Abstract
The pharmacokinetics of imipenem were studied in adult intensive care unit (ICU) patients during continuous venovenous hemofiltration (CVVH; n = 6 patients) or hemodiafiltration (CVVHDF; n = 6 patients). Patients (mean ± standard deviation age, 50.9 ± 15.9 years; weight, 98.5 ± 15.9 kg) received imipenem at 0.5 g every 8 to 12 h (total daily doses of 1 to 1.5 g/day) by intravenous infusion over 30 min. Pre- and postmembrane blood (plasma) and corresponding ultrafiltrate or dialysate samples were collected 1, 2, 4, and 8 or 12 h (depending on dosing interval) after completion of the drug infusion. Drug concentrations were measured using validated high-performance liquid chromatography methods. Mean systemic clearance (CLS) and elimination half-life (t1/2) of imipenem were 145 ± 18 ml/min and 2.7 ± 1.3 h during CVVH versus 178 ± 18 ml/min and 2.6 ± 1.6 h during CVVHDF, respectively. Imipenem clearance was substantially increased during both CVVH and CVVHDF, with membrane clearance representing 25% and 32% of CLS, respectively. The results of this study indicate that CVVH and CVVHDF contribute to imipenem clearance to a greater degree than previously reported. Imipenem doses of 1.0 g/day appear to achieve concentrations adequate to treat most common gram-negative pathogens (MIC up to 2 μg/ml) during CVVH or CVVHDF, but doses of 2.0 g/day or more may be required to adequately treat and prevent resistance in pathogens with higher MICs (MIC = 4 to 8 μg/ml). Higher doses should only be used after consideration of potential central nervous system toxicities or other risks of therapy in these severely ill patients.
Imipenem-cilastatin is frequently used in critically ill patients due to its broad spectrum of potent antimicrobial activity against many common gram-positive and gram-negative pathogens, including Pseudomonas aeruginosa, that has been maintained through many years of clinical use (8, 20). A number of published studies have evaluated the pharmacokinetics of imipenem and cilastatin in critically ill patients with severe acute renal failure undergoing continuous renal replacement therapies (CRRT) (14, 19, 25, 32, 38, 43). However, there are important questions regarding the applicability of these previous studies to current clinical practice. These older studies are probably no longer useful as guides in the dosing of imipenem-cilastatin due to the current use of newer high-flux, large-surface-area hemofilters that were not available when these studies were performed (34). In addition, several of these older studies evaluated drug dosing during continuous arteriovenous procedures that are no longer routinely used in favor of the better tolerated and less invasive venovenous techniques, such as continuous venovenous hemofiltration (CVVH) (19, 32, 43). Even more recently published studies may no longer be adequate to guide imipenem-cilastatin dosing due to rapid advances in CRRT techniques (14, 38). For example, a study of imipenem pharmacokinetics during continuous venovenous hemodiafiltration (CVVHDF) utilized ultrafiltration rates of 60 to 180 ml/h (14). However, in two recently published studies of patients receiving CVVH at our institution, ultrafiltration rates of 700 to 1,400 ml/h were utilized (23, 24). Since ultrafiltration rate is one of the primary determinants of drug clearance during CRRT (7, 12, 17), it is unknown whether the results of previous studies can be used to make accurate imipenem dosing recommendations during CRRT using current methods.
Current CRRT techniques have the potential to increase systemic drug clearance to a greater degree than anticipated based on previously published recommendations. Critically ill patients also often have larger volumes of distribution for antimicrobials than less severely ill or healthy persons due to a number of factors, including alterations in protein binding and increased total fluid volumes (1, 31, 40). The net result of these changes in critically ill patients may be plasma concentrations of antimicrobials that are substantially lower than expected. The lack of clinical data regarding imipenem-cilastatin dosing in patients receiving modern CRRT is thus of concern due to the potential for unanticipated pharmacokinetic alterations with associated poor clinical and microbiological outcomes.
The primary objective of the present study was therefore to more fully characterize the pharmacokinetic disposition of imipenem in critically ill adult intensive care unit (ICU) patients during currently used CVVH or CVVHDF regimens. The present study also evaluated the pharmacodynamic adequacy of prescribed dosing regimens by comparing imipenem concentrations achieved with MICs of pathogens isolated from study patients as well as those reported in the medical literature for typical ICU pathogens.
MATERIALS AND METHODS
Patient eligibility.
This was a prospective open-label study of imipenem-cilastatin administered as the combination product in a fixed 1:1 ratio (Merck & Co., Inc., Whitehouse Station, NJ). All adult patients greater than 18 years of age who were hospital inpatients in a medical, surgical or burn/trauma ICU, who were prescribed imipenem-cilastatin as part of their required medical care, and who were receiving CRRT for treatment of severe renal failure were eligible for inclusion in this study. Exclusion criteria included age less than 18 years or use of conventional hemodialysis rather than CRRT. The study was approved by the Institutional Review Board of the hospital where the study was performed, and written informed consent was obtained from all patients or their legally designated representatives prior to study entry.
Medications.
Patients enrolled into the study received imipenem-cilastatin as part of their medical care. Dosing regimens were determined by the physicians caring for the patients and selected based on clinical indication, institutional dosing guidelines, and published dosing guides available to the medical house staff (10). Imipenem-cilastatin regimens thus consisted of 0.5-g doses administered intravenously every 8 to 12 h for total daily doses ranging from 1.0 to 1.5 g/day. Imipenem-cilastatin doses were infused over 30 min. Specific dosing regimens and times of administration were recorded for each study patient. Complete medical histories were obtained for each enrolled patient, and complete physical examinations and laboratory review of blood chemistry and hematology profiles were performed and reviewed prior to collection of samples for pharmacokinetic analysis.
CRRT technique.
For all patients, CRRT was administered using a Hospal BSM-22SC machine (CGH Medical, Lakewood, CO) with a Multiflow60 AN69HF 0.60-m2 polyacrylonitrile hollow fiber membrane (Hospal Industrie, Meyzieu, France). Vascular access was obtained by introduction of a 12 French, 20-cm double-lumen central venous catheter (Arrow, Reading, PA) into a femoral vein. CRRT was managed by the renal consult service caring for the patient, and parameters such as blood flow rate (Qb) and dialysate flow rate (Qd) for those receiving CVVHDF were adjusted as therapeutically necessary. Replacement fluids usually consisted of 0.9% sodium chloride alternating with 0.45% sodium chloride plus 75 meq/liter of sodium bicarbonate; these fluids were delivered postmembrane via a volumetric pump. During CVVHDF, dialysate fluids (Premixed dialysate for hemodiafiltration; Baxter Healthcare, Deerfield, IL) were also delivered via a volumetric pump into the dialysate compartment of the filter in a direction countercurrent to the blood flow. Additional electrolytes such as calcium and potassium were added to replacement and dialysate fluids as required. The extracorporeal circuit was anticoagulated as clinically indicated with heparin sodium at rates ranging from 100 to 1,100 IU/h. Data for parameters such as Qb, Qd, and ultrafiltrate flow rate (Quf) were obtained from the CRRT hourly monitoring logs kept for each patient. Urine output data were obtained from routine ICU patient monitoring data sheets.
Sample collection and storage.
In order to obtain plasma imipenem concentrations at or near steady state, pharmacokinetic sampling was performed at least 24 h after initiation of the CRRT and drug therapy and after obtaining informed consent. Pre- and postmembrane venous blood samples were obtained at time zero (just before the beginning of the drug infusion), 1, 2, 4, and 8 h after the completion of the drug infusion in all patients. Samples were also obtained in all patients just before administration of the next dose (either 8 or 12 h after the previous dose, depending on specific dosing interval ordered) whenever possible. Samples (4 ml) were taken from the in-line blood access port in the extracorporeal circuit and collected into tubes containing EDTA as an anticoagulant. Because imipenem is rapidly hydrolyzed in plasma through a pH-dependent reaction (37), blood samples were centrifuged immediately after collection and aliquots of plasma were mixed 1:1 with 0.5 M morpholinoethanesulfonic acid (MES; Fisher Scientific, Pittsburgh, PA) buffer solution at a pH of 6.0 in labeled polyethylene vials. Dialysate and/or ultrafiltrate samples (20 ml) were obtained simultaneous with blood samples in order to determine sieving coefficients and filter clearances. Dialysate and/or ultrafiltrate samples were collected from the CRRT apparatus directly into labeled polyethylene containers containing 0.5 M morpholinopropanesulfonic acid (MOPS; Fisher Scientific, Pittsburgh, PA) buffer solution in a quantity sufficient to achieve a 1:20 buffer-sample mixture at pH 6.9. Buffered plasma, dialysate, and ultrafiltrate samples were frozen at −80°C immediately after processing and kept frozen until assayed.
Sample assay.
Imipenem concentrations in plasma and dialysate/ultrafiltrate were determined using reversed-phase high-performance liquid chromatography (HPLC) with UV detection according to previously published methods with minor adaptations (13, 38). The HPLC system utilized a Novapak C18 4.6-mm by 150-mm column with a guard column containing Novapak C18 inserts (Waters, Milford, MA), and the detector was set at a wavelength of 298 nm. The mobile phase consisted of 0.2 M boric acid-methanol (3:97 [vol/vol]) adjusted to a pH of 7.2. Analytical-grade imipenem powder for validation and quality control of the HPLC assay was supplied by Merck & Co., Inc. (Whitehouse Station, NJ). Ceftriaxone (Sigma, St. Louis, MO) was used as an internal standard. The extraction procedure for buffered plasma samples involved precipitation of proteins with methanol followed by centrifugation. After vortexing, the organic and aqueous phases were separated by centrifugation, and an aliquot of the aqueous phase was injected into the HPLC system. No extraction was performed on the dialysate/ultrafiltrate samples; these were injected directly into the system.
Coefficients of determination (r2) for the plasma imipenem assay over the standard curve concentration ranges (0.25 to 200.0 μg/ml) were 0.996 to 0.999 for the entire study. For this study, within-day coefficients of variation (CV) for plasma imipenem samples were 1.6%, 0.9%, and 1.1% at concentrations of 2, 50, and 150 μg/ml, respectively. Between-day CV for plasma samples were 2.2%, 1.7%, and 1.3% at concentrations of 2, 50, and 150 μg/ml, respectively. Coefficients of variation (r2) for the ultrafiltrate/dialysate imipenem assay over the standard curve concentration ranges (0.25 to 100.0 μg/ml) were in the range of 0.997 to 1.000 for the entire study. For this study, the within-day CV for dialysate/ultrafiltrate samples were 3.3%, 2.5%, and 2.1% at concentrations of 1, 10, and 50 μg/ml, respectively. Between-day CV for dialysate/ultrafiltrate samples were 3.0%, 2.6%, and 2.3% at concentrations of 1, 10, and 50 μg/ml, respectively. The lower limit of imipenem quantitation in both plasma and ultrafiltrate/dialysate samples for this study was 0.25 μg/ml, the lower limit of the standard curves.
Pharmacokinetic analysis.
Plasma concentration-time data for imipenem were analyzed by standard pharmacokinetic methods. Imipenem has previously been demonstrated to follow a two-compartment open model during CRRT (38); however, curve fitting of data points obtained in this study (GraphPad Prism version 4.01 for Windows; GraphPad Software, San Diego, CA) demonstrated that a one-compartment open model with first-order elimination performed equally well in describing imipenem pharmacokinetics. Premembrane plasma drug concentrations were used to determine pharmacokinetic parameters. The apparent terminal elimination rate constant (kel) was determined by least-squares regression analysis of the terminal portion (last three to four concentration-versus-time points) of the natural log concentration-time curve. Elimination half-life (t1/2) was calculated as 0.693/kel. Maximum plasma drug concentration (Cmax) was calculated as Cfirst/e−kt, where Cfirst is the first measured plasma concentration (approximately 1 h postinfusion), k is kel, and t is the time from the end of the drug infusion to Cfirst. Minimum plasma concentration (Cmin) was determined by direct measurement or, in some patients, calculated as Clast × e−kt, where Clast is the last measured plasma concentration, k is kel, and t is the time from Clast to the end of the dosing interval. The area under the concentration-time curve from time zero to the end of the 24-h dosing interval (AUC0-24) was calculated by the linear trapezoidal summation method. For patients in whom imipenem was administered every 12 h, the total 24-h AUC was calculated as AUC0-12 × 2; for patients receiving imipenem every 8 h, the total 24-h AUC was calculated as AUC0-8 × 3. Since true pharmacokinetic steady-state conditions could not be assumed in all patients, volume of distribution (V) was calculated by non-steady-state methods which take into account the number of doses previously administered (36). Total systemic clearance (CLS) was calculated by dose/AUC0-τ, the AUC from time zero to the end of the dosing interval. Since Cmax measured in this study represents total (bound plus free) drug, total drug concentrations were converted to maximum free concentrations (Cmax,free) by measured Cmax × 0.83, the approximate free fraction of imipenem in human plasma. The time during which plasma concentrations of free drug are above the MIC of the infecting pathogen (T > MIC) was then calculated as natural log (Cmax, free/CMIC)/kel, where CMIC is the MIC for the organism. The percent T > MIC was determined by (T > MIC/τ) × 100, where τ is the dosing interval. The targeted goal for percent T > MIC was >40 to 50% for clinical efficacy and prevention of resistance (3, 4, 6, 39). MICs of imipenem for isolated pathogens were determined by the clinical microbiology laboratory at the institution where the study was performed. Predicted T > MIC for dosing regimens not actually observed in study patients (i.e., imipenem at 0.5 g every 6 h) were calculated based on pharmacokinetic parameters derived from individual patients within the CVVH and CVVHDF groups.
Principles of calculating drug clearances during CRRT are reviewed elsewhere (2, 33, 35). During CVVH, the only mechanism of drug removal is convection, the removal of plasma solutes by ultrafiltration of plasma fluid. The sieving coefficient (S), the ability of a drug to pass through the hemofilter membrane, was calculated as 2 × Cuf/(Ca + Cv), where Cuf is the drug concentration in ultrafiltrate, Ca is the drug concentration in premembrane plasma, and Cv is the drug concentration in postmembrane plasma. Clearance of drug across the membrane during CVVH (CLCVVH) was calculated by S × Quf.
Drug clearance by CVVHDF occurs by diffusion across the filter as well as convection. The saturation coefficient (Sa), the ability of a drug to diffuse through the membrane to dialysate fluid, was calculated in the same manner as the sieving coefficient: 2 × Cuf/d/(Ca + Cv), where Cuf/d is the concentration of drug in combined ultrafiltrate/dialysate. Drug clearance by CVVHDF (CLCVVHDF) was calculated as Sa × (Quf + Qd).
The percentage of CLS contributed by CLCVVH or CLCVVHDF (%CLS) is calculated as either (CLCVVH/CLS) × 100 or (CLCVVHDF/CLS) × 100, respectively. Since renal clearance was considered to be negligible in these patients with acute renal failure and minimal urine output, non-renal clearance (CLNR) was calculated as CLS − CLCVVH or CLCVVHDF as appropriate.
All calculations were made by programming pharmacokinetic and CRRT clearance equations into Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA) spreadsheets. Also using Excel, measures of central tendency and variability were evaluated for all patient and CRRT characteristics, pharmacokinetic parameters, and CRRT clearances.
Statistical analysis.
Differences between demographic variables among patients receiving either CVVH or CVVHDF during administration of imipenem were assessed for statistical significance using the one-way analysis of variance fixed-effects model for continuous variables or two-way chi-square test for categorical variables. Differences among calculated pharmacokinetic parameters were assessed by two-tailed Mann-Whitney rank sum test for unpaired nonparametric data. Correlations between pharmacokinetic variables were determined using Spearman's rank correlation coefficient for nonparametric data. All statistical tests were performed using GraphPad InStat version 3.00 for Windows (GraphPad Software, San Diego, CA). P values of ≤ 0.05 were considered significant.
RESULTS
A total of 12 patients were enrolled into the study and completed the scheduled pharmacokinetic sampling. Detailed information regarding patient demographics and CRRT is given in Tables 1 and 2. There were no statistically significant differences in sex, age, weight, or APACHE II scores among patients receiving CVVH versus CVVHDF. Imipenem was apparently well tolerated in all patients, and no drug-related adverse effects were reported or detected during the study.
TABLE 1.
Demographic and clinical characteristics of 12 critically ill study patients treated with imipenem-cilastatin
| Patient | Age (yr) | Ht (cm) | Wt (kg) | Sexd | APACHE II scorea | Principal diagnosisb | Infectious diagnosis | Isolated pathogen (imipenem MIC in μg/ml) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 185 | 69.9 | F | 36 | End-stage liver disease, sepsis | Intra-abdominal sepsis | E. coli (0.5) | Died |
| 2 | 48 | 182 | 79.4 | M | 29 | Necrotizing pancreatitis, ARDS | Necrotizing pancreatitis | None | Died |
| 3 | 55 | 162 | 89.0 | M | 31 | AAA repair with complications | Abdominal surgical wound infection | None | Survived |
| 4 | 53 | 172 | 111.3 | F | 28 | End-stage liver disease, intra-abdominal sepsis | Intra-abdominal sepsis | Klebsiella oxytoca (0.5), E. coli (0.5) | Survived |
| 5 | 34 | 170 | 95.6 | F | 25 | Acute liver failure, sepsis | Intra-abdominal sepsis | Enterobacter aerogenes (2), E. coli (0.5), Bacteroides fragilisc | Died |
| 6 | 47 | 169 | 91.8 | M | 23 | Sepsis, ARDS | Pneumonia | Klebsiella pneumoniae (1), P. aeruginosa (1) | Died |
| 7 | 71 | 169 | 88.4 | M | 26 | Intra-abdominal sepsis | Intra-abdominal sepsis | None | Survived |
| 8 | 72 | 178 | 125.3 | F | 28 | Intra-abdominal sepsis | Intra-abdominal sepsis | None | Survived |
| 9 | 59 | 175 | 86.0 | F | 30 | End-stage liver disease, sepsis | Pneumonia | P. aeruginosa (1), S. aureusc | Died |
| 10 | 24 | 178 | 84.2 | M | 34 | End-stage liver disease | Pneumonia | P. aeruginosa (4), E. cloacae (1) | Died |
| 11 | 60 | 173 | 99.3 | M | 29 | Liver transplant with chronic rejection | Pneumonia | Acinetobacter baumanii (1), K. pneumoniae (0.5) | Died |
| 12 | 58 | 170 | 108.0 | F | 31 | Idiopathic thrombocytopenia purpura, respiratory failure | Pneumonia | P. aeruginosa (2) | Survived |
Score on admission to the intensive care unit. APACHE, Acute Physiology and Chronic Health Evaluation.
AAA, abdominal aortic aneurysm; ARDS, acute respiratory distress syndrome.
Imipenem MICs not determined.
F, female; M, male.
TABLE 2.
Etiologies of renal failure and details of continuous renal replacement therapy
| Patient | Etiology of renal failurea | Urine output/ 24 h (ml)b | Type of CRRT | Blood flow rate (ml/min)b | Dialysis rate (ml/h)bcd | Ultrafiltration rate (ml/min)bd | Concomitant vasoactive drug(s) |
|---|---|---|---|---|---|---|---|
| 1 | Hepatorenal syndrome | 11 | CVVH | 150 | 19 ± 5 | Dopamine | |
| 2 | Sepsis with MODS | 43 | CVVH | 150 | 13 ± 10 | Norepinephrine | |
| 3 | Ischemic ATN | 0 | CVVH | 150 | 15 ± 7 | None | |
| 4 | Hepatorenal syndrome | 25 | CVVH | 150 | 20 ± 2 | Dopamine | |
| 5 | Sepsis with MODS | 29 | CVVH | 150 | 24 ± 4 | Dopamine, norepinephrine | |
| 6 | ATN 2° sepsis with MODS | 0 | CVVH | 150 | 22 ± 4 | Dopamine, phenylephrine | |
| 7 | Sepsis with MODS | 135 | CVVHDF | 150 | 956 ± 58 | 21 ± 2 | Dopamine, norepinephrine |
| 8 | Sepsis with MODS | 54 | CVVHDF | 200 | 1,018 ± 48 | 23 ± 4 | Norepinephrine |
| 9 | Sepsis with MODS | 0 | CVVHDF | 150 | 986 ± 46 | 17 ± 11 | Dopamine, norepinephrine |
| 10 | ATN 2° unknown etiology | 60 | CVVHDF | 150 | 973 ± 34 | 17 ± 8 | Norepinephrine, vasopressin |
| 11 | Sepsis with MODS | 0 | CVVHDF | 150 | 944 ± 147 | 20 ± 2 | Dopamine, norepinephrine |
| 12 | Idiopathic thrombocytopenia purpura | 118 | CVVHDF | 150 | 961 ± 85 | 18 ± 6 | None |
ATN, acute tubular necrosis; MODS, multiple organ dysfunction syndrome.
During time of pharmacokinetic sampling.
Applicable only to patients receiving CVVHDF.
Rates shown as mean ± standard deviation.
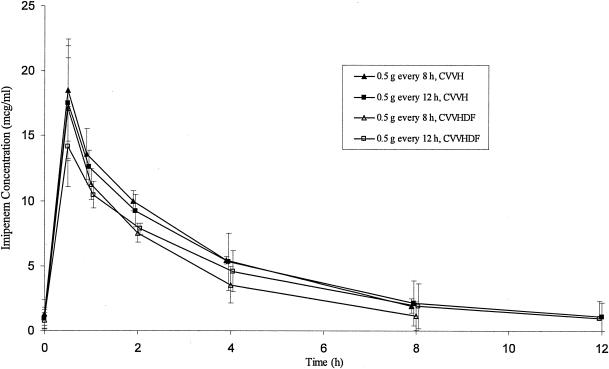
All patients were on imipenem for a minimum of 24 h before samples for pharmacokinetic analysis were obtained; five patients had samples drawn beginning ≥48 h after the start of imipenem therapy. Plasma pharmacokinetic parameters determined during administration of imipenem during CRRT are given in Table 3. Sampling of imipenem under steady-state conditions was confirmed by comparing the concentrations at time zero (just before the dose was administered) with observed or calculated Cmin values, taking into account potential assay error and minor variations in ultrafiltration rates. Mean plasma concentration-versus-time profiles for imipenem with each different dosing regimen during CVVH and CVVHDF are shown in Fig. 1. Although certain pharmacokinetic parameters appeared to be influenced by whether patients were receiving CVVH or CVVHDF, no statistically significant differences were observed for most parameters. Patients receiving CVVHDF had significantly higher CLS compared to patients receiving CVVH (P = 0.01), but this difference was not significant when normalized for total body weight (P = 0.477). Although CLS was different between groups and was also significantly correlated with t1/2 (Spearman's rank correlation coefficient of −0.685; P = 0.014), the observed t1/2 was overall similar between patients receiving CVVH versus CVVHDF (P = 0.860). Likewise, no significant differences in V (P = 0.884), S or Sa (P = 0.417), or ultrafiltration rates (P = 1.0) were noted between the CVVHDF and CVVH groups. Although drug clearance during CRRT (CLCRRT) and %CLS were increased 60% and nearly 30%, respectively, during CVVHDF compared to CVVH, these differences were also not significant (P = 0.109 and P = 0.364, respectively). It is interesting to note that differences in CLS were observed despite the fact that neither CLCRRT nor CLNR (P = 0.505) were significantly different. These observations are likely explained by the small number of patients as well as the high degree of pharmacokinetic variability observed in this critically ill population. Values for Cmax, Cmin, and AUC0-24 during each dosage regimen also appeared to be somewhat dependent on whether patients were receiving CVVH versus CVVHDF, but these differences were not consistently observed between regimens and the patient numbers in each group were very small.
TABLE 3.
Summary of imipenem pharmacokinetic parameters for patients receiving CRRT
| Patient and therapy | Dosing regimen | Cmax (μg/ml) | Cmin (μg/ml) | AUC0-24 (μg · h/ml) | t1/2 (h) | V (liter/kg) | CLSa
|
CLNRa
|
CRRT CLa
|
% CLSb | S/Sab | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ml/min | ml/min/kg | ml/min | ml/min/kg | ml/min | ml/min/kg | |||||||||
| CVVH | ||||||||||||||
| 1 | 0.5 g every 12 h | 22.8 | 0.1c | 99.2 | 1.31 | 0.28 | 173 ± 14 | 2.47 ± 0.20 | 147 ± 12 | 2.10 ± 0.17 | 26 ± 11 | 0.37 ± 0.14 | 15 | 1.16 |
| 2 | 0.5 g every 12 h | 18.9 | 0.3 | 112.6 | 1.87 | 0.32 | 157 ± 18 | 1.98 ± 0.23 | 102 ± 17 | 1.29 ± 0.21 | 55 ± 19 | 0.69 ± 0.21 | 35 | 1.31 |
| 3 | 0.5 g every 12 h | 16.0 | 1.8 | 160.5 | 3.65 | 0.43 | 121 ± 16 | 1.36 ± 0.18 | 73 ± 18 | 0.83 ± 0.20 | 47 ± 12 | 0.53 ± 0.16 | 39 | 1.18 |
| 4 | 0.5 g every 12 h | 12.3 | 2.3 | 145.7 | 4.72 | 0.51 | 139 ± 14 | 1.25 ± 0.12 | 115 ± 15 | 1.04 ± 0.13 | 24 ± 10 | 0.21 ± 0.09 | 17 | 1.34 |
| 5 | 0.5 g every 8 h | 15.7 | 2.3 | 175.5 | 2.72 | 0.36 | 146 ± 13 | 1.53 ± 0.14 | 116 ± 12 | 1.22 ± 0.12 | 29 ± 9 | 0.31 ± 0.16 | 20 | 1.03 |
| 6 | 0.5 g every 8 h | 21.3 | 1.5 | 191.0 | 1.98 | 0.25 | 134 ± 7 | 1.46 ± 0.08 | 101 ± 9 | 1.11 ± 0.10 | 32 ± 9 | 0.35 ± 0.10 | 24 | 1.25 |
| Mean ± SD | 17.8 ± 3.9 | 1.4 ± 1.0 | 147.4 ± 35.8 | 2.71 ± 1.27 | 0.36 ± 0.10 | 145 ± 18d | 1.67 ± 0.46d | 109 ± 24d | 1.30 ± 0.44d | 36 ± 13d | 0.41 ± 0.17d | 25 ± 10 | 1.21 ± 0.11 | |
| CVVHDF | ||||||||||||||
| 7 | 0.5 g every 12 h | 16.2 | 0.2c | 91.5 | 1.75 | 0.33 | 192 ± 21 | 2.17 ± 0.24 | 84 ± 22 | 0.96 ± 0.25 | 108 ± 31 | 1.22 ± 0.32 | 56 | 1.26 |
| 8 | 0.5 g every 12 h | 10.6 | 2.5 | 137.6 | 5.56 | 0.58 | 151 ± 16 | 1.21 ± 0.13 | 84 ± 18 | 0.67 ± 0.14 | 66 ± 10 | 0.53 ± 0.15 | 44 | 1.39 |
| 9 | 0.5 g every 12 h | 15.6 | 0.3 | 101.7 | 2.09 | 0.37 | 176 ± 19 | 2.05 ± 0.22 | 146 ± 22 | 1.70 ± 0.26 | 30 ± 11 | 0.35 ± 0.12 | 17 | 1.28 |
| 10 | 0.5 g every 8 h | 18.5 | 0.4 | 125.7 | 1.39 | 0.29 | 203 ± 17 | 2.41 ± 0.20 | 160 ± 15 | 1.90 ± 0.18 | 43 ± 9 | 0.51 ± 0.15 | 21 | 1.03 |
| 11 | 0.5 g every 8 h | 12.7 | 2.4 | 153.5 | 3.09 | 0.45 | 167 ± 6 | 1.68 ± 0.06 | 113 ± 7 | 1.14 ± 0.07 | 53 ± 13 | 0.54 ± 0.24 | 32 | 1.17 |
| 12 | 0.5 g every 8 h | 20.1 | 0.6 | 142.7 | 1.46 | 0.21 | 179 ± 11 | 1.66 ± 0.10 | 136 ± 10 | 1.26 ± 0.09 | 43 ± 16 | 0.40 ± 0.13 | 24 | 1.52 |
| Mean ± SD | 15.6 ± 3.5 | 1.1 ± 1.1 | 125.4 ± 24.3 | 2.56 ± 1.59 | 0.37 ± 0.13 | 178 ± 18d | 1.86 ± 0.43d | 120 ± 32d | 1.30 ± 0.46d | 57 ± 28d | 0.59 ± 0.32d | 32 ± 15 | 1.28 ± 0.17 | |
| P valuee | 0.309 | 0.916 | 0.392 | 0.860 | 0.884 | 0.010 | 0.477 | 0.505 | 1.00 | 0.109 | 0.252 | 0.364 | 0.417 | |
Values are means ± standard deviations (SD) calculated from parameter values determined during each of the three or four postdose sampling intervals within each patient: 1 to 2 h, 2 to 4 h, 4 to 8 h, and 8 to 12 h (except with 8-h-dosing-interval regimens).
Calculated from mean CRRT clearance for each patient.
Calculated values rather than directly measured.
Means ± standard deviations calculated from means of the three or four parameter values determined within each patient.
Values determined during CVVH regimens versus CVVHDF regimens.
FIG. 1.
Mean plasma concentrations of imipenem (μg/ml) with various dosage regimens during CVVH and CVVHDF. The x axis represents postinfusion times. Error bars represent standard deviation.
Mean Cmax, Cmin, and AUC0-24 values in patients receiving 0.5 g twice daily (1.0 g/day) during CVVH versus CVVHDF were 17.5 μg/ml versus 14.1 μg/ml, 1.1 μg/ml versus 1.0 μg/ml, and 129.5 μg · h/ml versus 110.3 μg · h/ml, respectively. Mean Cmax, Cmin, and AUC0-24 values in patients receiving 0.5 g three times daily (1.5 g/day) during CVVH versus CVVHDF were 18.5 μg/ml versus 17.1 μg/ml, 1.9 μg/ml versus 1.1 μg/ml, and 183.3 μg · h/ml versus 140.6 μg · h/ml, respectively. Differences in these parameters again suggest potential differences in drug elimination between CVVH and CVVHDF despite the small patient numbers and lack of statistical differences.
Pharmacokinetic and CRRT parameters for imipenem are given in Table 2. The mean imipenem S during CVVH and Sa during CVVHDF were estimated at 1.21 ± 0.11 and 1.28 ± 0.17, respectively, indicating that imipenem is extensively cleared across the CRRT membrane. These calculated values were quite consistent throughout the sampling periods, among all patients and across various ultrafiltration rates. Approximately 25% and 32% of imipenem CLS were attributed to membrane clearance during CVVH and CVVHDF, respectively, indicating that the clearance of imipenem was substantially enhanced during both CRRT techniques.
Calculated T > MIC during each dosing regimen is shown in Table 4. Most pathogens (12 of 13) isolated from patients in this study had imipenem MICs of ≤2 μg/ml. Doses of 0.5 g twice daily (1.0 g/day) and 0.5 g three times daily (1.5 g/day) generally provided T > MIC of at least 40 to 50% and 50 to 60%, respectively, levels of drug exposure that would be predicted to be favorable for achieving clinical efficacy against these pathogens. One pathogen, a P. aeruginosa strain, had an MIC of 4 μg/ml to imipenem. The predicted T > MIC of free imipenem for this organism in this patient was only approximately 34% while receiving 1.5 g/day. This level of drug exposure would be considered suboptimal, and the patient infected with this pathogen in fact died with an unresolved pneumonia. Imipenem doses of 1.0 to 1.5 g/day during CRRT would achieve a T > MIC of only 11 to 16% (<10% in one patient) against intermediately susceptible organisms (MIC = 8 μg/ml) and would not be considered adequate for successful treatment.
TABLE 4.
Calculated pharmacodynamic parameters for imipenem with various dosage regimens during CRRT
| T > MIC | Median % (range) for regimen:
|
|||||
|---|---|---|---|---|---|---|
| 0.5 g/12 h
|
0.5 g/8 h
|
0.5 g/6 h
|
||||
| CVVH (n = 4) | CVVHDF (n = 3) | CVVH (n = 2) | CVVHDF (n = 3) | CVVH modeled | CVVHDF modeled | |
| MIC ≤ 1 μg/ml | 62 (46-100) | 64 (56-100) | (100) | 74 (68-100) | 97 (78-100) | 84 (75-100) |
| MIC = 2 μg/ml | 46 (35-92) | 47 (40-99) | (78-92) | 56 (51-93) | 88 (61-100) | 76 (68-100) |
| MIC = 4 μg/ml | 35 (31-53) | 29 (26-53) | (53-58) | 38 (34-54) | 71 (49-81) | 61 (51-73) |
| MIC = 8 μg/ml | 14 (14-22) | 11 (6-12) | (24-28) | 16 (15-19) | 44 (35-62) | 36 (34-46) |
Doses of 0.5 g every 6 h (2.0 g/day) were modeled based on the pharmacokinetic parameters observed in this study and the T > MIC calculated (Table 4). At 2.0 g/day, organisms with a MIC of ≤4 μg/ml would be predicted to be adequately treated with a T > MIC of at least 50 to 80%. However, intermediately susceptible organisms (MIC = 8 μg/ml) were still not likely to be adequately treated in many patients with a predicted T > MIC ranging from 34 to 62%.
DISCUSSION
The primary objective of this study was to determine whether recent advances in CRRT techniques have resulted in the need for new dosing recommendations for imipenem. This study demonstrates that CRRT contributes substantially to imipenem elimination in patients with severe acute renal failure. The mean imipenem half-life of approximately 2.6 h observed during CRRT in the present study was considerably shorter than half-lives of 3.7 h to 4.8 h previously reported in patients with severe renal insufficiency (creatinine clearance, <10 ml/min) (9, 41, 42). This difference can be at least partially explained by enhanced imipenem clearance during CRRT. Previous studies conducted before 1998 reported CLCRRT ranging from 9 to 27 ml/min (14, 19, 25, 38, 43). In contrast, CLCRRT observed in the present study ranged from 24 to 55 ml/min during CVVH and 30 to 108 ml/min during CVVHDF. Rates of CRRT clearance observed in this study are thus increased by up to two- to threefold compared to those reported in the most recent previous studies. The proportion of total clearance contributed by CRRT (%CLS) was also increased by approximately 25% and 60% in patients receiving CVVH and CVVHDF, respectively, compared to these previous studies (14, 38).
Despite the apparently increased efficiency of drug removal of current CRRT techniques, the elimination t1/2 and actual imipenem concentrations observed in the present study are similar to those previously reported (38). It thus appears that the substantially increased CLCRRT and %CLS observed with current CRRT techniques do not necessarily directly translate into increased dosing requirements for imipenem. This is perhaps explained by the fact that the contribution of CRRT to overall CLS is still relatively small, representing only approximately 25 to 32% of CLS. Nonrenal mechanisms of drug elimination thus accounted for 70 to 75% of CLS compared to only 25 to 45% of CLS in healthy normal volunteers (5, 28). Increased proportions of imipenem clearance due to nonrenal mechanisms have been previously reported in anuric patients and patients receiving CRRT (25, 38, 43), as well as for other antimicrobials in patients with severe renal insufficiency (16, 23). Although critically ill patients with severe acute renal failure are typically fluid overloaded, no apparent increase in the V of imipenem was noted in this study compared to that previously described in healthy volunteers and patients with renal insufficiency (14, 15, 22, 43). This may perhaps be explained by the fact that subjects in this study had already received at least 24 to 48 h of CRRT with removal of a large fluid volume prior to measurement of imipenem concentrations. Maximum concentrations of imipenem (Cmax) observed in the present study were also within the range of reported concentrations obtained after 0.5-g doses in other populations (22, 38, 43).
Previous studies have determined that the most important pharmacodynamic predictor of clinical efficacy and risk of development of microbial resistance with β-lactam-type drugs is the time during which free plasma drug concentrations are above the MIC of the infecting pathogen (T > MIC) (3, 4, 6, 39). Studies indicate that T > MIC for carbapenems should be at least 40 to 50% of the dosing interval for maximum killing effects and reliable clinical efficacy (3, 4, 6, 39). Imipenem usually displays MICs of ≤2 μg/ml against most common gram-negative aerobic pathogens found in the ICU setting, including most β-lactamase-producing strains of Klebsiella, Escherichia coli, Enterobacter, Citrobacter, and Serratia (8, 20). This study suggests that imipenem doses of 1.0 to 1.5 g/day during either CVVH or CVVHDF would be expected to achieve favorable plasma drug concentrations and good clinical efficacy against susceptible pathogens with a MIC of ≤2 μg/ml, as judged by predicted values for T > MIC greater than 40% and often as high as 90 to 100% (Table 4). While increased drug clearance during CVVHDF compared to CVVH has been reported elsewhere for other antimicrobials (11, 23, 24), the present study included too few subjects and too much variability was observed within the data to demonstrate this conclusively for imipenem. Based on available data, dosing of imipenem for treatment of most susceptible gram-negative pathogens should be similar during CVVHDF and CVVH.
Certain other pathogens, particularly P. aeruginosa and Acinetobacter, are more frequently less susceptible (MIC at which 90% of the isolates tested are inhibited [MIC90] of ≥4 μg/ml (8, 20) or resistant to imipenem (18, 20, 21, 26, 27, 29, 30). As shown in Table 4, imipenem doses of ≥1.5 g/day would usually be required to achieve a T > MIC of at least 40% during CRRT for organisms with a MIC of 4 μg/ml. For infections caused by intermediately susceptible pathogens with a MIC of 8 μg/ml, doses of even 2.0 g/day would achieve a T > MIC, which would, in many patients, be only marginally adequate for successful treatment.
Based on these considerations, we would recommend imipenem doses of 2.0 g/day under most circumstances in critically ill patients receiving CRRT. This recommendation is due to frequent use of imipenem as empirical therapy, higher MICs of common nosocomial pathogens such as P. aeruginosa, unavailability of specific MICs in many institutions, and the variability in imipenem pharmacokinetics observed during CRRT. Imipenem regimens of ≤1.0 g/day, as often recommended by the manufacturer (Primaxin I.V. [imipenem and cilastatin], prescribing information; Merck & Co., Inc.) and other published sources for patients with severe renal dysfunction, with or without CRRT, would likely be subtherapeutic against all but the most highly susceptible pathogens (MIC, ≤2 μg/ml) when administered to patients receiving CRRT (10, 14, 43). Based on pharmacodynamic considerations discussed above, doses of 1.0 g/day would only be appropriately recommended for treatment of organisms with known or likely MIC of ≤2 μg/ml. Previous recommendations would be inappropriate for infections caused by pathogens with higher MICs, for which approximately 2.0 g/day would be required to achieve optimal T > MIC. A recent study by Tegeder et al. (38) also suggested that mean imipenem doses of 2.0 g/day are required during CVVH in order to effectively treat infections caused by pathogens with MICs of up to 8 μg/ml. It is noteworthy that no central nervous system toxicities were observed in patients actually receiving imipenem at 2.0 g/day during CVVH in that study. However, such high doses of imipenem during CRRT should be used only after careful consideration of potential risks associated with such aggressive therapy. Whenever possible, it would be advisable to use alternative antibiotics in such patients due to variability in imipenem pharmacokinetics during CRRT, the possibility of concentration-related imipenem toxicities (specifically central nervous system toxicities), and the relative lack of clinical experience with high doses in patients with severe renal impairment with or without CRRT. Alternatively, if no other agents are considered more suitable, imipenem doses of 2.0 g/day should be considered for empirical therapy in patients with severe nosocomial infections while awaiting results of culture and susceptibility testing. This may be particularly true in institutions with a high incidence of nosocomial infections due to P. aeruginosa, Acinetobacter, or other pathogens with an imipenem MIC90 of ≥4 μg/ml.
Limitations to this study include the relatively small number of subjects that received each dosage regimen during the different types of CRRT. This prevented more complete evaluations of relative drug clearances by CVVH versus CVVHDF as well as adequacy of each of the observed dosing regimens. However, this study reflects more current CRRT techniques and is the only study thus far to evaluate both CVVH and CVVHDF. Another limitation is that this study did not include a control group of patients not receiving CRRT; thus relative alterations in pharmacokinetics must be compared with historical rather than study-derived data. In addition, the potential adsorption of drug to CRRT membranes and false increases in apparent drug elimination rate were not evaluated. Because differences in ultrafiltration rates influence drug removal rates, failure to control CRRT parameters by strict protocol may perhaps be seen as a further limitation to this study. However, these results are directly applicable to the clinical setting because subjects were studied as they actually received CRRT and antibiotics for clinical indications without protocol-driven alterations in practice. Finally, errors in pharmacokinetic calculations are possible since sample collection took place over relatively short periods of time in relation to the slow drug elimination rates and long half-lives.
Imipenem elimination in patients with acute renal failure is substantially enhanced by CRRT. Imipenem dosing regimens currently recommended for anuric patients or patients receiving CRRT are likely to be subtherapeutic in many patients. Imipenem should be dosed at 1.0 to 1.5 g/day for infections due to susceptible gram-negative pathogens with a MIC of ≤2 μg/ml, including most Enterobacteriaceae. However, 2.0 g/day of imipenem appears to be required for pathogens such as P. aeruginosa with potentially higher MICs (4 to 8 μg/ml) or for empirical treatment of serious nosocomial infections in patients receiving CVVH or CVVHDF. The MICs for suspected pathogens, desired plasma drug concentrations, and potential risks to the patient should in all cases be considered when choosing an appropriate dosing regimen.
Acknowledgments
This investigator-initiated study was supported by a grant from Merck & Co., Inc.
REFERENCES
- 1.Bodenham, A., M. P. Shelly, and G. R. Park. 1988. The altered pharmacokinetics and pharmacodynamics of drugs commonly used in critically ill patients. Clin. Pharmacokinet. 14:347-373. [DOI] [PubMed] [Google Scholar]
- 2.Bressolle, F., J. Kinowski, J. E. de la Coussaye, N. Wynn, J. Eledjam, and M. Galtier. 1994. Clinical pharmacokinetics during continuous haemofiltration. Clin. Pharmacokinet. 26:457-471. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Drusano, G. L., H. C. Standiford, C. Bustamante, A. Forrest, G. Rivera, J. Leslie, B. Tatem, D. Delaportas, R. R. MacGregor, and S. C. Schimpff. 1984. Multiple-dose pharmacokinetics of imipenem-cilastatin. Antimicrob. Agents Chemother. 26:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drusano, G. L. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36(Suppl. 1):S42-S50. [DOI] [PubMed] [Google Scholar]
- 7.Forni, L. G., and P. J. Hilton. 1997. Continuous hemofiltration in the treatment of acute renal failure. N. Engl. J. Med. 336:1303-1309. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2001. In vitro activities of ertapenem (MK-0826) against clinical bacterial isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1915-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, T. P., J. L. Demetriades, and J. A. Bland. 1985. Imipenem/cilastatin: pharmacokinetic profile in renal insufficiency. Am. J. Med. 78(Suppl. 6A):54-61. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert, D. N., R. C. Moellering Jr., G. M. Eliopoulos, and M. A. Sande (editors). 2004. The Sanford guide to antimicrobial therapy, 34th ed. Antimicrobial Therapy, Inc., Hyde Park, Vt.
- 11.Giles, L. J., A. C. Jennings, A. H. Thomson, G. Creed, R. J. Beale, and A. McLuckie. 2000. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit. Care Med. 28:632-637. [DOI] [PubMed] [Google Scholar]
- 12.Golper, T. A. 1991. Drug removal during continuous hemofiltration or hemodialysis. Contrib. Nephrol. 93:110-116. [DOI] [PubMed] [Google Scholar]
- 13.Gravallese, D. A., D. G. Musson, L. T. Pauliukonis, and F. W. Bayne. 1984. Determination of imipenem (N-formimidoyl thienamycin) in human plasma and urine by high-performance liquid chromatography, comparison with microbiological methodology and stability. J. Chromatogr. 310:71-84. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto, S., M. Honda, M. Yamaguchi, M. Sekimoto, and Y. Tanaka. 1997. Pharmacokinetics of imipenem and cilastatin during continuous venovenous hemodialysis in patients who are critically ill. ASAIO J. 43:84-88. [PubMed] [Google Scholar]
- 15.Janmohamed, R. M., M. J. Leyland, J. Kelly, and I. Farrell. 1990. Pharmacokinetics of imipenem/cilastatin in neutropenic patients with haematological malignancies. J. Antimicrob. Chemother. 25:407-412. [DOI] [PubMed] [Google Scholar]
- 16.Jones, E. M., C. M. McMullin, A. J. Hedges, A. M. Lovering, L. O. White, D. S. Reeves, and A. P. MacGowan. 1997. The pharmacokinetics of intravenous ciprofloxacin 400 mg every 12 hours in patients with severe sepsis: the effect of renal function and intra-abdominal disease. J. Antimicrob. Chemother. 40:121-124. [DOI] [PubMed] [Google Scholar]
- 17.Joy, M. S., G. R. Matzke, D. K. Armstrong, M. A. Marx, and B. J. Zarowitz. 1998. A primer on continuous renal replacement therapy for critically ill patients. Ann. Pharmacother. 32:362-375. [DOI] [PubMed] [Google Scholar]
- 18.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. F. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller, E., H. Fecht, J. Bohler, and P. Schollmeyer. 1989. Single-dose kinetics of imipenem/cilastatin during continuous arteriovenous haemofiltration in intensive care patients. Nephrol. Dial. Transplant. 4:640-645. [PubMed] [Google Scholar]
- 20.Livermore, D. M., M. W. Carter, S. Bagel, B. Wiedemann, F. Baquero, E. Loza, H. P. Endtz, N. van den Braak, C. J. Fernandes, L. Fernandes, N. Frimodt-Moller, L. S. Rasmussen, H. Giamarellou, E. Giamarellous-Bourboulis, V. Jarlier, J. Nguyen, C.-E. Nord, M. J. Struelens, C. Nonhoff, J. Turnidge, J. Bell, R. Zbinden, S. Pfister, L. Mixson, and D. L. Shungu. 2001. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob. Agents Chemother. 45:1860-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 22.MacGregor, R. R., G. A. Gibson, and J. A. Bland. 1986. Imipenem pharmacokinetics and body fluid concentrations in patients receiving high-dose treatment for serious infections. Antimicrob. Agents Chemother. 29:188-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malone, R. S., D. N. Fish, E. Abraham, and I. Teitelbaum. 2001. Pharmacokinetics of levofloxacin and ciprofloxacin during continuous renal replacement therapy in critically ill patients. Antimicrob. Agents Chemother. 45:2949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malone, R. S., D. N. Fish, E. Abraham, and I. Teitelbaum. 2001. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob. Agents Chemother. 45:3148-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller, B. A., S. K. Scarim, and W. L. Macias. 1993. Comparison of imipenem pharmacokinetics in patients with acute or chronic renal failure treated with continuous hemofiltration. Am. J. Kidney Dis. 21:172-179. [DOI] [PubMed] [Google Scholar]
- 26.National Nosocomial Infections Surveillance System 1999. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1990-May 1999, issued June 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 27.Neuhauser, M. M., R. A. Weinstien, R. Rydman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson-Ehle, I., M. Hutchison, S. J. Haworth, and S. R. Norrby. 1991. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur. J. Clin. Microbiol. Infect. Dis. 10:85-88. [DOI] [PubMed] [Google Scholar]
- 29.Obritsch, M. D., D. N. Fish, R. MacLaren, and R. Jung. 2004. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 48:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., R. N. Jones, and D. J. Biedenbach. 2001. Antimicrobial resistance trends in medical centers using carbapenems: report of 1999 and 2000 results from the MYSTIC program (USA). Diagn. Microbiol. Infect. Dis. 41:177-182. [DOI] [PubMed] [Google Scholar]
- 31.Power, B. M., A. M. Forbes, P. V. Heerden, and K. F. Ilett. 1998. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharmacokinet. 34:25-56. [DOI] [PubMed] [Google Scholar]
- 32.Przechera, M., D. Bengel, and T. Risler. 1991. Pharmacokinetics of imipenem/cilastatin during continuous arteriovenous hemofiltration. Contrib. Nephrol. 93:131-134. [DOI] [PubMed] [Google Scholar]
- 33.Reetze-Bonorden, P., J. Bohler, and E. Keller. 1993. Drug dosing in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin. Pharmacokinet. 24:362-379. [DOI] [PubMed] [Google Scholar]
- 34.Ronco, C., R. Bellomo, and Z. Ricci. 2001. Continuous renal replacement therapy in critically ill patients. Nephrol. Dial. Transplant. 16(Suppl. 5):67-72. [DOI] [PubMed] [Google Scholar]
- 35.Schetz, M., P. Ferdinande, R. Van den Berghe, C. Verwaest, and P. Lauwers. 1995. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 21:612-620. [DOI] [PubMed] [Google Scholar]
- 36.Shargel, L., and A. B. C. Yu. 1999. Applied biopharmaceutics and pharmacokinetics, 4th ed. Appleton & Lange, Stamford, Conn.
- 37.Smith, G. B., G. C. Dezeny, and A. W. Douglas. 1990. Stability and kinetics of degradation of imipenem in aqueous solution. J. Pharm. Sci. 79:732-740. [DOI] [PubMed] [Google Scholar]
- 38.Tegeder, I., F. Bremer, R. Oelkers, H. Schobel, J. Schüttler, K. Brune, and G. Geisslinger. 1997. Pharmacokinetics of imipenem-cilastatin in critically ill patients undergoing continuous venovenous hemofiltration. Antimicrob. Agents Chemother. 41:2640-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnidge, J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 40.Van Dalen, R., and T. B. Vree. 1990. Pharmacokinetics of antibiotics in critically ill patients. Intensive Care Med. 16(Suppl. 3):S235-S238. [DOI] [PubMed] [Google Scholar]
- 41.Verbist, L., G. A. Verpooten, R. A. Guiliano, M. E. Debroe, A. P. Buntinx, L. A. Entwistle, and K. H. Jones. 1986. Pharmacokinetics and tolerance after repeated doses of imipenem/cilastatin in patients with severe renal failure. J. Antimicrob. Chemother. 18(Suppl. E):115-120. [DOI] [PubMed] [Google Scholar]
- 42.Verpooten, G. A., L. Verbist, A. P. Buntinx, L. A. Entwistle, K. H. Jones, and M. E. De Broe. 1984. The pharmacokinetics of imipenem (thienamycin-formamidine) and the renal dehydropeptidase inhibitor cilastatin sodium in normal subjects and patients with renal failure. Br. J. Clin. Pharmacol. 18:183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vos, M. C., H. H. Vincent, and E. P. F. Yzerman. 1992. Clearance of imipenem/cilastatin in acute renal failrure patients treated by continuous hemodiafiltration (CAVHD). Intensive Care Med. 18:282-285. [DOI] [PubMed] [Google Scholar]