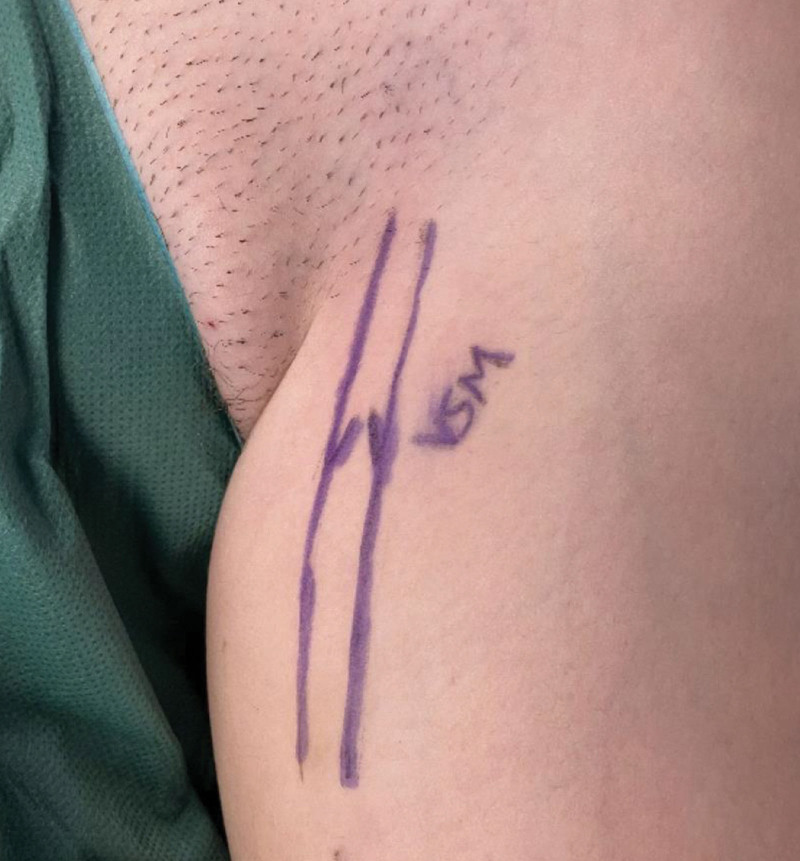Abstract
Background:
Recent advances in robotic microsurgery have enabled the application of robotic technology in central lymphatic reconstruction. Although the use of microsurgical robots demands careful consideration of associated costs and potentially prolonged operating times, it may offer improved surgical approaches and enhanced accessibility to deeper anatomical structures such as the thoracic duct (TD).
Methods:
We report on successful reconstruction of the central lymphatic system using the Symani Surgical System in four patients with lesions of the central lymphatic system. The patients were of different age (range: 8 mo–60 y) and had variable conditions, including central conducting lymphatic anomaly and other rare anomalies of the central lymphatic pathways.
Results:
Depending on the underlying pathology, a cervical access (n = 1) or median laparotomy (n = 3) was chosen to access the TD and perform anastomosis with a nearby vein. In all patients, anastomoses were patent, and chyle leakage decreased postoperatively. From a surgical perspective, the Symani Surgical System improved the precision of the microsurgeon and accessibility to the deep-lying TD.
Conclusion:
Considering the high morbidity and rarity of pathologies of the central lymphatic system, robotic-assisted microsurgery holds substantial promise in expanding and improving the microsurgical treatment for central lymphatic anomalies.
Takeaways
Question: Can the Symani Surgical System be used for reconstruction of the central lymphatic system?
Findings: The robotic technology allowed for thoracic duct-vein anastomosis in four patients with rare anomalies of the central lymphatic system. The Symani Surgical System improved precision and access to the deep-lying structures. All anastomoses were patent, and clinical symptoms improved in all patients.
Meaning: The Symani Surgical System has great potential to expand the microsurgical treatment possibilities for central lymphatic disorders.
INTRODUCTION
Advancements in robotic technology have only recently made it possible to use robotic systems in reconstructive lymphatic surgery. During the last years, the number of preclinical and clinical studies demonstrating the feasibility of robotic-assisted (super) microsurgical procedures is rapidly increasing.1–14 Although the application of microsurgical robots requires careful consideration of the associated costs and potentially longer operating times, they can enable smaller surgical approaches and provide easier access to anatomically deeper structures.3 The latter is particularly important when performing surgery of the central lymphatic system. Central lymphatic surgery is not only rare, but also technically difficult due to the anatomical location of the thoracic duct (TD). Recently, we have published the benefits of using the Symani Surgical System for the reconstruction of the central lymphatic pathways.1
Although lesions and anomalies of the central lymphatic system are rare, they are associated with a high morbidity and mortality. Depending on the anatomical location involved, patients may exhibit chyle leakage, recurrent chylothorax, or chylous ascites, all of which predispose to infections and additional complications due to an ongoing protein and fluid loss, resulting in a mortality rate of up to 50%.15,16 As described previously by our group, initial treatment relies on conservative approaches, which include a diet consisting of medium-chain triglycerides or a full parenteral diet.17,18 This regimen may be supplemented with drugs aimed at reducing chyle production, such as octreotide. If conservative treatment fails, lymphangiography-guided interventions represent the second line of therapeutic approach, with good success rates and a low risk for complications.19–22 Considering, that an occlusion of the TD carries the risk of protein losing enteropathy, lower extremity lymphedema, or worsening of lymphatic reflux with a fistula at a different location, microsurgical reconstruction of the central lymphatic system has opened new frontiers to treat rare central lymphatic lesions by TD-vein anastomoses.17,18,23
METHODS
We report on the four patients with a central lymphatic lesion who received microsurgical reconstruction with the aid of the Symani Surgical System (Medical Microinstruments, Inc., Wilmington, Del.) at the department of plastic surgery and hand surgery between June 2023 and March 2024. All patients were operated on by the senior author. Approval was granted by the Cantonal Ethics Committee of Zurich, Switzerland (ethical approval no.: 2021-02351). Written consent was obtained from all patients or from their parents in the case of minors. Additional consent was acquired from patients whose pre-, intra-, or postoperative images are shown.
CASE PRESENTATION
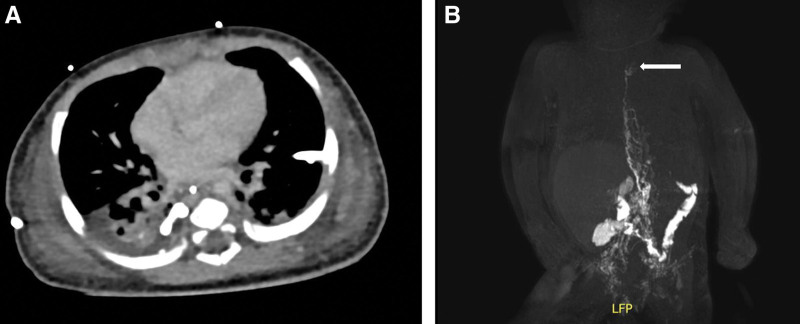
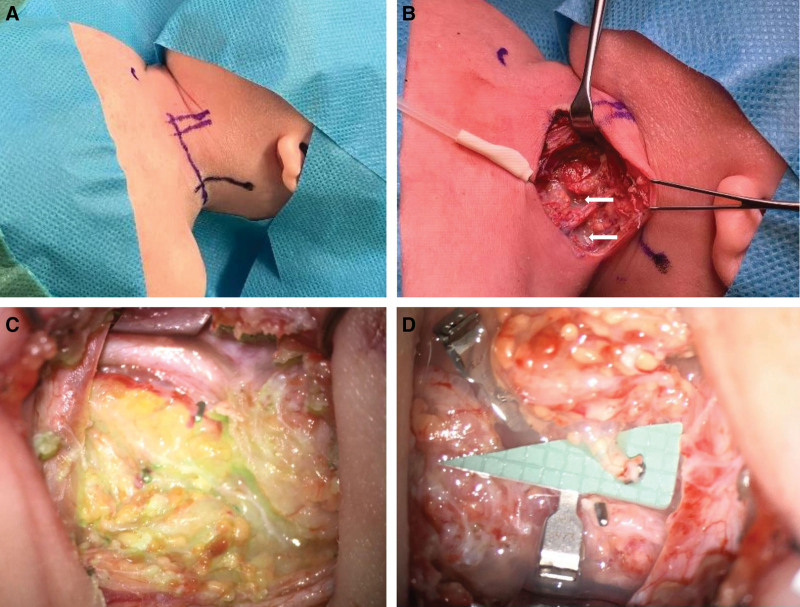
An 8-month-old infant was referred to our department with a history of severe bilateral chylothorax and protein-losing enteropathy due a catheter-associated venous thrombosis following asphyxia and reanimation after birth (Fig. 1A). Magnetic resonance lymphangiography (MRL) showed an occlusion of the TD at the level of the left venous angle (Fig. 1B). Both recanalization using conventional lymphangiography and conservative treatment were unsuccessful, so the decision was made to perform a lymphatic reconstruction. At the beginning of the surgery, an interventional radiologist performed an intranodal inguinal indocyanine green (ICG) injection at both groins (Fig. 2). Afterwards a cervical access was chosen to reach the TD at its confluence with the venous angle (Fig. 3A, B). The enrichment of ICG enabled the TD to be quickly identified in the fluorescence mode of the microscope (Fig. 3C). The TD and a branch of the left external jugular vein were dissected and prepared for anastomosis with conventional microinstruments (Fig. 3D). The wrapped Symani Surgical System with its NanoWrist microinstruments was placed. Visualization was performed with a 3D exoscope integrated into an optical microscope (KINEVO 900, Carl Zeiss AG, Oberkochen, Germany). End-to-end anastomosis of the TD and a branch of the left external jugular vein was performed to reconstruct the lymphatic drainage into the venous system . [See Video (online), which displays robotically-assisted TD-vein anastomosis with the Symani Surgical System. The 8-month-old patient had a history of severe bilateral chylothorax, protein-losing enteropathy and multiple venous thromboses since birth. Preoperative MRL showed an occlusion of the TD at the level of the left venous angle. End-to-end anastomosis of the TD and a branch of the left external jugular vein was performed to reconstruct the lymphatic drainage into the venous system.] Patency of the anastomosis was confirmed by ICG-flow. In this particular case, the use of the robotic system was of great advantage. In contrast to a manual approach, the robotic-assisted setup makes the operation independent from the operating table. This will prevent repetitive focus changes or intrinsic movements of the operation field under the high magnification when, for example, resting the hands within the operative field and operating on a small patient. In addition, the use of the Symani Surgical System minimizes the incision of the sternocleidomastoid muscle covering the TD, because its movements are significantly smaller and more precise and therefore only require a small surgical field.
Fig. 1.
Congenital central lymphatic flow disorder. A, Thoracic computer tomography of an 8-month-old boy with bilateral chylothorax and bilateral chest drains. B, MR-lymphangiography indicating the stenosis of the TD at the left venous angle (white arrow).
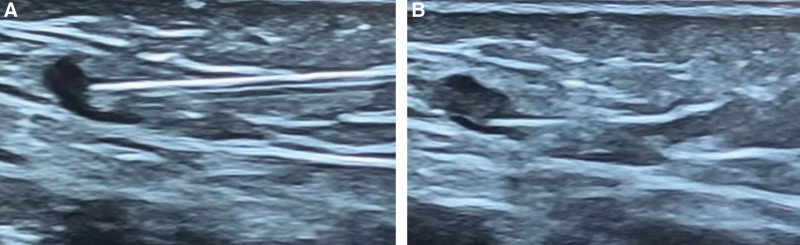
Fig. 2.
Ultrasound-guided intranodal injection of ICG. A, Preoperative sonographic-assisted intranodal injection of ICG at the groin. Visualization of the technique. The needle is placed at the cortical-medullary junction and 1.5 mL of ICG is injected. B, Microbubbles contained within the ICG solution can be visualized to confirm accurate injection.
Fig. 3.
Intraoperative findings in the neck. A, Preoperative view with skin markings, showing the cervical supraclavicular access, carotid artery, and external jugular vein. B, After incision of the skin, a continuous leakage of chyle was observed (white arrows). C, ICG overlay mode confirms a diffuse leakage of chyle. D, Preparation of the TD.
Video 1. displays robotically-assisted TD-vein anastomosis with the Symani Surgical System. The 8-month-old patient had a history of severe bilateral chylothorax, protein-losing enteropathy and multiple venous thromboses since birth. Preoperative MRL showed an occlusion of the TD at the level of the left venous angle. End-to-end anastomosis of the TD and a branch of the left external jugular vein was performed to reconstruct the lymphatic drainage into the venous system.
RESULTS
Four patients with anomalies of the central lymphatic system received microsurgical central lymphatic reconstruction (Table 1). The age of the patients varied from 8 months to 60 years. Preoperative MRL was performed in all patients to localize the underlying anomaly of the central lymphatics. In two patients who were diagnosed with a central conducting lymphatic anomaly, MRL showed an abruption of the TD, causing bilateral chylothorax and even chylopericardium in one patient. The third patient was diagnosed with a retroperitoneal central lymphatic cyst, which led to lower extremity edema, abdominal pain, and reduced physical capacity. The youngest patient had severe bilateral chylothorax as described above.
Table 1.
Patient Characteristics and Operative Details
| Patient | Preoperative Condition | MRL | Lymph- angiography |
Surgical Access | Anastomosis | Duration of Surgery (min) | Duration of Anastomosis (min) | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 (47 y) | Lower extremity edema, abdominal pain and reduced physical capacity | Aneurysmal dilatation of the left paramedian lymphatic duct and dilated lymphatic cisterns along the iliac lymphatic pathways | Embolization | Median laparotomy | Intraabdominal end-to-side anastomosis of left OV with lymphatic cyst | 508 | 23 | 11 mo | Complete regression of symptoms; MRL proved drainage from the aneurysmal dilatation into the ovarian vein |
| 2 (10 y) |
CCLA with bilateral chylothorax |
TD abruption, thoracic lymphatic malformation on the right and lymphatic reflux on the left | Embolization | Median laparotomy | LVA between subdiaphragmal lymph vessel and omental vein; MLL | 410 | 35 | 7 mo | Subsequent interventional embolization |
| 3 (60 y) | CCLA with bilateral chylothorax and lower extremity lymphedema | TD not detectable, abruption of retroperitoneal lymphatic pathways at the level of lumbar vertebra 2 | None | Median laparotomy | Intraabdominal end-to-end anastomosis of TD and right GEV, para-aortal and iliac LVA on the left | 480 | 23 | 5 mo | Regression of chylothorax |
| 4 (8 mo) | Bilateral chylothorax | TD stenosis at the level of the angulus venosus sinister, multiple venous thrombosis | Recanalization | Neck | End-to-end anastomosis of TD and branch of the left EJ | 273 | 19 | 2 mo | Partial regression of chylothorax |
CCLA, central conducting lymphatic anomaly; EJ, external jugular vein; OV, ovarian vein; GEV, gastroepiploic vein.
Regardless of the etiology, all patients, except the patient with the retroperitoneal central lymphatic cyst, underwent initial conservative treatment for at least 6 weeks. In addition, a conventional lymphangiography was performed to aim for recanalization or embolization depending on the etiology of the lesion.
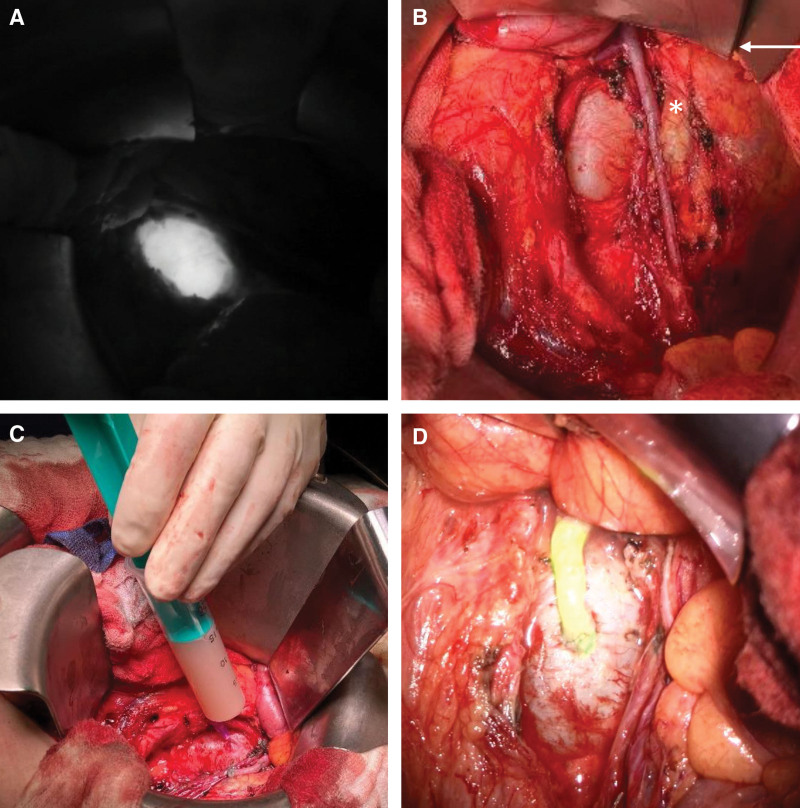
The surgical treatment was carefully planned on the basis of the preoperative MRL and in interdisciplinary collaboration with radiologists and visceral surgeons. For an underlying central lymphatic lesion, a cervical access (n = 1) (Fig. 3) or median laparotomy (n = 3; Fig. 4) was used to reach the TD. At the beginning of the surgery, an interventional radiologist performed an intranodal inguinal indocyanine green (ICG) injection, which enables the central lymphatic pathways to be localized with a near infrared camera and the ICG mode of the microscope. In all patients, the TD (Fig. 3D and Fig. 5A) could be identified successfully. After preparation of a nearby vein (Fig. 5B), end-to-end TD vein anastomosis (Fig. 5C) was performed using the Symani Surgical System. A patent anastomosis with washout was documented in all patients (Fig. 5D). Complete regression of symptoms was observed in the patient with the retroperitoneal cyst (Fig. 4) with a visible drainage over the anastomosis on postoperative MRL. In the remaining patients, chyle leakage decreased steadily.
Fig. 4.
Central lymphatic dilatation. A, Localisation of the cyst using a near-infrared camera. B, Macroscopic view of the central lymphatic cyst (white asterisk) and adjacent left ovarian vein (white arrow). C, Puncture with partial drainage of the cyst. D, Successfull anastomosis between cyst and left ovarian vein. The near-infrared overlay mode of the exoscope showed ICG flow over the anastomosis which confirmed its patency.1 Reproduced with permission from Wolters Kluwer Health from Weinzierl A, Puippe GD, et al. First-in-human use of a microsurgical robotic system for central lymphatic reconstruction. Plast Reconstr Surg Glob Open. 2023;11:e5484. © 2023 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
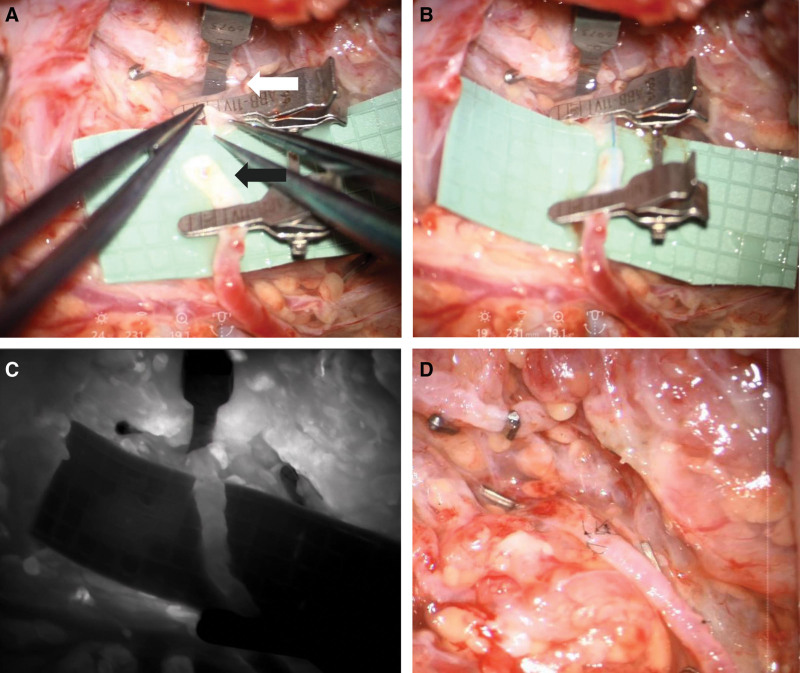
Fig. 5.
A, Preparation of the TD (white arrow) and a nearby branch of the external jugular vein (black arrow). B, An inserted blue 6-0 suture acts as intravascular stent (IVAS) to stabilize the vessels. C, Patency of the anastomosis is confirmed by ICG-flow. D, Completed thoracic duct-vein anastomosis with chylous washout into the vein.
DISCUSSION
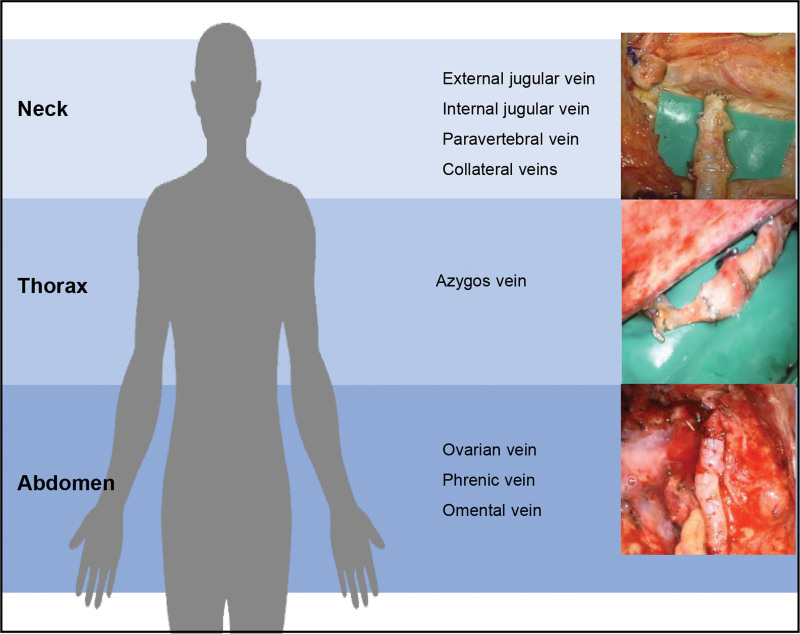
In this work, we demonstrate the successful use of the Symani Surgical System for microsurgical anastomosis of the TD in different anatomical locations. In general, the central lymphatic system can be accessed via three different locations: the neck, the thorax, and the abdomen (Fig. 6).24 The choice of access requires a thorough interdisciplinary evaluation to define the area in which the reconstruction of lymphatic flow shall be performed. This will be influenced by the pathophysiology of the underlying condition and the specific pattern of lymphatic flow disorder and leakage, for example, into the pleural or abdominal cavities. In particular, the venous pressure has to be considered. An elevated venous pressure (eg, in Fontan patients) may impair lymphatic drainage in general.25 However, additional surgical injury to the central lymphatic system may significantly worsen the lymphatic congestion. Although in a healthy patient the central venous pressure will be between 3 and 8 mm Hg,26 it can be significantly higher in a patient with a Fontan circulation.27 Ideally, valve-bearing veins are primarily chosen as recipient veins (V. jug. ext., V. ovarica, etc.) to prevent backflow. Venous interposition grafts, including valves are options to prevent venous backflow into the central lymphatic vessel and can be sonographically marked and harvested from the proximal thigh (Figure 7).
Fig. 6.
The central lymphatic system can be accessed via neck, thorax, and abdomen. Depending on the location of the underlying lesion, various recipient vessels may be considered.50 Reproduced from Weinzierl A, Grünherz L, Puippe GD, et al. Microsurgical central lymphatic reconstruction-the role of thoracic duct lymphovenous anastomoses at different anatomical levels. Front Surg. 2024;11:1415010. © 2024 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Fig. 7.
Preoperative marking on the saphenous vein including a valve based on sonography at the level of the proximal thigh. Harvesting of the vein and its use as an interposition graft can prevent venous backflow into the central lymphatic vessel.
Apart from the technical advantages offered by the microsurgical robotic system, our case series adds to the growing body of literature on TD vein anastomosis to treat central lymphatic obstruction of different etiologies. In our experience, treatment by TD vein anastomosis is successful in about 70%–80% of patients, which is also reflected in the current literature and in our previous publications.23,28–40 Success rates particularly depend on accurate determination of the etiology of the central lymphatic obstruction. In fact, isolated iatrogenic lesions of the TD are much easier to reconstruct compared with congenital anomalies in which multiple lesions are usually present in addition to an impaired lymphatic peristalsis.23 The postoperative course will also depend on whether the lymphovenous anastomosis was performed caudal to a potential leakage site, for example, within the abdomen for chylothorax in which the distal part of the TD may be ligated physically preventing lymphatic flow into the pleura. In this case the chyle leakage may stop immediately due to the interruption of flow and shunting of chyle into the venous system via the newly created LVA. However, in congenital malformations, multiple lymphatic collaterals and aberrant pathways may be present supporting the flow of chyle and have to be treated by additional sclerotherapy and pharmacological treatment (eg, sirolimus).15,41 If the anastomosis is performed distal to the presumed leakage site (eg, in a thrombotic occlusion within the neck), the antegrade flow of chyle into the vein must be provoked by intermittent clamping of existing drains. Otherwise, the chyle will take the way of least resistance in the beginning after lymphovenous reconstruction [eg, via the body cavity into an existing drain (often additionally under suction)]. It therefore requires an experienced team to surveil the drainage amounts postoperatively and to find an equilibrium in which the chyle will drain into the venous system rather than into the existing pathways and cavities, which may usually take several weeks.
The Symani Surgical System proved to be particularly beneficial in patients in whom the main obstruction of the TD was located in the abdomen. The Symani Surgical System not only simplifies access by laparotomy but also makes it easier to operate with microinstruments in a plane that is otherwise difficult to reach. In contrast, in most of the currently reported cases, TD vein anastomosis is performed at the level of the left neck,28,29,31,32,34–36,42 whereas only a few authors chose an access by thoracotomy30,33,37 or laparotomy.23,38 Considering that the access to the anatomically deep central lymphatic system remains challenging, other surgical treatments, such as multiple LVAs or lymph node to vein anastomosis have been proposed to bypass proximal lymphatic impairment.43,44 Also, in recurrent chylous and lymphatic ascites, performing multiple LVAs on the thigh or at different levels of the leg has been suggested to bypass proximal lymphatic impairment.44–46 Other treatment approaches for chylous ascites represent the lymphatic cable flap by Chen47 or a microsurgical peritoneal-venous anastomosis as described by Kovach and coauthors.48 Coupler anastomoses may be feasible to connect lymphatic trunks and veins, if there is one vessel and the lumen is larger than 1 mm. However, depending on the location, this is not necessarily easier than suture anastomosis (narrow access, eg, epigastrium) and the coupler handle (current models) may be too short in the abdomen.
We have previously demonstrated that the Symani Surgical System, with flexible, free-moving robotic arms and seven degrees of freedom, allows for smaller surgical approaches and simplifies access to anatomically deeper structures.3 Even if additional muscle has to be incised, the Symani Surgical System with its very precise movements requires a much smaller surgical field and thus favors a less traumatic procedure. Other advantages include superior precision for microsurgical anastomosis in vessels with a diameter below 1 mm while simultaneously improving the ergonomic position of the surgeon, who sits at the comfortable console next to the operating table.49 Hence, there is enough space for a second team of microsurgeons to operate in parallel with a second microscope at a nearby anatomic region. With its potential to enable teleoperation, the Symani Surgical System holds immense promise for the future, particularly addressing challenges in treating rare diseases. In fact, the microsurgeon may operate the Symani Surgical System from distant locations, transcending geographical barriers and expanding access to specialized surgical techniques such as central lymphatic reconstruction. In addition, we have noticed that the robotic-assisted setup makes the operation independent from the operating table (eg, in infants and small children). This will prevent repetitive focus changes or intrinsic movements of the operation field under the high magnification when, for example, resting the hands within the operative field. Regarding the time to perform anastomosis, we have recently shown that times for robotic-assisted anastomosis approach anastomotic times after a steep learning curve, in particular, in lymphovenous anastomoses.4 Similarly, the preparation and handling of the Symani Surgical System should not require any additional time if the surgical team has been trained accordingly. However, the difficult setup of operating deep within the body on small vessels with long microinstruments may prolong anastomotic and operating times in general in these cases, independent from using a robotic system.
Present constraints of the Symani Surgical System for central lymphatic reconstruction involve predetermined angles in relation to the alignment of its two robotic arms, meaning that as the arms are lowered, the gap between them increases at the skin level, potentially necessitating longer incisions for surgery at even greater depths. Nevertheless, advancements in the technology, such as the introduction of flexible arms, may address this issue in the future.
In conclusion, the Symani Surgical System has been successfully used for TD vein anastomosis at different anatomic locations. Considering the high morbidity coupled with the rarity of the central lymphatic system pathologies, microsurgical reconstruction necessitates meticulous planning by a specialized and interdisciplinary team of surgeons. We believe that the Symani Surgical System holds promise for broadening the scope of microsurgical reconstruction for central lymphatic anomalies in the future.
DISCLOSURES
Dr. Lindenblatt acts as a consultant and scientific advisor for Medical Microinstruments (MMI). All the other authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 16 September 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Grunherz L, Weinzierl A, Puippe GD, et al. First-in-human use of a microsurgical robotic system for central lymphatic reconstruction. Plast Reconstr Surg Glob Open. 2023;11:e5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindenblatt N, Grünherz L, Wang A, et al. Early experience using a new robotic microsurgical system for lymphatic surgery. Plast Reconstr Surg Glob Open. 2022;10:e4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinzierl A, Barbon C, Gousopoulos E, et al. Benefits of robotic-assisted lymphatic microsurgery in deep anatomical planes. JPRAS Open. 2023;37:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbon C, Grunherz L, Uyulmaz S, et al. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open. 2022;34:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Mulken TJM, Schols RM, Scharmga AMJ, et al. ; MicroSurgical Robot Research Group. First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun. 2020;11:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Mulken TJM, Boymans C, Schols RM, et al. Preclinical experience using a new robotic system created for microsurgery. Plast Reconstr Surg. 2018;142:1367–1376. [DOI] [PubMed] [Google Scholar]
- 7.van Mulken TJM, Schols RM, Qiu SS, et al. Robotic (super) microsurgery: feasibility of a new master-slave platform in an in vivo animal model and future directions. J Surg Oncol. 2018;118:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Mulken TJM, Wolfs J, Qiu SS, et al. One-year outcomes of the first human trial on robot-assisted lymphaticovenous anastomosis for breast cancer-related lymphedema. Plast Reconstr Surg. 2022;149:151–161. [DOI] [PubMed] [Google Scholar]
- 9.Ballestin A, Malzone G, Menichini G, et al. New robotic system with wristed microinstruments allows precise reconstructive microsurgery: preclinical study. Ann Surg Oncol. 2022;29:7859–7867. [DOI] [PubMed] [Google Scholar]
- 10.Innocenti M, Malzone G, Menichini G. First-in-human free flap tissue reconstruction using a dedicated microsurgical robotic platform. Plast Reconstr Surg. 2023;151:1078–1082. [DOI] [PubMed] [Google Scholar]
- 11.Schafer B, Bahm J, Beier JP. Nerve transfers using a dedicated microsurgical robotic system. Plast Reconstr Surg Glob Open. 2023;11:e5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beier JP, Hackenberg S, Boos AM, et al. First series of free flap reconstruction using a dedicated robotic system in a multidisciplinary microsurgical center. Plast Reconstr Surg Glob Open. 2023;11:e5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunherz L, Gousopoulos E, Barbon C, et al. [Robotics in plastic surgery]. Chirurgie (Heidelb). 2023;94:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gousopoulos E, Grünherz L, Giovanoli P, et al. Robotic-assisted microsurgery for lymphedema treatment. Plast Aesthet Res. 2023;10:7. [Google Scholar]
- 15.Trenor CC, III, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23:186–190. [DOI] [PubMed] [Google Scholar]
- 16.Lagarde SM, Omloo JM, de Jong K, et al. Incidence and management of chyle leakage after esophagectomy. Ann Thorac Surg. 2005;80:449–454. [DOI] [PubMed] [Google Scholar]
- 17.Lindenblatt N, Puippe G, Broglie MA, et al. Lymphovenous anastomosis for the treatment of thoracic duct lesion: a case report and systematic review of literature. Ann Plast Surg. 2020;84:402–408. [DOI] [PubMed] [Google Scholar]
- 18.Grünherz L, Lindenblatt N. Central lymphatic surgery. Plast Aesthet Res. 2023;10:20. [Google Scholar]
- 19.Morikawa K, Takenaga S, Hasumi J, et al. Retrograde transvenous lymphatic embolization for postoperative chylous ascites: a report of three cases and literature review. Radiol Case Rep. 2020;15:1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieper CC, Hur S, Sommer CM, et al. Back to the future: lipiodol in lymphography-from diagnostics to theranostics. Invest Radiol. 2019;54:600–615. [DOI] [PubMed] [Google Scholar]
- 21.Schild HH, Pieper CC. Where have all the punctures gone? An analysis of thoracic duct embolizations. J Vasc Interv Radiol. 2020;31:74–79. [DOI] [PubMed] [Google Scholar]
- 22.Schild HH, Naehle CP, Wilhelm KE, et al. Lymphatic interventions for treatment of chylothorax. Rofo. 2015;187:584–588. [DOI] [PubMed] [Google Scholar]
- 23.Lindenblatt N, Gutschow CA, Vetter D, et al. Lympho-venous anastomosis for the treatment of congenital and acquired lesions of the central lymphatic system: a multidisciplinary treatment approach. Eur J Plast Surg. 2022;45:841–849. [Google Scholar]
- 24.Weinzierl A, Grunherz L, Puippe GD, et al. Microsurgical central lymphatic reconstruction-the role of thoracic duct lymphovenous anastomoses at different anatomical levels. Front Surg. 2024;11:1415010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RocheRodriguez M, DiNardo JA. The lymphatic system in the fontan patient-pathophysiology, imaging, and interventions: what the anesthesiologist should know. J Cardiothorac Vasc Anesth. 2022;36:2669–2678. [DOI] [PubMed] [Google Scholar]
- 26.Shah P, Louis MA. Physiology, central venous pressure. In StatPearls. Treasure Island, FL: 2024. [PubMed] [Google Scholar]
- 27.Ohuchi H. Where is the “optimal” fontan hemodynamics? Korean Circ J. 2017;47:842–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dortch JD, Eck D, Hakaim AG, et al. Management of cervical thoracic duct cyst with cyst-venous anastomosis. Int J Surg Case Rep. 2014;5:1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veziant J, Sakka L, Galvaing G, et al. Lymphovenous anastomosis for recurrent swelling syndrome and chylous effusion due to cervical thoracic duct cyst. J Vasc Surg. 2015;62:1068–1070. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Y, Chen LQ, Zhao Y. Anastomosis between thoracic duct and azygos vein during esophagectomy: a novel technique with 3-year follow-up. World J Surg. 2016;40:2984–2987. [DOI] [PubMed] [Google Scholar]
- 31.Miller TJ, Gilstrap JN, Maeda K, et al. Correction of complete thoracic duct obstruction with lymphovenous bypass: a case report. Microsurgery. 2019;39:255–258. [DOI] [PubMed] [Google Scholar]
- 32.Weissler JM, Cho EH, Koltz PF, et al. Lymphovenous anastomosis for the treatment of chylothorax in infants: a novel microsurgical approach to a devastating problem. Plast Reconstr Surg. 2018;141:1502–1507. [DOI] [PubMed] [Google Scholar]
- 33.Tang H, Zhou X, Xu M, et al. Recurrent chylothorax treated with thoracic duct-venous anastomosis: a retrospective review of medical records. JTCVS Tech. 2022;15:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taghinia AH, Upton J, Trenor CC, 3rd, et al. Lymphaticovenous bypass of the thoracic duct for the treatment of chylous leak in central conducting lymphatic anomalies. J Pediatr Surg. 2019;54:562–568. [DOI] [PubMed] [Google Scholar]
- 35.Reisen B, Kovach SJ, Levin LS, et al. Thoracic duct-to-vein anastomosis for the management of thoracic duct outflow obstruction in newborns and infants: a CASE series. J Pediatr Surg. 2020;55:234–239. [DOI] [PubMed] [Google Scholar]
- 36.Rodi T, Tung Nguyen B, Fritsche E, et al. Direct repair of iatrogenic thoracic duct injury through lymphovenous anastomosis (LVA): a case report. J Surg Oncol. 2020;121:224–227. [DOI] [PubMed] [Google Scholar]
- 37.de Beco G, Van Winghem J, Lengele B, et al. Thoracic duct-azygos vein anastomosis in an infant with superior vena cava syndrome and recurrent chylothorax. Interact Cardiovasc Thorac Surg. 2020;31:280–281. [DOI] [PubMed] [Google Scholar]
- 38.Chu CF, Wu CT, Hsieh WC, et al. Management of intractable post-adrenalectomy chylous ascites with microsurgical intra-abdominal lymphaticovenous anastomosis: a case report and literature review. Microsurgery. 2021;41:480–487. [DOI] [PubMed] [Google Scholar]
- 39.Ishiura R, Mitsui K, Danno K, et al. Successful treatment of large abdominal lymphatic malformations and chylous ascites with intra-abdominal lymphovenous anastomosis. J Vasc Surg Venous Lymphat Disord. 2020;9:499–503. [DOI] [PubMed] [Google Scholar]
- 40.Othman S, Azoury SC, DiBardino D, et al. Respiratory failure in noonan syndrome treated by microsurgical thoracic duct-venous anastomosis. Ann Thorac Surg. 2022;113:e219–e221. [DOI] [PubMed] [Google Scholar]
- 41.Ozeki M, Endo S, Yasue S, et al. Sirolimus treatment for intractable lymphatic anomalies: an open-label, single-arm, multicenter, prospective trial. Front Med (Lausanne). 2024;11:1335469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Othman S, Azoury SC, Klifto K, et al. Microsurgical thoracic duct lymphovenous bypass in the adult population. Plast Reconstr Surg Glob Open. 2021;9:e3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Kim HB, Pak CJ, et al. Using lymphovenous anastomosis and lymph node to vein anastomosis for treatment of posttraumatic chylothorax with increased thoracic duct pressure in 3-year-old child. Arch Plast Surg. 2022;49:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hattori Y, Yamashita S, Furuse K, et al. Lymphatic-venous anastomosis for the treatment of refractory lymphatic ascites following radiation therapy: a case report. Microsurgery. 2022;42:376–380. [DOI] [PubMed] [Google Scholar]
- 45.Kadota H, Shimamoto R, Fukushima S, et al. Lymphaticovenular anastomosis for lymph vessel injury in the pelvis and groin. Microsurgery. 2021;41:421–429. [DOI] [PubMed] [Google Scholar]
- 46.Tsuzaka S, Aiyama T, Kamachi H, et al. Lymphaticovenous anastomosis for treatment of refractory chylous ascites: a case report. Microsurgery. 2023;43:606–610. [DOI] [PubMed] [Google Scholar]
- 47.Chen SH, Yeh LF, Ciudad P, et al. Successful surgical treatment of intractable chylous ascites using the lymphatic cable flap: a retrospective review study. World J Surg. 2017;41:3100–3104. [DOI] [PubMed] [Google Scholar]
- 48.Klifto KM, Card EB, Itkin M, et al. Microsurgical peritoneovenous bypass for the treatment of recalcitrant chylous ascites. Plast Reconstr Surg. 2023;152:433–439. [DOI] [PubMed] [Google Scholar]
- 49.Weinzierl A, Barbon C, Gousopoulos E, et al. Benefits of robotic-assisted lymphatic microsurgery in deep anatomical planes. JPRAS Open. 2023;37:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinzierl A, Grünherz L, Puippe GD, et al. Microsurgical central lymphatic reconstruction-the role of thoracic duct lymphovenous anastomoses at different anatomical levels. Front Surg. 2024;11:1415010. [DOI] [PMC free article] [PubMed] [Google Scholar]