To the Editor: Immune checkpoint inhibitors (ICIs), anticancer agents that enhance antitumor response, can cause autoimmune toxicities, including ICI-associated acute kidney injury (ICI-AKI). The most common histopathologic lesion in patients with ICI-AKI is acute tubulointerstitial nephritis (ATIN); however, a definitive diagnosis of ATIN requires a kidney biopsy (1). This represents a frequently encountered clinical challenge for providers, as AKI is very common among cancer patients, many of whom have contraindications to kidney biopsy (e.g., solitary kidney, therapeutic anticoagulation). Accordingly, noninvasive methods of diagnosing ICI-AKI are urgently needed, as treatment involves glucocorticoids and discontinuation of potentially life-saving immunotherapy.
Case reports and one case series explored the utility of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography–computed tomography (F18-FDG PET-CT) for diagnosing ICI-AKI and reported mixed findings (2, 3); however, these studies did not have clear inclusion and exclusion criteria to carefully phenotype the patients, did not use rigorous techniques to minimize sampling error, and, most importantly, in some cases did not include a control group. We sought to address these key knowledge gaps and define the role of F18-FDG PET-CT in diagnosing ICI-AKI.
We used data from a retrospective, multicenter cohort study of 429 patients with ICI-AKI treated at 30 sites across 10 countries (1). Patients were diagnosed with ICI-AKI between 2012 and 2023 and had either biopsy-proven or clinically adjudicated ICI-AKI (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI182275DS1), specifically ICI-ATIN.
We also assembled two control groups, each consisting of patients with cancer treated at Mass General Brigham (MGB). The first comprised patients with AKI from non-ICI etiologies, and the second comprised patients treated with ICIs who did not have AKI at the time of a follow-up F18-FDG PET-CT.
For all three groups, patients were included if they had F18-FDG PET-CT scans at baseline and within 14 days of AKI onset (or, for the second control group, a follow-up scan between 90 and 365 days following ICI initiation). Patients were excluded from all three groups if they had genitourinary cancer, lymphomatous infiltration of the kidneys, or received 7 or more days of glucocorticoids prior to the follow-up scan.
Radiologists at each site reviewed the F18-FDG PET-CTs. They were unaware of group assignment at the time of review. Five 0.5 cm diameter regions of interest (ROIs) were drawn in the cortex of each kidney, avoiding the collecting system and space-occupying lesions, such as cysts. The ROIs were selected to represent each kidney’s upper, mid, and lower poles. The mean standardized uptake value (SUVmean) for each ROI was recorded.
Fifty-three patients were included (9 with ICI-AKI, 24 with AKI from non-ICI causes, and 20 ICI-treated without AKI; Supplemental Figure 1). Baseline characteristics were largely similar among the three groups (Supplemental Table 2), as were F18-FDG PET-CT scan technical parameters (Supplemental Table 3).
Detailed characteristics of the 9 ICI-AKI patients are shown in Supplemental Table 4. Three had biopsy-proven ATIN, whereas the remaining 6 had clinically adjudicated ICI-ATIN. All had clinical features supporting a diagnosis of ATIN (Supplemental Table 5). Those with AKI from non-ICI causes had prerenal AKI (n = 10), ischemic or septic acute tubular necrosis (n = 10), or other AKI etiologies (n = 4) (Supplemental Table 6).
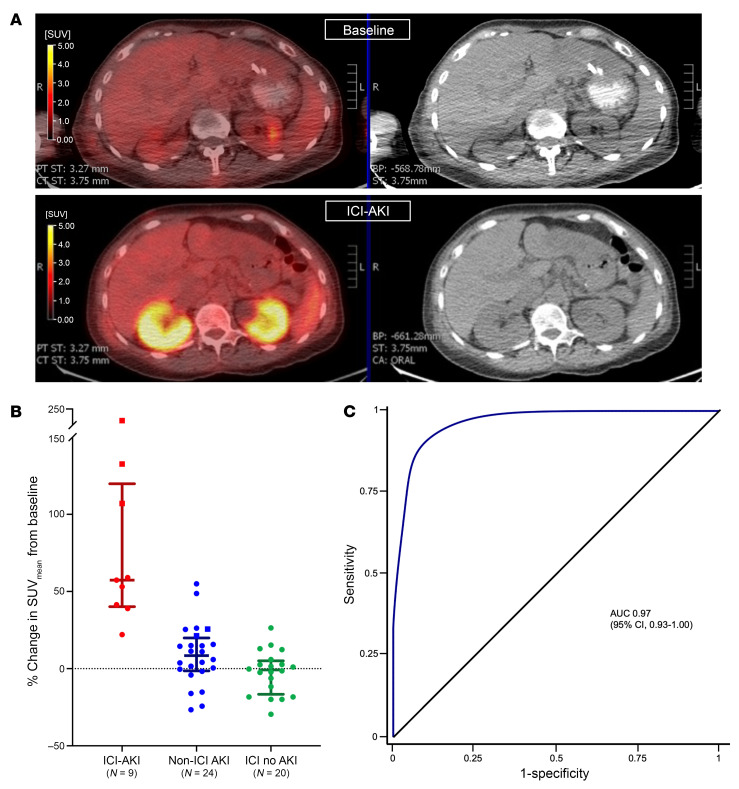
Representative images from baseline and follow-up F18-FDG PET-CTs from an ICI-AKI patient (no. 1) are shown in Figure 1A. Among those with ICI-AKI, the SUVmean increased by a median of 57.4% (IQR, 40.3 to 119.8) from baseline to follow-up. In contrast, it increased by 8.5% (IQR, 1.4 to 19.9) among patients with AKI from non-ICI causes and decreased by 0.8% (IQR, -16.6 to 5.1) among patients receiving ICIs without AKI (P < 0.001; Figure 1B). The increase in SUVmean in patients with ICI-AKI was also greater compared with that of patients with AKI from non-ICI causes when stratified by AKI etiology (Supplemental Figure 2). The AUC for the differentiation of ICI-AKI from the two control groups according to percentage change in SUVmean was 0.97 (95% CI, 0.93 to 1.00) (Figure 1C). In a sensitivity analysis (described in the Supplemental Methods), the AUC was unchanged at 0.97 (95% CI, 0.92 to 1.00).
Figure 1. F18-FDG PET-CT and ICI-AKI.
(A) Representative F18-FDG PET-CT images at baseline (top panels) and at the time of ICI-AKI (lower panels). (B) Percentage change in SUVmean from baseline to the time of AKI among patients with ICI-AKI (red), AKI from other causes (blue), and patients receiving ICI therapy without AKI (green). Biopsy-proven patients are represented by squares, and clinically adjudicated patients with circles. (C) ROC curve of percentage change in SUVmean for differentiation of ICI-AKI from AKI from other causes.
In the ICI-AKI cohort, there was little intraindividual variability in the ROIs at each time point (Supplemental Figure 3), though overall precision improved monotonically with a greater number of ROIs (Supplemental Figure 4).
We found that patients with ICI-AKI had a considerable increase in SUVmean on F18-FDG PET-CT from baseline to the time of AKI compared with two groups of control patients. These findings suggest that, when a baseline F18-FDG PET-CT is available, these scans have diagnostic utility in differentiating ICI-AKI from AKI caused by other etiologies and could offer a noninvasive alternative to kidney biopsy.
Though predominantly used for cancer staging and assessing treatment response, F18-FDG PET-CTs have also been used to examine autoimmune toxicity resulting from ICIs. Patients with suspected ICI-associated colitis had increased radiotracer uptake in the colon, whereas uptake decreased with treatment with glucocorticoids (4). Another study found that patients with positive F18-FDG PET-CTs of the thyroid were more likely to develop ICI-associated hypothyroidism (5).
Fewer data are available on the role of F18-FDG PET-CT imaging for ICI-AKI (2, 3). A single-center study examined F18-FDG PET-CT scans in 14 patients with ICI-AKI and reported an increase in FDG activity in the renal parenchyma and a decrease in the collecting system (2). However, the study did not exclude patients with genitourinary cancer or those who had received prolonged courses of glucocorticoids prior to the follow-up F18-FDG PET-CT scan, nor did the authors compare their findings with controls without ICI-AKI. Further, only a single ROI in the renal cortex was obtained in each patient, which could have resulted in sampling error.
In our study, we compared changes in FDG uptake from baseline to the time of AKI among patients with and without ICI-AKI while also incorporating rigorous inclusion and exclusion criteria. We acknowledge as a limitation that not all patients had biopsy-proven ICI-AKI; however, this reflects clinical practice, where a diagnosis is often made based on established risk factors, clinical features, and an absence of alternative etiologies (1).
In summary, we found that F18-FDG PET-CT may be a useful adjunctive test for diagnosing ICI-AKI in patients with baseline imaging available. Larger prospective studies are needed to validate these findings.
Supplementary Material
Version 1. 08/08/2024
In-Press Preview
Version 2. 09/17/2024
Electronic publication
Funding Statement
This study is not funded by any entity.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2024, Gupta et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2024;134(18):e182275. https://doi.org/10.1172/JCI182275.
Contributor Information
Shruti Gupta, Email: sgupta21@bwh.harvard.edu.
Olivia Green-Lingren, Email: ogreen-lingren@mgb.org.
Sudhir Bhimaniya, Email: sbhimaniya@bwh.harvard.edu.
Aleksandra Krokhmal, Email: akrokhmal@mgb.org.
Heather Jacene, Email: hjacene@bwh.harvard.edu.
Marlies Ostermann, Email: Marlies.Ostermann@gstt.nhs.uk.
Sugama Chicklore, Email: sugama.chicklore@gstt.nhs.uk.
Ben Sprangers, Email: ben.sprangers@zol.be.
Christophe M. Deroose, Email: christophe.deroose@uzleuven.be.
Sandra M. Herrmann, Email: Herrmann.Sandra@mayo.edu.
Sophia L. Wells, Email: swells8@bwh.harvard.edu.
Sarah A. Kaunfer, Email: skaunfer@bwh.harvard.edu.
Jessica L. Ortega, Email: jlortega@bwh.harvard.edu.
Clara García-Carro, Email: cgcarro@salud.madrid.org.
Michael Bold, Email: bold.michael@mayo.edu.
Kevin L. Chen, Email: kevinleechen@g.harvard.edu.
Meghan E. Sise, Email: msise@partners.org.
Pedram Heidari, Email: heidari.pedram@mgh.harvard.edu.
Wai Lun Will Pak, Email: pakw@mskcc.org.
Meghan D. Lee, Email: mlee@mgh.harvard.edu.
Pazit Beckerman, Email: pazit.beckerman@sheba.health.gov.il.
Yael Eshet, Email: yael.eshet@sheba.health.gov.il.
Raymond K. Hsu, Email: raymond.hsu@ucsf.edu.
Arash Rashidi, Email: arash.rashidi@uhhospitals.org.
Norbert Avril, Email: norbert.avril@uhhospitals.org.
Vicki Donley, Email: vicki.donley@uhhospitals.org.
Zain Mithani, Email: zmithani@med.miami.edu.
Russ Kuker, Email: rkuker2@med.miami.edu.
Muhammad O Awiwi, Email: mohammad_owiwi@hotmail.com.
Mindy X. Wang, Email: mwang12@mdanderson.org.
Sujal I. Shah, Email: sishah@bwh.harvard.edu.
Michael D. Weintraub, Email: mweintraub@bwh.harvard.edu.
Heiko Schoder, Email: schoderh@mskcc.org.
Raad B. Chowdhury, Email: rbchowdhury@bwh.harvard.edu.
Harish Seethapathy, Email: hseethapathy@mgh.harvard.edu.
Kerry L. Reynolds, Email: kreynolds7@mgb.org.
Maria Jose Soler, Email: mjsoler01@gmail.com.
Ala Abudayyeh, Email: aabudayyeh@mdanderson.org.
Ilya Glezerman, Email: glezermi@mskcc.org.
David E. Leaf, Email: deleaf@bwh.harvard.edu.
References
- 1.Gupta S, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(10):e003467. doi: 10.1136/jitc-2021-003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awiwi MO, et al. Imaging features of immune checkpoint inhibitor-related nephritis with clinical correlation: a retrospective series of biopsy-proven cases. Eur Radiol. 2023;33(3):2227–2238. doi: 10.1007/s00330-022-09158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qualls D, et al. Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J Immunother Cancer. 2019;7(1):356. doi: 10.1186/s40425-019-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang N, et al. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy. 2019;11(8):667–676. doi: 10.2217/imt-2018-0146. [DOI] [PubMed] [Google Scholar]
- 5.Galligan A, et al. Increased thyroidal activity on routine FDG-PET/CT after combination immune checkpoint inhibition: temporal associations with clinical and biochemical thyroiditis. Cancers (Basel) 2023;15(24):5803. doi: 10.3390/cancers15245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.