Abstract
The distribution of abacavir into the cerebrospinal fluid (CSF) was assessed by use of a population pharmacokinetic analysis. Plasma and CSF abacavir concentrations in 54 subjects were determined. The abacavir CSF/plasma ratio averaged 36% and increased throughout the dose interval. Abacavir penetrates into the CSF in adequate concentrations to inhibit local human immunodeficiency virus replication.
Nucleoside reverse transcriptase inhibitors, in combination with potent nonnucleoside reverse transcriptase inhibitors or protease inhibitors, can decrease viral replication, improve immune function, and prolong survival. Combination antiretroviral regimens may also reduce viral replication in the central nervous system (CNS). Nucleosides appear to penetrate into the CNS better than protease inhibitors and have been useful in treating patients with human immunodeficiency virus (HIV)-associated dementia (5, 12). Many of these agents are substrates for active transporter systems that effectively pump drugs out of the CNS. Cerebrospinal fluid (CSF) and plasma exhibit different viral dynamics, indicating that the CNS may serve as an independent reservoir for HIV replication (4, 6). Optimal antiretroviral concentrations in the CNS are necessary to limit local HIV replication and prevent the development of drug-resistant virus and overall treatment failure (11). A few pharmacokinetic evaluations of abacavir in primates and humans have reported limited penetration into the CNS, as characterized by CSF/plasma ratios, ranging from less than 20% to 35% (3, 8). However, most of these evaluations were derived from measurements collected early in the dose interval, which may have biased the estimates of CSF penetration (4, 5, 7). The CSF/plasma concentration ratio is a dynamic measure, and single measurements may not accurately estimate exposure in this potential sanctuary. The ratio of the areas under the CSF and plasma concentration-time curves (AUCCSF/AUCplasma) is a better estimate of drug exposure, as it accounts for the variability over the entire dosing interval, thus giving a more accurate estimate of CNS penetration (2, 10). Since multiple CSF samples for individual AUC determinations are difficult to collect, we used sparse CSF and plasma concentrations with population pharmacokinetic analysis to estimate the population abacavir AUCCSF/AUCplasma ratio in HIV-infected patients.
Plasma and CSF samples were obtained from 54 male adult HIV-infected patients receiving an abacavir-containing regimen. All subjects were consenting participants in prospective research studies conducted at the University of California San Diego HIV Neurobehavioral Research Center and approved by the University of California San Diego Institutional Review Board. Fifty-one subjects (94%) were taking abacavir according to a conventional dosing schedule, (300 mg twice daily), while three received 150 mg, 400 mg, or 600 mg twice daily, respectively. All patients were at steady state and free of opportunistic infection at the time of sample collection.
Several subjects contributed multiple samples, resulting in 70 CSF and 64 plasma abacavir measurements available for analysis. No attempts to standardize the dose to sample collection time were made. This resulted in samples that were obtained throughout the dosing interval. Most samples were collected in pairs (CSF and plasma) and obtained within 1 h of each other during a single visit. The average number of CSF samples/patient was 1.2 (range, 1 to 4). Abacavir plasma and CSF levels were measured by use of validated high-performance liquid chromatography assays for each matrix. The assay limit of detection was 40 ng/ml for plasma and 8.3 ng/ml for CSF, and the intra-assay and interassay variabilities were <12% for both matrices.
Population pharmacokinetic (PPK) parameters were estimated by use of NONMEM software (version V.1) using the FOCE (first-order conditional estimation) subroutine with interaction. A two-compartment physiologic model with first-order absorption and elimination was used to fit the data (9, 13, 15). A “bioavailability fraction” corresponding to the AUCCSF/AUCplasma ratio was estimated for the population. Thirteen plasma concentrations were below the assay limit of detection (20 ng/ml) and were set to half the assay limit value and given an additional residual error equal to their assigned value. All CSF samples had measurable abacavir concentrations. The data were well described by the pharmacokinetic model, with no significant biases, and the estimated systemic pharmacokinetic parameters were similar to those previously published (1). The calculated residual model errors were 49% for concentrations in plasma and 36% for CSF.
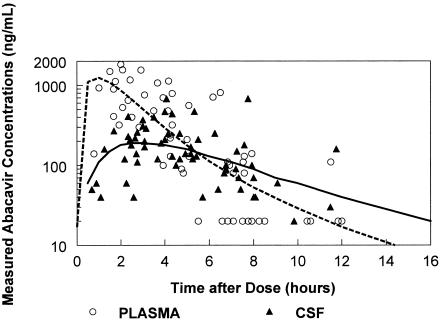
The median abacavir concentrations were 139 ng/ml (range, <40 to 1,130) in plasma and 128 ng/ml (range, 37 to 384) in CSF. PPK modeling (Fig. 1) estimated the maximum CSF abacavir concentration to be 215 ng/ml. The CSF curve was flatter than the plasma curve, likely due to slow influx and efflux of abacavir from the CSF compartment. The estimated half-life in CSF was 2.5 h and was significantly longer than the half-life seen for the drug in plasma. Table 1 summarizes the pharmacokinetic parameters and intersubject variability for the model. The fraction of abacavir that penetrated into the CSF from plasma was 36% (95% confidence interval, 0.28 to 0.46), similar to that previously reported with intensive sampling with three subjects (0.35) (8). The PPK model predicted CSF and plasma troughs of 46 and 17 ng/ml, exceeding the 50% inhibitory concentration of 70 ng/ml for 85 and 60% of the dose interval, respectively. The median abacavir CSF/plasma ratio increased over the dosing interval. For paired samples, the abacavir CSF/plasma ratio correlated with sample collection time postdose (r2 = 0.36; P < 0.001). The median CSF/plasma ratio for samples collected during the first half of the dose interval was one-third of that collected during the second half of the dose interval.
FIG. 1.
Abacavir CSF and plasma concentrations.
TABLE 1.
Pharmacokinetic parameters and intersubject variability
| Pharmacokinetic parametera | Estimated mean pharmacokinetic value and SE | Intersubject variability (%) |
|---|---|---|
| Vol of distribution (liters) | 135 + 32 | 43 |
| AUCCSF/AUCplasma ratio (%) | 36 + 5 | |
| KPlasma-to-CSF (h−1) | 0.075 ± 0.036 | |
| KCSF-to-plasma (h−1) | 0.28 ± 0.06 | |
| Plasma half-life (h) | 1.2 ± 0.2 | 50 |
| CSF half-life (h) | 2.5 ± 0.6 |
KPlasma-to-CSF, rate constant from plasma into the CSF; KCSF-to-plasma, rate constant out of the CSF to plasma.
Optimal antiretroviral therapy requires achieving adequate drug concentrations in all sites where viral replication occurs, including the CNS. Initial studies indicated that abacavir reduces CSF viral load, suggesting significant CNS penetration (B. J. Brew, S. J. Brown, J. Catalan, N. Sacktor, M. Halman, W. T. Symonds, C. Romero, and CNAB 3001 Study Team, Abstr. 12th World AIDS Conf., abstr. 32192, 1998). We found that a sparse sample collection approach could be used to construct a population pharmacokinetic model of abacavir distribution in CSF. This model demonstrated different CSF and plasma abacavir concentration profile shapes, and thus, the CSF/plasma ratio from paired samples is a function of collection time. The estimated CSF AUCCSF/AUCplasma ratio avoids the influence of collection time on isolated CSF/plasma ratios. van Praag et al. found that average CSF abacavir concentrations collected 1 h postdose were 75 ng/ml, about 5% of peak abacavir plasma concentrations, and less than the typical abacavir CSF concentrations in the current study (14). This suggests that the early sampling may have missed the peak CSF abacavir concentration. Although abacavir demonstrated greater CSF penetration than most other antiretrovirals, its CSF penetration is less than that of its free fraction, suggesting active transport out of the CNS. The slow CSF distribution results in prolonged drug exposure above the 50% inhibitory concentration compared to the plasma compartment. While intracellular carbovir triphosphate, the active moiety of abacavir, was not measured and the study was not designed to assess viral replication in the CNS, these results suggest that abacavir penetration may be sufficient to reduce viral replication in sanctuary sites such as the central nervous system.
Acknowledgments
S. L. Letendre received salary support from a grant from the National Institute of Mental Health (K23-MH-01779). The HIV Neurobehavioral Research Center is funded by the National Institute of Mental Health (P30-MH-62512) and by a grant from the National Institute of Mental Health (RO1-MH-58076).
The San Diego HIV Neurobehavioral Research Center group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the San Diego Veterans Affairs Healthcare System and includes Igor Grant (director); J. Hampton Atkinson and J. Allen McCutchan (co-directors); Thomas D. Marcotte (center manager); (Naval Hospital San Diego) Mark R. Wallace (principal investigator [P.I.]); (Neuromedical Component) J. Allen McCutchan (P.I.), Mariana Cherner, Joseph Sadek, and Steven Paul Woods; (Imaging Component) Terry Jernigan (P.I.), John Hesselink, and Michael J. Taylor; (Neuropathology Component) Eliezer Masliah (P.I.), Ian Everall, and Dianne Langford; (Clinical Trials Component) J. Allen McCutchan, J. Hampton Atkinson, Ronald J. Ellis, and Scott Letendre; (Data Systems Management Unit) Daniel R. Masys (P.I.) and Michelle Frybarger (Data Systems Manager); (Statistics Unit) Ian Abramson (P.I.), Deborah Lazzaretto, and Tanya Wolfson.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
REFERENCES
- 1.Beal, S. L., and L. B. Sheiner (ed.). 1992. NONMEM user guides. NONMEM Project Group, University of California, San Francisco.
- 2.Cherubin, C. E., R. H. Eng, R. Norrby, J. Modai, G. Humbert, and G. Overturf. 1989. Penetration of newer cephalosporins into cerebrospinal fluid. Rev. Infect. Dis. 11:526-548. [DOI] [PubMed] [Google Scholar]
- 3.Daluge, S. M., S. S. Good, M. B. Faletto, W. H. Miller, M. H. St. Clair, L. R. Boone, M. Tisdale, N. R. Parry, J. E. Reardon, R. E. Dornsife, D. R. Averett, and T. A. Krenitsky. 1997. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob. Agents Chemother. 41:1082-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis, R. J., A. C. Gamst, E. Capparelli, S. A. Spector, K. Hsia, T. Wolfson, I. Abramson, I. Grant, and J. A. McCutchan. 2000. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology 54:927-936. [DOI] [PubMed] [Google Scholar]
- 5.Foudraine, N. A., R. M. Hoetelmans, J. M. Lange, F. de Wolf, B. H. van Benthem, J. J. Maas, I. P. Keet, and P. Portegies. 1998. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet 351:1547-1551. [DOI] [PubMed] [Google Scholar]
- 6.Haas, D. W., B. W. Johnson, P. Spearman, S. Raffanti, J. Nicotera, D. Schmidt, T. Hulgan, R. Shepard, and S. A. Fiscus. 2003. Two phases of HIV RNA decay in CSF during initial days of multidrug therapy. Neurology 61:1391-1396. [DOI] [PubMed] [Google Scholar]
- 7.Lutsar, I., G. H. McCracken, Jr., and I. R. Friedland. 1998. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 27:1117-1129. [DOI] [PubMed] [Google Scholar]
- 8.McDowell, J. A., G. E. Chittick, J. R. Ravitch, R. E. Polk, T. M. Kerkering, D. S. Stein, N. A. Foudraine, R. M. Hoetelmans, J. M. Lange, F. de Wolf, B. H. van Benthem, J. J. Maas, I. P. Keet, P. Portegies, D. M. Burger, C. L. Kraaijeveld, P. L. Meenhorst, J. W. Mulder, C. H. Koks, A. Bult, and J. H. Beijnen. 1999. Pharmacokinetics of [14C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob. Agents Chemother. 43:2855-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDowell, J. A., Y. Lou, W. S. Symonds, and D. S. Stein. 2000. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 44:2061-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nau, R., G. Zysk, A. Thiel, and H. W. Prange. 1993. Pharmacokinetic quantification of the exchange of drugs between blood and cerebrospinal fluid in man. Eur. J. Clin. Pharmacol. 45:469-475. [DOI] [PubMed] [Google Scholar]
- 11.Reddy, Y. S., A. Kashuba, J. Gerber, and V. Miller. 2003. Roundtable report: importance of antiretroviral drug concentrations in sanctuary sites and viral reservoirs. AIDS Res. Hum. Retrovir. 19:167-176. [DOI] [PubMed] [Google Scholar]
- 12.Simpson, D. M. 1999. Human immunodeficiency virus-associated dementia: review of pathogenesis, prophylaxis, and treatment studies of zidovudine therapy. Clin. Infect. Dis. 29:19-34. [DOI] [PubMed] [Google Scholar]
- 13.van Praag, R. M., R. P. van Heeswijk, S. Jurriaans, J. M. Lange, R. M. Hoetelmans, and J. M. Prins. 2001. Penetration of the nucleoside analogue abacavir into the genital tract of men infected with human immunodeficiency virus type 1. Clin. Infect. Dis. 33:e91-e92. [DOI] [PubMed] [Google Scholar]
- 14.van Praag, R. M., E. C. van Weert, R. P. van Heeswijk, X. J. Zhou, J. P. Sommadossi, S. Jurriaans, J. M. Lange, R. M. Hoetelmans, and J. M. Prins. 2002. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob. Agents Chemother. 46:896-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller, S., K. M. Radomski, Y. Lou, and D. S. Stein. 2000. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 44:2052-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]