Abstract
The present study tested the ability of linezolid to penetrate soft tissues in healthy volunteers. Ten healthy volunteers were subjected to linezolid drug intake at a dose of 600 mg twice a day for 3 to 5 days. The first dose was administered intravenously. All following doses were self-administered orally. The tissue penetration of linezolid was assessed by use of in vivo microdialysis. In the single-dose experiments the ratios of the area under the concentration-time curve from 0 to 8 h (AUC0-8) for tissue to the AUC0-8 for free plasma were 1.4 ± 0.3 (mean ± standard deviation) and 1.3 ± 0.4 for subcutaneous adipose and muscle tissue, respectively. After multiple doses, the corresponding mean ratios were 0.9 ± 0.2 and 1.0 ± 0.5, respectively. The ratios of the AUC from 0 to 24 h (AUC0-24) for free linezolid in tissues to the MIC were between 50 and 100 for target pathogens with MICs between 2 and 4 mg/liter. In conclusion, the present study showed that linezolid penetrates rapidly into the interstitial space fluid of subcutaneous adipose and skeletal muscle tissues in healthy volunteers. On the basis of pharmacokinetic-pharmacodynamic calculations, we suggest that linezolid concentrations in soft tissues can be considered sufficient to inhibit the growth of many clinically relevant bacteria.
The worldwide increase of gram-positive, antimicrobial-resistant pathogens accelerates the search for novel anti-infective agents (28). Linezolid is the first approved substance of a new class of antibiotics called the oxazolidinones. Linezolid has been demonstrated to have excellent in vitro and in vivo activities against glycopeptide-resistant gram-positive bacteria (30). These in vivo and in vitro characteristics of linezolid have triggered the conduct of a large body of scientific research in animals and humans. However, most of the currently available pharmacokinetic (PK) studies about linezolid are based on measurements of total plasma concentrations (3, 26). This may be misleading for two reasons: first, only the free drug concentration of an antimicrobial agent exerts antibacterial activity (12, 16). Second, most relevant pathogens reside in the interstitial space fluid (ISF) of the infected tissues but do not reside in the bloodstream (22-24). Therefore, regulatory authorities such as the U.S. Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products recommend that the concentration of an antibiotic needs to be determined at the target site (5, 6). It is generally accepted that this site is represented by the ISF of the tissue for most bacterial infections (22). A clinical technique that can be used to assess target site concentrations in the ISF is in vivo microdialysis (MD) (4, 20).
The present study was performed to evaluate the ability of linezolid to penetrate soft tissues by use of the MD technique as a means to meet the regulatory requirements. The unbound, i.e., microbiologically active, concentration-versus-time profile of linezolid was determined in skeletal muscle, subcutaneous adipose tissue, and plasma after single and multiple doses.
MATERIALS AND METHODS
The study took place at the Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria. The study protocol was approved by the local ethics committee and was performed in accordance with the Declaration of Helsinki (1964) in the revised version of 2000 (Edinburgh), the Guidelines of the International Conference of Harmonization, the Good Clinical Practice Guidelines, and the Austrian drug law. All volunteers were given a detailed description of the study, and their written consent was obtained prior to their enrollment in the study.
Subjects.
Ten healthy volunteers (five males and five females; age, 55 ± 10 years; weight, 66 ± 8 kg; height, 168 ± 8 cm; means ± standard deviations [SDs]) were included in the present study. Prior to inclusion in the study, the volunteers were subjected to a screening examination, which comprised a medical history, physical examination, 12-lead electrocardiogram, blood pressure measurement, complete blood count with differential blood analysis, urine analysis, urine drug screen, a urine human chorionic gonadotropin test to exclude pregnant female volunteers, clinical blood chemistry analysis, blood coagulation tests, HBs antigen test, and human immunodeficiency virus antibody tests.
Microdialysis.
The principles of MD have previously been described in detail (17, 19). In brief, MD is based on sampling of analytes from the extracellular space of tissues by means of a semipermeable membrane at the tip of an MD probe. The probe is constantly perfused with a physiologic solution at a flow rate of 1.5 μl/min. Once the probe is implanted into the tissue, substances present in the extracellular fluid at a certain concentration (Ctissue) diffuse through the membrane into the perfusate, resulting in a concentration (Cdialysate) in the perfusion medium. For most analytes, equilibrium between the extracellular fluid and the perfusion medium is incomplete, and therefore, Ctissue is greater than Cdialysate. The calibration factor by which the concentrations are interrelated is termed recovery. For calibration of the microdialysis probes, in vivo recovery was assessed in each experiment by the retrodialysis method (18). The principle of this method relies on the fact that the diffusion process is quantitatively equal in both directions along the semipermeable membrane (2). For in vivo probe calibration, linezolid was added to the perfusate (Ringer's solution; Mayrhofer Pharmazeutika, Linz, Austria) at a concentration of approximately 100 mg/liter in the single-dose experiment and at a concentration of 150 mg/liter in the steady-state experiment. The disappearance rate (delivery) through the membrane was taken as in vivo recovery. Retrodialysis was performed at the beginning of the single-dose experiment and at the end of the steady-state experiment over a period of 30 min. In vivo recovery values for linezolid were calculated as follows: in vivo recovery (%) = 100 - [100 × (linezoliddialysate/linezolidperfusate)].
Study protocol.
On study day 1, a plastic cannula was inserted into an antecubital vein for the intravenous (i.v.) administration of linezolid and a second plastic cannula was inserted in the contralateral upper limb to monitor blood concentrations of linezolid at defined time intervals. Two commercially available microdialysis probes (CMA 60; CMA, Solna, Sweden) with molecular weight cutoffs of 20,000 and a membrane length of 30 mm were inserted into the subcutaneous adipose tissue and a skeletal muscle of the thigh without prior anesthesia by a previously described procedure (11). The microdialysis system was connected to a perfusion precision pump (CMA 100, CMA) and was perfused with Ringer's solution at a flow rate of 1.5 μl/min. After a 30-min baseline sampling period and in vivo calibration of the probe, 600 mg of linezolid (Zyvoxid; 2 mg/ml; Infusionsbeutel; Pharmacia, Vienna, Austria) was administered intravenously over 30 min. Sampling of microdialysates and plasma was performed at defined time intervals for up to 8 h postdosing. All samples were frozen at −80°C until analysis.
After withdrawal of the plastic cannulas and the MD probes, the volunteers left the research ward and were subjected to oral linezolid (Zyvoxid; 600 mg; Filmtabletten; Pharmacia) drug intake at a dose of 600 mg twice a day for three to five consecutive days. MD experiments on study day 2 were started simultaneously with the final drug intake, and the MD probes were calibrated at the end of the sampling procedure of 8 h.
Bioanalysis.
Linezolid concentrations in plasma and microdialysates were measured by high-performance liquid chromatography (HPLC) (2). Total concentrations of linezolid in plasma were corrected for individual plasma protein binding (PPB) values assessed by ultrafiltration to determine free plasma concentrations. The limit of quantification was 0.2 mg/liter in plasma and 0.8 mg/liter in microdialysates. Overall, the accuracy and the coefficient of variation ranged from −2.7 to 6.1% for microdialysates and plasma samples.
Determination of individual plasma protein binding.
To evaluate the individual PPB, a commercially available ultrafiltration membrane (Centrifree ultrafiltration device; Millipore, Eschborn, Germany) with a regenerated cellulose membrane (molecular weight cutoff, 30,000) was used. A total of 200 μl of a plasma sample was transferred into the ultrafiltration device, which was placed in a centrifuge (Megafuge; Heraeus, Hanau, Germany) and centrifuged at 1,064 × g for 10 min at ambient temperature. The ultrafiltrate was then subjected to HPLC for quantification of linezolid.
Prior to analysis of the human samples, the characteristics of the adsorption of linezolid to the ultrafiltration device were investigated in vitro. Six aqueous linezolid solutions of 10 mg/liter were prepared. Three of six solutions were ultrafiltrated, and the concentrations in the ultrafiltrate were compared to those in nonfiltrated aqueous solutions. No relevant differences were detected. In addition, the unbound fractions (fus) in pooled plasma containing concentrations of linezolid at 2, 10, and 20 mg/liter (n = 3 each) were determined. The unweighted linear correlation of the unbound fraction versus the total concentrations of linezolid yielded y = 0.331x + 71.4 (R = 0.611). Thus, linezolid did not bind to the ultrafiltration membrane, and binding can be considered independent from the concentrations used.
Two plasma samples per volunteer and study day were selected to determine the individual protein binding representing specific time points: an early time point (≤3 h) and a late time point (8 h), respectively. fu was calculated as 100 · (unbound plasma concentration/total plasma concentration) (at the same time point). The mean of both fu values per study day and volunteer was calculated and was used for calculation of the free fraction of linezolid in plasma. The resulting individual unbound fractions of linezolid ranged from 79.5 to 95.8%, with a mean of 89.5% (coefficient of variation = 4.8%; n = 20).
Calculations and data analysis.
The individual in vivo recovery values were used to calculate interstitial linezolid concentrations according to the following equation: tissue concentration = 100 · (sample concentration/in vivo recovery).
PK analysis was performed by using commercially available computer software (Kinetica version 3.0, InnaPhase, Philadelphia, Pa.), and the values of the PK parameters were calculated by a noncompartmental approach. The area under the concentration-time curve (AUC) values for plasma and the interstitium were calculated from nonfitted data by using the linear trapezoidal rule. The AUC from 0 to 24 h (AUC0-24) was determined by use of the concentration at 24 h (C24) value, which was calculated by extrapolation of the last measured concentration (Clast) to the concentration at 24 h by the formula C24 = Clast × e(−kel × t), where t is the time interval between Clast and C24.
The values of the following main PK parameters were determined: maximum concentration (Cmax) and the time to Cmax (Tmax). The half-life for the terminal slope (t1/2) was calculated by the equation t1/2 = ln 2/kel. The apparent total body clearance (CL) and the apparent volume of distribution (V) after a single i.v. administration of 600 mg of linezolid were determined for total plasma by use of standard formulae, as follows: V = dose × F/(AUC0-∞ × kel) and CL = dose/AUC0-∞, where AUC0-∞ represents the AUC from 0 h to infinity. Since the oral bioavailability (F) of linezolid is considered to be 100%, correction of V and CL for this parameter was not necessary (27).
Statistical analysis.
Wilcoxon matched pairs tests and Bonferroni adjustments were performed with commercially available computer software (Statistica, version 5.0; Statsoft Inc., Tulsa, Okla.) for statistical comparison of the main outcome PK parameter (AUCtissue to AUCfree plasma). All data are presented as means ± SDs. A two-sided P value of <0.05 was considered the level of significance.
RESULTS
The present study tested the ability of linezolid to penetrate soft tissues in healthy volunteers by means of in vivo microdialysis. Linezolid was well tolerated by all volunteers. The mean in vivo recovery values for linezolid in single- and multiple-dose experiments were 40.5% ± 16.8% and 47.5% ± 12.9%, respectively (P > 0.05, which was not significant [ns]). In contrast to the reported PPB value of approximately 30% (15, 21), we found mean PPB values of 8.7% ± 3.1% in the single-dose experiment and 12.4% ± 4.7% after the administration of multiple doses (P > 0.05; ns).
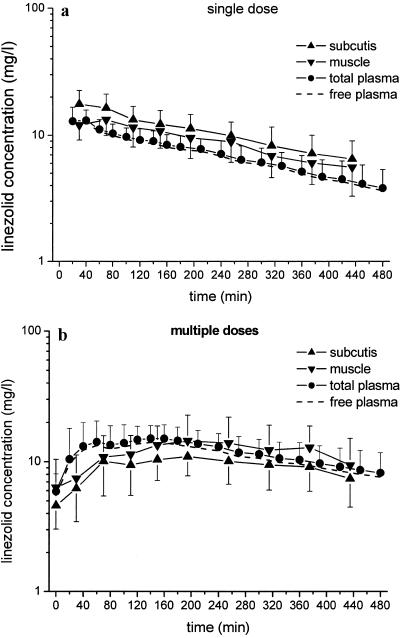
After a single intravenous dose of linezolid, the AUC0-8 for soft tissues was significantly higher than that for free plasma (P = 0.007 for adipose tissue and P = 0.02 for skeletal muscle) (Fig. 1a). This is pointed out by the mean ratios of the AUCtissue 0-8 to the AUCfree plasma 0-8 of 1.4 ± 0.3 for adipose tissue and 1.3 ± 0.4 for skeletal muscle.
FIG. 1.
Time-concentration curves (means ± SDs) of linezolid in plasma (total and free) and ISF of subcutaneous adipose tissue and skeletal muscle. (a) Profile after administration of a single intravenous dose of 600 mg (n = 10); (b) concentration-versus-time profile after multiple doses of oral linezolid intake (600 mg twice a day; n = 9).
Noncompliance rendered the PK profile of linezolid at steady state invalid for one volunteer. Therefore, multiple-dose PK data were available from only nine volunteers. After multiple doses, the concentration-versus-time profiles of linezolid for plasma (total and free) and tissues clearly demonstrated that the unbound plasma fraction of linezolid equilibrated completely with the ISF concentrations in adipose tissue and skeletal muscle (Fig. 1b). This is also indicated by the mean ratios of the AUCtissue 0-8 to the AUCfree plasma 0-8, with values of 0.9 ± 0.2 for adipose tissue and 1.0 ± 0.5 for skeletal muscle.
The values of the main pharmacokinetic parameters of linezolid after single-dose administration (Table 1) and at steady state (Table 2) were in excellent agreement with previously published results (15).
TABLE 1.
Pharmacokinetic parameters (means ± SDs) of linezolid in plasma, muscle, and subcutaneous adipose tissue following single intravenous dose administration of 600 mg
| Compartment | AUC0-8 (mg · h/liter) | AUCtissue/AUCfree plasma | AUC0-24 (mg · h/liter) | Cmax (mg/liter) | Tmax (h) | t1/2 (h) | CL (liters/h) | V (liters) |
|---|---|---|---|---|---|---|---|---|
| Plasma total | 58.1 ± 12.7 | 88.1 ± 34.0 | 14.1 ± 2.8 | 0.5 ± 0.2 | 5.1 ± 2.6 | 7.9 ± 3.2 | 46.3 ± 9.1 | |
| Plasma free | 53.0 ± 11.6 | 80.3 ± 30.9 | 12.8 ± 2.6 | 0.5 ± 0.2 | 5.1 ± 2.6 | NDa | ND | |
| Subcutis | 75.8 ± 24.2b | 1.4 ± 0.3 | 129.7 ± 57.8 | 18.1 ± 4.8 | 0.9 ± 0.3 | 6.1 ± 1.9 | ND | ND |
| Muscle | 65.4 ± 18.2b | 1.3 ± 0.4 | 94.1 ± 35.5 | 13.5 ± 3.3 | 1.1 ± 0.3 | 3.9 ± 1.2 | ND | ND |
ND, not determined.
P < 0.05 compared with the results for free plasma.
TABLE 2.
Pharmacokinetic parameters (means ± SDs) of linezolid for plasma, muscle, and subcutaneous adipose tissue after multiple oral doses of 600 mg twice a day over a period of three to five consecutive days
| Compartment | AUC0-8 (mg · h/liter) | AUCtissue/AUCfree plasma | AUC0-24 (mg · h/liter) | Cmax (mg/liter) | Tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|---|
| Plasma total | 94.9 ± 24.3 | 244.9 ± 72.3 | 19.5 ± 4.6 | 1.4 ± 1.0 | 6.9 ± 2.6 | |
| Plasma free | 83.9 ± 23.9 | 217.1 ± 71.5 | 17.1 ± 4.0 | 1.4 ± 1.0 | 6.9 ± 2.6 | |
| Subcutis | 71.5 ± 15.3 | 0.9 ± 0.2 | 200.8 ± 53.2 | 12.9 ± 2.4 | 2.2 ± 1.2 | 7.5 ± 2.7 |
| Muscle | 80.2 ± 42.3 | 1.0 ± 0.5 | 235.5 ± 132.1 | 15.4 ± 8.0 | 2.6 ± 1.1 | 6.4 ± 1.8 |
Table 3 shows the ratios of the AUC0-24 to the MIC for pathogens with MICs of 2 mg/liter and 4 mg/liter, respectively, for plasma and soft tissues at steady state.
TABLE 3.
AUC0-24/MIC ratios for plasma and soft tissues at steady state
| Compartment | AUC0-24/MIC for pathogens with an MIC ofa
|
|
|---|---|---|
| 2 mg/liter | 4 mg/liter | |
| Plasma total | 122.5 ± 36.2 (71.9-183.0) | 61.2 ± 18.1 (36.0-91.5) |
| Plasma free | 108.5 ± 35.8 (62.4-168.8) | 54.3 ± 17.9 (31.2-84.4) |
| Subcutis | 93.3 ± 31.8 (43.4-147.9) | 46.6 ± 15.9 (21.7-73.9) |
| Muscle | 117.7 ± 66.1 (35.5-236.1) | 58.9 ± 33.0 (17.7-118.1) |
Data are presented as means ± SDs (ranges).
DISCUSSION
Most of the recent PK data for linezolid available in the literature focused on plasma concentrations (15), skin blister studies (8), and tissue biopsy specimens (14). Nevertheless, none of these techniques are capable of the assessment of the free concentrations of linezolid in tissues, which more accurately reflect the site of infection and drug action. Free tissue concentrations are crucial for the prediction of antimicrobial and clinical efficacy (10). Thus, in the present study we used the in vivo microdialysis technique to selectively analyze the unbound, i.e., pharmacologically active, concentration of linezolid in the ISF.
The key finding of our study is the complete equilibration of linezolid between plasma and the ISF of soft tissues after multiple doses, i.e., at steady state, as indicated by the ratio of the AUCtissue/AUCfree plasma of about 1. This is in excellent accordance with the previously published data by Gee et al. (8).
In contrast, the concentrations of linezolid in soft tissues after a single i.v. administration were significantly higher than the free plasma concentrations (P = 0.007 for adipose tissue and P = 0.02 for skeletal muscle). The corresponding ratios of the AUCtissue to AUCfree plasma were greater than 1.
This finding is hard to explain, although different factors are well known to markedly influence the plasma-to-tissue equilibration of an antibiotic. Chief among them are the physicochemical properties of the substance, such as the molecular weight, the pKa value, the octanol/water partition coefficient, the pH of the compartment, and the presence of active transport pumps, which markedly determine penetration characteristics of antibiotics into tissues. However, all these parameters cannot explain the discrepancy between the results of the single-dose part and the multiple-dose part of the present study because no significant change in pKa, the pH of the tissue, or the octanol/water partition coefficient is expected to occur between single and multiple doses in our healthy study population. Thus, our observation is not yet fully understood, but it should be mentioned that similar behaviors were found by our group for gemifloxacin (9) and telithromycin (7). This underlines the need for novel strategies for the investigation of the tissue pharmacokinetics of antibiotics. These strategies should allow the simultaneous assessment of the intra- and extracellular concentrations of the compound. One promising approach that can be used to overcome the current limitations is the use of the positron emission tomography combined with the microdialysis technique. The suitability and applicability of the combined use of these techniques have recently been demonstrated for fluorine-18-labeled ciprofloxacin (13).
As tissue homogenization leads to an underestimation of the concentration of linezolid in tissues, it is not unexpected that there are marked differences between the results of the present study and data derived by use of the traditional biopsy method (14). Hence, the measurement of linezolid concentrations by use of biopsy specimens might be regarded as inappropriate, as the concentrations at the actual site of bacterial infection are not adequately determined. This is particularly relevant for antibiotics that have a low volume of distribution of about 20 to 30 liters and that distribute predominantly within the extracellular fluid. In the present study the calculated volume of distribution was approximately 50 liters and indicated that linezolid distributed moderately into cells. The AUC0-24 and the Cmax for free linezolid in plasma at steady state were significantly higher than those after a single dose of linezolid (P < 0.05) and confirmed the assumption of Burkhardt et al. (3) of a limited accumulation of linezolid after multiple doses.
The clinical efficacy of linezolid is shown to correlate with the ratio of the AUC0-24 to the MIC (15). On the basis of the generally accepted breakpoint for efficient dosing at an AUC0-24/MIC ratio of 50 to 100 (1), the calculated mean ratio of the AUC0-24 to the MIC (Table 3) for plasma and soft tissues suggests that linezolid reaches tissue concentrations at steady state effective against pathogens with MICs up to 4 mg/liter, which covers bacteria such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium (25). However, this is only true for the mean ratios of the AUC0-24 to the MIC, since huge interindividual differences in the pharmacokinetics of linezolid were detected, which may result in the risk that pathogens with MICs higher than 2 mg/liter will be not optimally inhibited in some individuals. In addition, in recent studies we were able to demonstrate that the tissue penetration of antimicrobial agents is markedly decreased in the presence of local or systemic inflammation (11, 29), and hence, the ability of linezolid to penetrate inflamed tissues needs to be explored in distinct patient populations.
In conclusion, in the present study we showed that the penetration of linezolid into the interstitial space fluid of subcutaneous adipose tissue and skeletal muscle might be sufficient for the treatment of soft tissue infections. However, additional data from studies with patients with local or systemic infections are required to confirm this conclusion.
Acknowledgments
We are indebted to our study nurse, Petra Zeleny, for her essential contribution to this study.
This work was supported by a research grant (grant 10286) from the “Oesterreichische Nationalbank Fonds.”
REFERENCES
- 1.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buerger, C., C. Joukhadar, M. Muller, and C. Kloft. 2003. Development of a liquid chromatography method for the determination of linezolid and its application to in vitro and human microdialysis samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796:155-164. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt, O., K. Borner, N. von der Hoh, P. Koppe, M. W. Pletz, C. E. Nord, and H. Lode. 2002. Single- and multiple-dose pharmacokinetics of linezolid and co-amoxiclav in healthy human volunteers. J. Antimicrob. Chemother. 50:707-712. [DOI] [PubMed] [Google Scholar]
- 4.Elmquist, W. F., and R. J. Sawchuk. 1997. Application of microdialysis in pharmacokinetic studies. Pharm. Res. 14:267-288. [DOI] [PubMed] [Google Scholar]
- 5.European Agency for the Evaluation of Medicinal Products. 2000. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. [Online.] www.emea.eu.int/pdfs/human/ewp/265599en.pdf.
- 6.Food and Drug Administration. 1998. Guidance for industry. Developing antimicrobial drugs—general considerations for clinical trials. [Online.] www.fda.gov/cder/guidance/2580dft.pdf.
- 7.Gattringer, R., E. Urbauer, F. Traunmüller, M. Zeitlinger, P. Dehghanyar, P. Zeleny, W. Graninger, M. Muller, and C. Joukhadar. 2004. Pharmacokinetics of telithromycin in plasma and soft tissues after single-dose administration to healthy volunteers. Antimicrob. Agents Chemother. 48:4650-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gee, T., R. Ellis, G. Marshall, J. Andrews, J. Ashby, and R. Wise. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islinger, F., R. Bouw, M. Stahl, E. Lackner, P. Zeleny, M. Brunner, M. Muller, H. G. Eichler, and C. Joukhadar. Concentrations of gemifloxacin at the target site after a single oral dose in healthy volunteers. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 10.Joukhadar, C., and M. Muller. Microdialysis: current applications to clinical pharmacokinetic studies and its role in the future. Clin. Pharmacokinet., in press. [DOI] [PubMed]
- 11.Joukhadar, C., H. Stass, U. Muller-Zellenberg, E. Lackner, F. Kovar, E. Minar, and M. Muller. 2003. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob. Agents Chemother. 47:3099-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunin, C. M., W. A. Craig, M. Kornguth, and R. Monson. 1973. Influence of binding on the pharmacologic activity of antibiotics. Ann. N. Y. Acad. Sci. 226:214-224. [DOI] [PubMed] [Google Scholar]
- 13.Langer, O., M. Brunner, U. Muller, G. Dobrozemsky, C. Joukhadar, M. Mitterhauser, W. Wadsak, K. Kletter, R. Dudczak, and M. Muller. 2004. Positron emission tomography (PET) and microdialysis (MD) for in vivo assessment of intracellular drug concentrations in humans. Int. J. Clin. Pharmacol. Ther. 42:395. (Abstract.) [Google Scholar]
- 14.Lovering, A. M., J. Zhang, G. C. Bannister, B. J. Lankester, J. H. Brown, G. Narendra, and A. P. MacGowan. 2002. Penetration of linezolid into bone, fat, muscle and haematoma of patients undergoing routine hip replacement. J. Antimicrob. Chemother. 50:73-77. [DOI] [PubMed] [Google Scholar]
- 15.MacGowan, A. P. 2003. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with gram-positive infections. J. Antimicrob. Chemother. 51(Suppl. 2):17-25. [DOI] [PubMed] [Google Scholar]
- 16.Merrikin, D. J., J. Briant, and G. N. Rolinson. 1983. Effect of protein binding on antibiotic activity in vivo. J. Antimicrob. Chemother. 11:233-238. [DOI] [PubMed] [Google Scholar]
- 17.Muller, M. 2002. Science, medicine, and the future: microdialysis. BMJ 324:588-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller, M., R. Schmid, A. Georgopoulos, A. Buxbaum, C. Wasicek, and H. G. Eichler. 1995. Application of microdialysis to clinical pharmacokinetics in humans. Clin. Pharmacol. Ther. 57:371-380. [DOI] [PubMed] [Google Scholar]
- 20.Muller, M., H. Stass, M. Brunner, J. G. Moller, E. Lackner, and H. G. Eichler. 1999. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob. Agents Chemother. 43:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawsey, S. D., P. T. Daley-Yates, C. P. Wajszczuk, and D. J. Stalker. 1996. U-100766 Safety, toleration and pharmacokinetics after oral and intravenous administration. Abstr. First Eur. Congr. Chemother., abstr. F151. Federation for the Societies for European Chemotherapy and Infection, London, United Kingdom.
- 22.Ryan, D. M. 1993. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 31(Suppl. D):1-16. [DOI] [PubMed] [Google Scholar]
- 23.Ryan, D. M., and O. Cars. 1983. A problem in the interpretation of beta-lactam antibiotic levels in tissues. J. Antimicrob. Chemother. 12:281-284. [DOI] [PubMed] [Google Scholar]
- 24.Ryan, D. M., O. Cars, and B. Hoffstedt. 1986. The use of antibiotic serum levels to predict concentrations in tissues. Scand. J. Infect. Dis. 18:381-388. [DOI] [PubMed] [Google Scholar]
- 25.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin- intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stalker, D. J., G. L. Jungbluth, N. K. Hopkins, and D. H. Batts. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 51:1239-1246. [DOI] [PubMed] [Google Scholar]
- 27.Welshman, I. R., D. J. Stalker, and C. P. Wajszczuk. 1998. Assessment of absolute bioavailability and evaluation of the effect of food on oral bioavailability of linezolid. Anti-Infective Drugs Chemother. 54(Suppl. 1):16. [Google Scholar]
- 28.Wilcox, M. H. 2003. Efficacy of linezolid versus comparator therapies in gram-positive infections. J. Antimicrob. Chemother. 51(Suppl. 2):27-35. [DOI] [PubMed] [Google Scholar]
- 29.Zeitlinger, M. A., P. Dehghanyar, B. X. Mayer, B. S. Schenk, U. Neckel, G. Heinz, A. Georgopoulos, M. Muller, and C. Joukhadar. 2003. Relevance of soft-tissue penetration by levofloxacin for target site bacterial killing in patients with sepsis. Antimicrob. Agents Chemother. 47:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]