Abstract
Biosynthesized tea polyphenols showed antichlamydial activity against Chlamydia trachomatis D/UW-3/Cx and L2/434/Bu using cell culture. The most active compounds were (−)-epigallocatechin gallate and (−)-epicatechin gallate, followed by (−)-epicatechin (EC). (+)-Epicatechin and (−)-epigallocatechin were intermediate. EC was the least toxic. These results warrant evaluation of tea polyphenols as topical antichlamydial agents.
Chlamydia trachomatis is one of the most common causes of sexually transmitted diseases (5). Results obtained with topical antichlamydial agents such as nonoxynol-9 (3, 8, 10), monocaprin (4), C31G (14), cecropin peptides (1), and protegrins (17) have been reported. Disinfectants containing products such as chlorhexidine gluconate gel (9) and the spermicide benzalkonium chloride (2) have also been shown to inactivate C. trachomatis. We have previously reported that tea extracts have antichlamydial activity in vitro and that the active compounds are polyphenols (16).
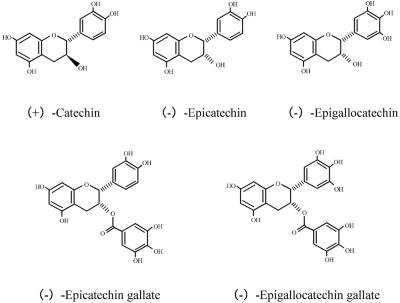
Five tea polyphenols (Fig. 1), (+)-catechin (Catech), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECg), and (−)-epigallocatechin gallate (EGCg), were tested in this study. These chemicals were biosynthesized naturally and supplied by Mitsui Norin Co., Ltd. (Tokyo, Japan).
FIG. 1.
Structural formula of the biosynthesized tea polyphenols. Five biosynthesized compounds, (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, and (−)-epigallocatechin gallate, were evaluated. (−)-Epigallocatechin is produced by hydroxylation of (−)-epicatechin. (−)-Epicatechin gallate and (−)-epigallocatechin gallate are synthesized by esterification with gallic acid.
The chlamydial strains tested were C. trachomatis D/UW-3/Cx and L2/434/Bu. HeLa 229 cells were used for growing C. trachomatis. A preinoculation minimal cidal concentration method, previously described by Lampe et al. (9), was used to test the in vitro susceptibility of C. trachomatis to tea polyphenols. A total of 1 ml of culture medium containing 2 × 105 HeLa 229 cells per ml was dispensed into each well of a plastic 24-well culture plate and incubated in 5% CO2 at 37°C for 24 h to form a confluent monolayer. Then, 1.0 × 104 inclusion-forming units of C. trachomatis were incubated with serial dilutions of tea polyphenols at 35°C for 90 min. Controls were incubated with sucrose phosphate glutamate (SPG) buffer containing no test compounds. Pretreated inocula were centrifuged onto the cells at 1,500 rpm for 60 min. After centrifugation, the inocula were removed. Culture medium containing no tea polyphenols was added and incubated for 72 h. Eagle's minimum essential medium containing 10% fetal calf serum and 0.6 μg/ml cycloheximide was used as the culture medium. The cells were fixed with methanol and stained with fluorescein isothiocyanate-conjugated antichlamydial monoclonal antibody (Denka Seiken Co., Ltd., Tokyo, Japan). Inclusions were counted by a fluorescent microscope. At least three wells per dilution were tested, and each experiment was repeated at least three times.
The toxic effects of EC, ECg, and EGCg on HeLa 229 cells were examined by using a CK01 cell counting kit (Dojindo Laboratory Co., Ltd., Kumamoto, Japan), a colorimetric assay for cell proliferation and viability. Cell activity was determined according to the manufacturer's instructions. Briefly, serial dilutions of tea polyphenols in SPG buffer were dispensed into 96-well microtiter plates containing a monolayer of HeLa 229 cells and incubated for 60 min at 35°C. After removal of tea polyphenols, cell activity was determined. After being fixed with methanol and stained using Giemsa stain, the condition of the cells was assessed using a microscope. The integrity of the cell monolayer and morphological changes in the cells, such as a round shape, were evaluated.
All tea polyphenols tested had an inhibitory effect on chlamydial proliferation (Table 1). ECg and EGCg completely inhibited the proliferation of C. trachomatis serovar D at concentrations of 1.6 and 0.8 mg/ml, respectively. Complete inactivation was also noted for C. trachomatis serovar L2 after incubation with EC, ECg, and EGCg at concentrations of 0.4, 0.4, and 0.8 mg/ml, respectively.
TABLE 1.
Inhibitory effect of tea polyphenols on serovars D/UW-3/Cx and L2/434/Bu of C. trachomatis in the preinoculation minimal cidal concentration method
| Compounda | Drug concn (mg/ml) for complete (100%) inhibition
|
|
|---|---|---|
| D/UW-3/Cx | L2/434/Bu | |
| Catech | >6.4b | >3.2b |
| EC | >6.4b | 0.4 |
| EGC | >6.4b | >6.4b |
| ECg | 1.6 | 0.4 |
| EGCg | 0.8 | 0.8 |
Catech, (+)-catechin; EC, (−)-epicatechin; EGC, (−)-epigallocatechin; ECg, (−)-epicatechin gallate; EGCg, (−)-epigallocatechin gallate.
> indicates the highest concentration tested.
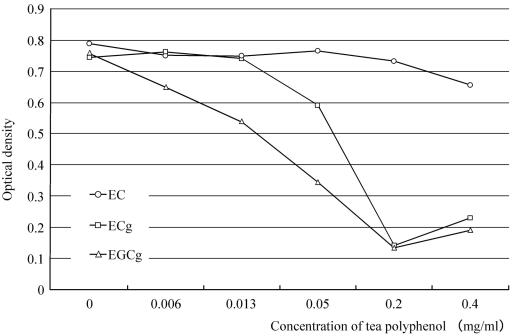
The activity of HeLa 229 cells incubated with EC, ECg, and EGCg is shown in Fig. 2. After 60 min of incubation with 0.4 mg/ml EC, cell activity did not decrease and no differences in the condition of the cells were observed after Giemsa staining. By contrast, after 60 min of incubation with increasing concentrations of ECg and EGCg, the activity of HeLa 229 cells decreased and at high concentrations of ECg and EGCg the cells had a round shape.
FIG. 2.
Activity of HeLa 229 cells incubated with (−)-epicatechin (EC), (−)-epicatechin gallate (ECg), and (−)-epigallocatechin gallate (EGCg) evaluated by a CK01 cell counting kit, a colorimetric assay for cell proliferation. Serial dilutions of tea polyphenols in SPG buffer were incubated with HeLa 229 cells for 60 min, and the cell activity was determined. Cell activity was assessed by the optical density. The activity of the HeLa 229 cells did not decrease after 60 min of incubation with 0.4 mg/ml EC but did decrease after 60 min of incubation with ECg and EGCg at the concentrations used.
Tea polyphenols have been shown to have in vitro antimicrobial effects (6, 11-13, 15). We previously reported that Polyphenon 70S, a mixed compound of tea polyphenols, had an in vitro inhibitory effect on C. trachomatis (16). Polyphenon 70S is composed of EGCg, EGC, ECg, EC, and (−)-gallocatechin gallate. Each individual constituent had an inhibitory effect at a lower concentration than that in Polyphenon 70S: the endpoint for C. trachomatis serovar D was 1.6 mg/ml when incubated with Polyphenon 70S and 0.8 mg/ml when incubated with EGCg. These data suggest that the individual constituents of tea polyphenols, such as EC, are potential candidates for antichlamydial drugs.
The concentration of tea polyphenols required for complete inhibition of chlamydial proliferation is relatively high compared with the MIC of antibiotics such as tetracyclines, macrolides, and fluoroquinolones (16). Therefore, oral administration of tea polyphenols is not suitable for treating systemic infection. Each tea polyphenol constituent might be used topically. The development of more-effective drugs for systemic use by modifying the structures of EC, ECg, and EGCg is expected in the future.
Nonoxynol-9 is an active spermicidal ingredient used in a wide variety of vaginal contraceptive preparations. Products containing nonoxynol-9 also inhibit the growth of C. trachomatis. Lampe et al. indicated that a chlorhexidine gluconate gel could remain in the vagina for hours after topical application and provided protection against C. trachomatis infection for that time period (9). Addition of tea polyphenols to contraceptive jelly could have potential clinical use in the prevention of cervical infection caused by C. trachomatis. Of note, EC was the least toxic among the tea polyphenols that inactivated chlamydial strains. Further studies are required to clarify the safety of tea polyphenols for clinical usage.
Ikigai et al. indicated that EGCg damaged bacterial membranes (7). The inhibitory mechanisms of tea polyphenols against C. trachomatis are unknown and should be investigated further.
REFERENCES
- 1.Ballweber, L. M., J. E. Jaynes, W. E. Stamm, and M. F. Lampe. 2002. In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 46:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bélec, L., C. Tevi-Benissan, A. Bianchi, S. Cotigny, M. Beumont-Mauviel, A. Si-Mohamed, and J.-E. Malkin. 2000. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J. Antimicrob. Chemother. 46:685-693. [DOI] [PubMed] [Google Scholar]
- 3.Benes, S., and W. M. McCormack. 1985. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob. Agents Chemother. 27:724-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergsson, G., J. Arnfinnsson, S. M. Karlsson, Ó. Steingrímsson, and H. Thormar. 1998. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 42:2290-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1997. Chlamydia trachomatis genital infections—United States, 1995. Morb. Mortal. Wkly. Rep. 46:193-198. [PubMed] [Google Scholar]
- 6.Diker, K. S., M. Akan, G. Hascelik, and M. Yurdakök. 1991. The bactericidal activity of tea against Campylobacter jejuni and Campylobacter coli. Lett. Appl. Microbiol. 12:34-35. [Google Scholar]
- 7.Ikigai, H., T. Nakae, Y. Hara, and T. Shimamura. 1993. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1147:132-136. [DOI] [PubMed] [Google Scholar]
- 8.Kelly, J. P., R. B. Reynolds, S. Stagno, W. C. Louv, and W. J. Alexander. 1985. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 27:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampe, M. F., L. M. Ballweber, and W. E. Stamm. 1998. Susceptibility of Chlamydia trachomatis to chlorhexidine gluconate gel. Antimicrob. Agents Chemother. 42:1726-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons, J. M., and J. I. Ito, Jr. 1995. Reducing the risk of Chlamydia trachomatis genital tract infection by evaluating the prophylactic potential of vaginally applied chemicals. Clin. Infect. Dis. 21(Suppl. 2):S174-S177. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama, M., M. Toda, S. Okubo, and T. Shimamura. 1990. Inhibition of influenza virus infection by tea. Lett. Appl. Microbiol. 11:38-40. [Google Scholar]
- 12.Nakayama, M., K. Suzuki, M. Toda, S. Okubo, Y. Hara, and T. Shimamura. 1993. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 21:289-299. [DOI] [PubMed] [Google Scholar]
- 13.Toda, M., S. Okubo, H. Ikigai, T. Suzuki, Y. Suzuki, and T. Shimamura. 1991. The protective activity of tea against Vibrio cholerae O1. J. Appl. Bacteriol. 70:109-112. [DOI] [PubMed] [Google Scholar]
- 14.Wyrick, P. B., S. T. Knight, D. G. Gerbig, Jr., J. E. Raulston, C. H. Davis, T. R. Paul, and D. Malamud. 1997. The microbicidal agent C31G inhibits Chlamydia trachomatis infectivity in vitro. Antimicrob. Agents Chemother. 41:1335-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yam, T. S., J. M. T. Hamilton-Miller, and S. Shah. 1998. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2′ synthesis, and β-lactamase production in Staphylococcus aureus. J. Antimicrob. Chemother. 42:211-216. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki, T., M. Inoue, N. Sasaki, T. Hagiwara, T. Kishimoto, S. Shiga, M. Ogawa, Y. Hara, and T. Matsumoto. 2003. In vitro inhibitory effects of tea polyphenols on the proliferation of Chlamydia trachomatis and Chlamydia pneumoniae. Jpn. J. Infect. Dis. 56:143-145. [PubMed] [Google Scholar]
- 17.Yasin, B., S. S. L. Harwig, R. I. Lehrer, and E. A. Wagar. 1996. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect. Immun. 64:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]