that the rate of killing by BodipyFL-VAN is at least a fast as vancomycin. C. The vancomycin MBCs for MN8Δica::tet, 10833Δica::tet, MN8m, and 10833 biofilms were 250- to 600-fold greater than the MBCs for planktonic cultures. All experiments were performed three times, and error bars representing standard deviations are shown.
We also tested the MBC for vancomycin of S. aureus growing in biofilms. It was not feasible to use concentrations of BodipyFL-VAN sufficient to kill the biofilm bacteria, so MBCs for biofilms were not determined with this agent. Vancomycin MBCs for biofilm bacteria were more than 200 times that for planktonic cultures, ranging from 750 to almost 2,000 μg/ml (Fig. 1C). Despite the important role played by PNAG in biofilm formation, its production did not increase vancomycin resistance of planktonic or sessile bacteria, and in fact, similar to planktonic bacteria, the PNAG-positive biofilms were significantly (P < 0.01 comparing each wild-type strain with its isogenic Δica::tet mutant by t test) more sensitive to vancomycin.
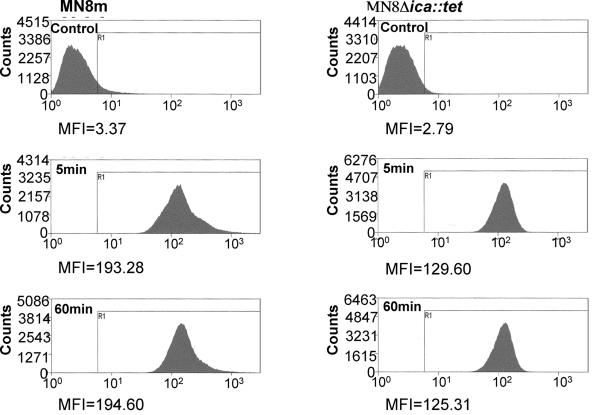
BodipyFL-vancomycin binds rapidly to planktonic bacteria.
We analyzed binding of BodipyFL-VAN to the cell wall of planktonically growing S. aureus by flow cytometry. Comparison of the relative mean fluorescence intensities (MFI) indicated that binding of BodipyFL-VAN to planktonic bacteria was very rapid and that maximum binding occurred within the time it took to wash the cells (approximately 5 min). Figure 2 shows that after 5 min of incubation with BodipyFL-VAN, the MFI of strain MN8m increased from 3.4 to 193.3. This MFI did not increase further over the next 60 min. Similarly, the MFI of PNAG-negative strain MN8Δica::tet at 5 min, 125.3, did not increase over the 60-min incubation. The experiment was repeated with the unrelated strains 10833 and 10833Δica::tet with similar results (data not shown). We noted that the level of fluorescence of BodipyFL-VAN-treated PNAG-producing strains (MFI of MN8m, 193.3; MFI of 10833, 169.2) was greater than the PNAG-negative strains (MFI of MN8Δica::tet, 129.6; MFI of 10833Δica::tet, 137.7), which is likely related to the lower vancomycin MBC of the PNAG-positive strains.
FIG. 2.
Flow cytometry of planktonic bacteria treated with BodipyFL-VAN demonstrates very rapid binding of drug to bacteria. Planktonic cells of S. aureus strains MN8m and MN8Δica::tet were treated with BodipyFL-VAN for 0 to 60 min, washed thoroughly, and analyzed by flow cytometry using a FITC filter. BodipyFL-VAN bound rapidly to the bacteria and increased the MFI of strain MN8m from 2.79 to 129.6 and the MFI of MN8Δica::tet from 3.37 to 193.3 within the time required to remove the antibiotic by washing the cells (5 min). There was no further increase in MFI after 60 min of incubation. Experiments were performed three times with comparable results.
Diffusion of vancomycin into biofilms of S. aureus strains MN8m and MN8Δica::tet.
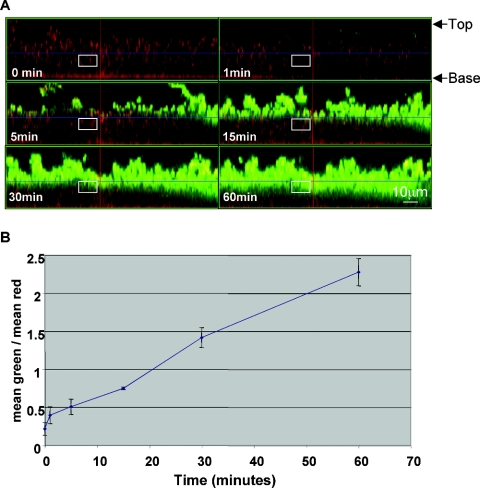
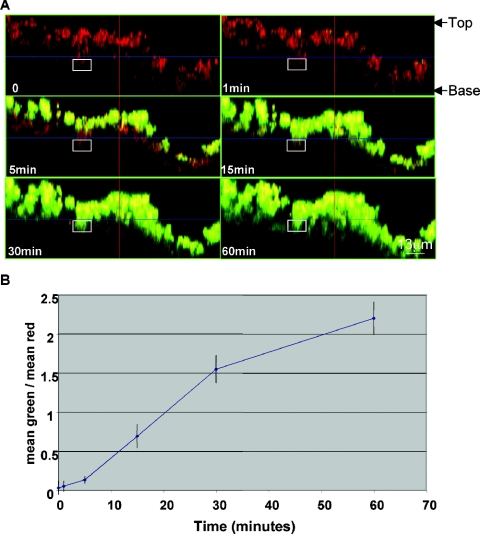
We next used confocal microscopy to characterize penetration of BodipyFL-VAN through S. aureus biofilms grown on collagen-coated glass. We found that the convective force applied to the antibiotic mixture over the biofilm was critical and that when the BodipyFL-VAN was added very gently to the water over the biofilm, the bacterial cells did not begin to exhibit fluorescence for 40 min. If, however, the antibiotic was mixed thoroughly with the water in the dish when it was added, then the surface of the biofilm became fluorescent within a minute. Therefore, after the addition of 15 μg BodipyFL-VAN/ml, the liquid over the biofilm was mixed by pipetting up and down once per minute for the duration of the experiment. Figures 3 and 4 (also refer to http://www.aureus.bwh.harvard.edu/) show that BodipyFL-VAN bound to the surfaces of the biofilms rapidly but that transport to deeper layers occurred relatively slowly over the course of 60 min. Even after incubation with the antibiotic for an hour, the bacteria within the deepest layers of the biofilm exhibited only minimal green fluorescence. Figures 3B and 4B display a semiquantitative measure of the rate of BodipyFL-VAN penetration through the biofilms. There was not a significant difference in the rate of BodipyFL-VAN penetration throughout the PNAG-negative and PNAG-positive biofilms.
FIG. 3.
Confocal microscopy of biofilms of S. aureus strain MN8m treated with BodipyFL-VAN shows the slow rate of penetration of antibiotic to cells deep within the biofilm. A. Side-view images of a PNAG-positive MN8m biofilm before and 1 to 60 min after the addition of 15 μg (8.7 μmol) BodipyFL-VAN/ml. The biofilms were characterized by peaks and valleys, and the maximum height of the peaks was approximately 45 μm. The top (liquid interface) and base (coverslip interface) of the biofilm are labeled. The white square indicates the region near the center of the biofilm that was converted to a histogram to obtain mean intensity values for green and red. Penetration of BodipyFL-VAN (green) to the bacterial cells (red) deeper in the biofilm layer was not even complete by 60 min. B. The rate of BodipyFL-VAN penetration is depicted as the ratio of the mean intensity value of green to red within the selected area (y axis) with respect to the time elapsed following addition of BodipyFL-VAN. The experiment was repeated four times with similar results. Symbols represent means, and error bars represent the standard deviations.
FIG. 4.
Confocal microscopy of S. aureus strain MN8Δica::tet biofilms treated with BodipyFL-VAN shows the slow rate of penetration of antibiotic to cells deep within the biofilm. A. Side-view images of a PNAG-negative MN8Δica::tet biofilm before and 1 to 60 min after the addition of 15 μg BodipyFL-VAN/ml. The height of the biofilm was approximately 30 μm. Penetration of BodipyFL-VAN (green color) to the bacterial cells deeper in the biofilm layer was not even complete by 60 min and was not different from that seen with PNAG-producing strain MN8m (see Fig. 3). B. The rate of BodipyFL-VAN penetration is depicted as the ratio of the mean intensity value of green to red within the selected area (y axis) with respect to the time elapsed following addition of BodipyFL-VAN. The experiment was repeated three times with similar results.
In order to rule out the possibility that the fluorescence at the bottom of the biofilm was low due to poor light penetration, we treated a biofilm with 15 μg/ml BodipyFL-VAN for 15 min, inverted the coverslip, and analyzed the upside-down biofilm by CSLM. In Fig. 5 green fluorescence at the top of the confocal image, which is the base of the biofilm, is weak and fluorescence at the bottom of the image, which is the top of the biofilm, is strong, indicating the 488-nm laser penetrates the biofilm effectively. We did note, however, that the red fluorescence reflected by the cells was brighter near the top of both upside-down (Fig. 5A, bottom of biofilm) and right-side-up images (Fig. 3A, 4A, and 6A, top of biofilm), suggesting that penetration of the longer-wavelength (633-nm) laser or the light emitted (>545 nm) was partially inhibited by the biofilm.
FIG. 5.
Confocal imaging of an upside-down biofilm shows that 488-nm excitation and 512-nm emission wavelengths penetrate the biofilm. A biofilm of S. aureus strain MN8m was treated for 15 min with 15 μg BodipyFL-VAN/ml after which the coverslip was inverted and imaged “upside down” by CSLM. In this orientation the laser light first penetrated the coverslip from the bottom of the biofilm to the top, producing an inverted image. The base (coverslip interface) and top of the biofilm are indicated.
FIG. 6.
Transport of Syto 9 through biofilms is similar to that of BodipyFL-VAN. A. Side-view images of an S. aureus strain MN8m biofilm before and 1 to 60 min after the addition of 3.5 μg (8.7 μmol) Syto 9/ml. The top (liquid interface) and base (coverslip interface) of the biofilm are labeled. The white square indicates the region near the center of the biofilm that was converted to a histogram to obtain mean intensity values for green and red. Penetration of Syto 9 (green) through the layers of bacterial cells (red) was slow and similar to that of BodipyFL-VAN. B. The rate of BodipyFL-VAN penetration is depicted as the ratio of the mean intensity value of green to red within the selected area (y axis) with respect to the time elapsed following addition of Syto 9. The experiment was performed in triplicate with similar results.
Finally, to test the possibility that BodipyFL-VAN was unable to bind the bacteria on the bottom of the biofilm for a reason other than slow transport (such as slow growth), we treated biofilms for 15 min with BodipyFL-VAN, then dislodged the bacteria by sonication in the presence of the antibiotic, and analyzed their MFI by flow cytometry. Flow cytometry revealed a relatively homogeneously fluorescent population of bacteria without any detectable unstained cells (data not shown), suggesting that, if it has access, BodipyFL-VAN is able to bind to cells on the bottom of the biofilm.
Influence of solute size on the rate of transport.
BodipyFL-VAN is relatively large (molecular weight [MW], 1,723) in comparison to other antibiotics. We therefore analyzed the transport of a small fluorescent nucleic acid stain, Syto 9 (MW, 400), through biofilms by CSLM. The overall dynamic of Syto 9 transport was similar to that of BodipyFL-VAN with binding to the superficial layers of the biofilms occurring rapidly andtransport to the deeper layers occurring gradually over the 60-min time course (Fig. 6). Also similar to BodipyFL-VAN, maximal binding of Syto 9 to planktonic bacteria occurred within 5 min (data not shown).
Roles of convective forces on BodipyFL-VAN transport.
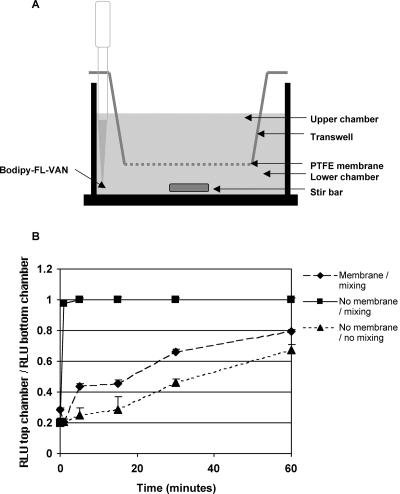
In order to test whether the reduced rate of BodipyFL-VAN transport was due to a specific characteristic of biofilms or whether any “barrier” with a small pore size would produce a similar effect, we analyzed the rate of BodipyFL-VAN transport from the bottom chamber of a transwell, through either water alone or through a membrane, to the upper chamber by fluorimetry. Figure 7 illustrates that in the absence of mixing with a magnetic stir bar, BodipyFL-VAN diffused very slowly into the upper chamber of the transwell, even in the absence of a transwell membrane. When a magnetic stir bar was added to the system to exert a mixing force in the absence of the membrane, the concentration of the fluorophore was equal in the upper and lower chambers within a minute. However, when the transwell membrane was left in place, transport of BodipyFL-VAN was relatively slow, even when the system was mixed vigorously and constantly. This finding suggests that the transwell membrane inhibited transport of BodipyFL-VAN by convective forces.
FIG. 7.
Influence of mixing and the presence of a PTFE membrane on transport of BodipyFL-VAN. A. Schematic representation of the transwell apparatus used to measure BodipyFL-VAN transport. B. The 0.4-μm-pore-size PTFE membrane in 12-well transwells was either left intact (diamonds) or carefully excised (squares and triangles). BodipyFL-VAN/ml (final concentration, 15 μg) was added to the base of the lower chamber either in the absence of a mixing force (triangles) or in the presence of a magnetic stirrer (diamonds and squares). Transport of BodipyFL-VAN was monitored by fluorimetric analysis of 10-μl aliquots taken from the upper chamber once per minute. The y axis represents the ratio of the RLU detected in the upper chamber versus RLU in the lower chamber where a ratio of 1 represents equilibrium between the chambers. The x axis represents the time elapsed following the addition of BodipyFL-VAN.
DISCUSSION
We used the fluorescently labeled vancomycin derivative BodipyFL-vancomycin, which has been previously shown to bind to the cell wall of S. aureus, to analyze antibiotic penetration through biofilms by confocal microscopy (7). We found in this study that binding of vancomycin to the bacterial cells within the deepest layers of a S. aureus biofilm was not detected for nearly 60 min, whereas maximal binding of the antibiotic to planktonic cells occurred within the time it took to wash the cells (about 5 min). Vancomycin concentrations in serum can range from 15 to 45 μg/ml, and the concentration of BodipyFL-VAN used in our experiments falls within this range, suggesting that the decreased rate of transport is likely to be physiologically relevant. The relatively slow and gradual accumulation of the antibiotic within the deeper layers of the biofilms suggests that the bacteria may encounter a subinhibitory dose of the drug before they are exposed to the full dose. As bacteria have been shown to mount a transcriptional stress response to low doses of cell wall-active antibiotics within an hour (21), it is conceivable that the resistance of bacteria in the deeper layers of the biofilm may actually increase after administration of the antibiotic. The cells may be able to mount a response at the level of transcription or protein secretion in time to defend themselves against the lethal effects of the antibiotic. For example, the alternative sigma factor σB, which controls a large regulon of genes that encode efflux pumps, multidrug resistance determinants, heat shock proteins, and other stress-related proteins, could be induced so that the cells become more resistant to the antibiotic challenge (2). In contrast to the popular belief that characteristics specific to bacterial biofilms make them “constitutively” resistant to antibiotics, this idea suggests that resistance may be at least partially inducible.
Our finding that fluorophore transport in biofilms is gradual over a period of at least 60 min is in contrast to those of Stone et al. who found, using CSLM, that all cells within an Escherichia coli biofilm are rapidly exposed to tetracycline (20). We considered the possibility that tetracycline may penetrate the biofilm more rapidly than vancomycin due to its smaller size (MW of BodipyFL-VAN, 1,723; MW of tetracycline, 500) and tested this by analyzing transport of the fluorescent nucleic acid stain Syto 9 (MW, 400). We found that the transport of Syto 9 through the biofilms was similar to that of BodipyFL-VAN and that it was also significantly slower than its transport through mixed water to planktonic bacteria. It is unlikely that our contrasting findings are due to the different bacterial species composing the biofilms because we found that a PTFE transwell membrane with a pore size and thickness similar to those of our biofilms was also an effective barrier against transport of the fluorescent molecule. The finding by Stone et al. that tetracycline is rapidly transported through biofilms may be related to the fact that the antibiotic was not fluorescent but rather triggered fluorescence of TetR9B, a bacterial protein involved in tetracycline resistance. It is therefore possible that exposure of the bacteria on the biofilm surface to tetracycline started a “chain reaction” through the biofilm bacteria. Another possibility is that tetracycline is somehow actively transported by the cells in the biofilm.
The intercellular adhesin locus (ica) encodes the proteins necessary for synthesis of the major polysaccharide component of an S. aureus biofilm matrix, PNAG. We used two ica deletion mutants in this study to characterize the effect of PNAG in vancomycin penetration. Strains MN8Δica::tet and 10833Δica::tet are very weak biofilm producers but will grow as a multilayered mat on the surface of an undisturbed polystyrene tissue culture dish. These bacterial mats are not durable like true biofilms and were therefore manipulated very gently. Despite the fact that the ica mutant strains do not produce a substantial biofilm, the bacterial mats are referred to in this work as biofilms for simplicity. We found that PNAG was not required for limiting antibiotic penetration and that penetration of the labeled vancomycin through PNAG-negative mats of bacteria occurred at a rate comparable to that in a PNAG-encased biofilm. We also tested the effect of a transwell membrane on BodipyFL-VAN penetration. PTFE membranes were chosen because they have a torturous pore path, similar to biofilms. The thickness of the membranes, 50 μm, approximated that of biofilms formed by strain MN8m, and the pore size, 0.4 μm, was chosen as a rough estimate of the pore size within biofilms. Similar to biofilms, the PTFE membranes inhibited the rate of BodipyFL-VAN penetration, suggesting that any barrier with a small pore size can inhibit convective forces and reduce the rate of solute transport and that the inhibition of BodipyFL-VAN transport by S. aureus biofilms is a nonspecific effect rather than a unique characteristic of biofilms.
Interestingly, we also noted that if BodipyFL-VAN was added very carefully to the biofilms, then no bacterial labeling, not even bacteria on the surface of the biofilm, occurred within the first 40 min of incubation. Together these results suggest that when BodipyFL-VAN is added to cells in suspension, the mixing velocity (convective forces), which is high due to the forces of pipetting and motion of the culture tube, plays a more important role than diffusion on transport of the antibiotic in solution. In the biofilm system, when the mixing velocity in the liquid above the biofilm was high, the antibiotic bound quickly to the surface of the biofilm. We hypothesize that within the biofilm itself, however, mixing is limited by the bacterial biomass, and diffusion, rather than mixing, becomes the predominant force. The idea that diffusion, rather than convection, is the predominant force behind transport through cell clusters within biofilms has been put forth by Stewart (19), but our results suggest that convective forces are low throughout the biofilm.
The relative roles of mixing and diffusion are represented in fluid dynamics by the Péclet number (Pe), which is equal to the length of distance traveled (L) times the mixing or convection velocity (V), divided by the diffusion coefficient (D) and is represented as Pe = LV/D (11). We hypothesize, therefore, that in planktonic cultures, under normal conditions, mixing is high (for example, pipetting or vortexing in vitro and blood flow in vivo), the Péclet number is high, and the diffusion constant does not play an important role in transport of the antibiotic. Within a biofilm, however, mixing is restricted by the bacterial cells, and the Péclet number is likely low, making diffusion the predominant force behind antibiotic transport (19). Our finding that, in the absence of convective forces, the concentration of BodipyFL-VAN in the upper chamber of a transwell in which the membrane had been excised was lower than the concentration in the bottom chamber even after 60 min indicates that transport by diffusion alone is quite slow. We hypothesize that, in agreement with previous reports, there is not a significant difference between the diffusion coefficient of vancomycin in the biofilm matrix with diffusion in water but that transport by diffusion is much slower than transport by mixing (19). In certain types of biofilm-related diseases, such as catheter infections, limitations on mixing within the biofilm may be considerably more important than the diffusion rate.
In addition to limitations of convective forces, another possible cause for the reduced rate of BodipyFL-VAN transport in biofilms is depletion of the drug solution by the bacterial cells in the superficial layer of the biofilm. Analysis of the BodipyFL-VAN solution by fluorimetry following exposure to the biofilm did indicate an approximately 30% reduction in fluorescence after 1 h (data not shown). Even though 2 μg/ml BodipyFL-VAN is enough to kill bacteria, it is still possible that the cells can bind considerably more of the antibiotic.
We were surprised to find in our studies that not only did PNAG elaboration fail to increase the level of vancomycin resistance of a given strain but that it actually decreased the resistance of S. aureus to the drug. The amount of BodipyFL-VAN that bound to PNAG-producing cells, a measure of fluorescence intensity, was greater than that for PNAG-negative strains, suggesting that the increase in vancomycin sensitivity is due to increased binding of the drug. The increased binding could be due to a decrease in carboxypeptidase or transpeptidase activity, both of which could result in a greater number of terminal d-Ala-d-Ala residues within the cell wall. Alternatively, the increased vancomycin binding and sensitivity could be due to a higher rate of cell wall turnover. It is also possible that vancomycin actually binds to PNAG. We are currently pursuing the basis for augmented vancomycin binding to PNAG-producing strains in an attempt to gain further insight into the interplay of the various factors involved in biofilm formation by staphylococci and resistance to key antibiotics.
Acknowledgments
We thank Mandana Farhadi for performing the FACS analysis and Michelle Ocana and Milan Bajmoczi for technical help with CSLM. We also thank Lakshminarayanan Mahadevan for invaluable advice about fluid dynamics.
This work was supported by NIH grants F32AI51892 and R21AI61590-01 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bartley, J. 2002. First case of VRSA identified in Michigan. Infect. Control Hosp. Epidemiol. 23:480. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff, M., P. Dunman, J. Kormanec, D. Macapagal, E. Murphy, W. Mounts, B. Berger-Bachi, and S. Projan. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 186:4085-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 4.Centers for Disease and Prevention. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 8.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 9.Dunne, W. M., Jr., E. O. Mason, Jr., and S. L. Kaplan. 1993. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 37:2522-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber, B. F., M. H. Kaplan, and A. G. Clogston. 1990. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J. Infect. Dis. 161:37-40. [DOI] [PubMed] [Google Scholar]
- 11.Hwang, C. W., D. Wu, and E. R. Edelman. 2001. Physiological transport forces govern drug distribution for stent-based delivery. Circulation 104:600-605. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson, K. K., S. E. Cramton, F. Gotz, and G. B. Pier. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 48:889-899. [DOI] [PubMed] [Google Scholar]
- 13.Konig, C., S. Schwank, and J. Blaser. 2001. Factors compromising antibiotic activity against biofilms of Staphylococcus epidermidis. Eur. J. Clin. Microbiol. Infect. Dis. 20:20-26. [DOI] [PubMed] [Google Scholar]
- 14.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monzon, M., C. Oteiza, J. Leiva, M. Lamata, and B. Amorena. 2002. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn. Microbiol. Infect. Dis. 44:319-324. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds, P. E., and E. A. Somner. 1990. Comparison of the target sites and mechanisms of action of glycopeptide and lipoglycodepsipeptide antibiotics. Drugs Exp. Clin. Res. 16:385-389. [PubMed] [Google Scholar]
- 17.Richet, H. M., M. Benbachir, D. E. Brown, H. Giamarellou, I. Gould, M. Gubina, P. Heczko, S. Kalenic, M. Pana, D. Pittet, S. B. Redjeb, J. Schindler, C. Starling, M. J. Struelens, W. R. Witte, and S. Jarvis. 2003. Are there regional variations in the diagnosis, surveillance, and control of methicillin-resistant Staphylococcus aureus? Infect. Control Hosp. Epidemiol. 24:334-341. [DOI] [PubMed] [Google Scholar]
- 18.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 19.Stewart, P. S. 1998. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol. Bioeng. 59:261-272. [DOI] [PubMed] [Google Scholar]
- 20.Stone, G., P. Wood, L. Dixon, M. Keyhan, and A. Matin. 2002. Tetracycline rapidly reaches all the constituent cells of uropathogenic Escherichia coli biofilms. Antimicrob. Agents Chemother. 46:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utaida, S., P. M. Dunman, D. Macapagal, E. Murphy, S. J. Projan, V. K. Singh, R. K. Jayaswal, and B. J. Wilkinson. 2003. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149:2719-2732. [DOI] [PubMed] [Google Scholar]
- 22.Zheng, Z., and P. S. Stewart. 2002. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 46:900-903. [DOI] [PMC free article] [PubMed] [Google Scholar]