Abstract
LBM415 activity against extracellular and intracellular Legionella pneumophila was studied. The LBM415 MIC50 for 20 Legionella sp. strains was 4 μg/ml, versus 0.06, 0.25, and ≤ 0.03 μg/ml for azithromycin, erythromycin, and levofloxacin, respectively. LBM415 (0.5 and 16 μg/ml) reversibly prevented intracellular growth of two L. pneumophila strains and was less active than erythromycin.
LBM415 (formerly NVP PDF713; Novartis Pharmaceuticals) is a novel peptide deformylase inhibitor antimicrobial agent with excellent activity against many gram-positive cocci but previously unknown activity against Legionella bacteria, the causative agents of Legionnaires' disease (7). This study examined the activity of LBM415 against extracellular and intracellular Legionella bacteria.
Bacterial strains and growth conditions.
A total of 20 different clinical isolates of five different Legionella sp. bacterial strains were used to determine the in vitro activity of the study compounds. The species tested included Legionella bozemanii (1 isolate), L. dumoffii (2 isolates), L. longbeachae (2 isolates), L. micdadei (2 isolates), and L. pneumophila (13 isolates). Staphylococcus aureus ATCC 29213 was used as the control organism for susceptibility testing. Legionellae were grown on MOPS (morpholinepropanesulfonic acid)-buffered charcoal yeast extract medium supplemented with 0.1% α-ketoglutarate (BCYEα) (3) or in n-(2-acetamido)-2-aminoethanesulfonic acid-buffered yeast extract broth supplemented with 0.1% α-ketoglutarate (BYEα), and non-legionellae were grown on commercial tryptic soy agar containing 5% sheep blood. Incubation of all media was at 35°C in humidified air for 24 to 48 h, depending on organism and growth rate. Broth media were shaken (150 rpm) during growth.
Antimicrobials.
LBM415 powder (Novartis Pharmaceuticals) was dissolved in ethanol (2 mg/ml) and then further diluted in M199 tissue culture medium, Mueller-Hinton broth, or BYEα broth as needed. Erythromycin powder (Sigma Chemicals) was dissolved in 95% ethanol and then diluted in tissue culture medium or bacterial culture broths. Lyophilized azithromycin for injection (Pfizer), which contains no preservatives, was reconstituted (100 mg/ml) with sterile water for injection (USP), aliquoted, and frozen at −20°C. Frozen azithromycin stock solution retains its expected biological activity for a several-month period (5). Further dilutions were made in the appropriate culture medium. Levofloxacin premixed IV solution (Ortho-McNeil) (5 mg/ml) was diluted as needed in the appropriate media.
Antimicrobial susceptibility testing.
Broth microdilution susceptibility testing was performed using BYEα (Legionella bacteria) or Mueller-Hinton broth (non-Legionella bacteria), with a final volume of 100 μl and final bacterial concentration of 5 × 105 CFU/ml (4). The Legionella and non-Legionella bacteria were incubated for 48 and 24 h, respectively, both at 35°C.
Growth inhibition in alveolar macrophages.
Guinea pig pulmonary alveolar macrophages were harvested and purified as described previously (1). The final concentration of macrophages was approximately 5 × 105 cells per well. Incubation conditions for all macrophage studies were 5% CO2 in air at 37°C.
Legionella pneumophila strains F889 and F2111 grown to early log phase in BYEα broth were used to infect the macrophages. Approximately 104 bacteria were added to each well. Bacteria were incubated with macrophages for 1 h in a shaking incubator and then for 1 day in stationary culture (1). One set of replicate wells was washed (500 μl) thrice with tissue culture medium and then sonicated at low energy to release intracellular bacteria, which were quantified using BCYEα agar. Antimicrobials were then added to the washed nonsonicated wells; several wells had no antimicrobial added to serve as growth controls. The infected tissue cultures were incubated for 2 days, after which supernatant samples were taken for quantitative culturing. For some experiments, the antimicrobials were then removed by washing, and the experiment continued for 4 more days, with daily quantification of L. pneumophila in well supernatants. All experiments were carried out in duplicate or triplicate, and quantitative plating was done in duplicate. All wells were observed microscopically daily to detect macrophage infection and to roughly quantify numbers of macrophages in the wells. To exclude LBM415 macrophage toxicity, control wells were set up that contained macrophages, tissue culture medium, and antimicrobial agents but no bacteria. The presence of macrophage toxicity was determined by microscopic observation of the cells. Prior studies have demonstrated no macrophage toxicity caused by levofloxacin, erythromycin, or azithromycin (2, 4). In this system there is no extracellular growth of L. pneumophila, so all increases in supernatant bacterial concentration are the result of intracellular growth (6).
Drug assay.
LBM415 concentrations were measured in tissue culture medium M199 plus 20% fetal calf serum, seeded with LBM415 (4 μg/ml), and incubated for up to 48 h (37°C, 5% CO2). The tissue culture medium containing drug was frozen at −70°C until analyzed by liquid chromatography/mass spectroscopy/mass spectroscopy at Novartis Pharmaceuticals. This analytical method had a linear range (r2 = 0.995) of 10 to 5,000 ng/ml and an accuracy of 97% when measuring 5,000 ng/ml and detects only nondegraded LBM415.
Broth dilution susceptibility results.
The LBM415 MIC50, MIC90 and range for the Legionella spp. tested were 8, 16, and 1 to 16 μg/ml; L. pneumophila strains F889 and F2111 were each inhibited by 8 μg/ml. The respective erythromycin MICs were 0.25, 0.50, and 0.125 to 0.5 μg/ml; strains F889 and F2111 were inhibited by 0.125 and 0.25 μg/ml, respectively. Azithromycin MICs were 0.06, 0.25, and 0.042 to 0.5 μg/ml; F889 and F2111 were inhibited by 0.06 and 0.5 μg/ml, respectively. Levofloxacin MICs were ≤0.03 μg/ml for all strains tested. The highest LBM415 MICs were observed for L. pneumophila strains (geometric mean, 9.1 μg/ml versus 3.4 μg/ml for non-L. pneumophila strains). In contrast, erythromycin and azithromycin were about 1.5 to 2 times more active against L. pneumophila than against the non-L. pneumophila strains tested. The LBM415 MIC for S. aureus tested in Mueller-Hinton broth was half of the MIC obtained using BYEα broth, indicating minimal drug inactivation by the test medium. Similar findings were made for erythromycin and levofloxacin. Azithromycin was the most inactivated by BYEα broth, with MICs for S. aureus being twofold to fourfold higher when using BYEα broth than when using Mueller-Hinton broth.
Antimicrobial inhibition of intracellular growth.
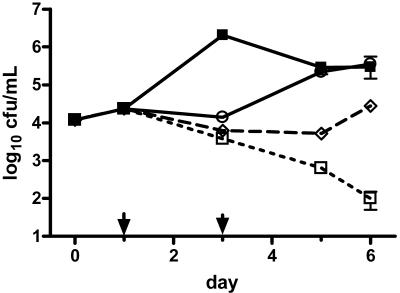
LBM415 inhibited the intracellular growth of L. pneumophila, but this inhibition was reversed after drug removal from the extracellular tissue culture medium (Fig. 1). The extracellular LBM415 concentrations that prevented intracellular growth were 0.25 to 0.5 and 8 to 16 μg/ml for strains F889 and F2111, respectively (Table 1). LBM415 extracellular concentrations greater than those inhibiting extracellular growth had no additional effect on intracellular bacterial concentration. Erythromycin, azithromycin, and levofloxacin were all more active against intracellular L. pneumophila than was LBM415, either in terms of growth inhibition or persistence of effect after drug removal (Fig. 1 and Table 1). Azithromycin and levofloxacin (both 1 μg/ml) were bactericidal and possessed a long-term postantibiotic effect (Fig. 1 for azithromycin, data not shown for levofloxacin). Erythromycin intracellular activity was solely inhibitory.
FIG. 1.
Growth of L. pneumophila serogroup 1 strain F889 in guinea pig alveolar macrophages versus day of incubation after initiation of infection. Drugs were added on day 1 and removed by washing on day 3, as designated by arrows. All points represent the mean of triplicate wells each plated in duplicate; error bars represent standard errors of the means, which unless shown were smaller than the height of the symbol representing the mean. ▪, no drug control; ○, LBM415 at 8 μg/ml; □, azithromycin at 1 μg/ml; ⋄, erythromycin at 1 μg/ml. Data for LBM415 (2 and 4 μg/ml) are not shown for greater clarity, as their effect was nearly identical to that of LBM415 (8 μg/ml).
TABLE 1.
Effect of LBM415 on L. pneumophila multiplication in guinea pig alveolar macrophages
| Strain or drug and concn (μg/ml) | Log10 (d3/d1ctl) CFU/mla
|
|||
|---|---|---|---|---|
| F889
|
F2111
|
|||
| expt 1 | expt 2 | expt 2 | expt 3 | |
| Control | 0.3 | 1.5 | 2.5 | 2.5 |
| LBM415 | ||||
| 0.125 | 0.7 | NDb | ND | ND |
| 0.25 | 0.2 | −0.1 | 2.5 | ND |
| 0.50 | ND | −0.4 | 2.4 | ND |
| 1 | ND | −0.4 | 2.4 | ND |
| 2 | ND | −0.4 | 2.5 | ND |
| 4 | ND | ND | 2.0 | ND |
| 8 | ND | ND | 1.2 | 0.6 |
| 16 | ND | ND | ND | −0.1 |
| 32 | ND | ND | ND | −0.2 |
| Levofloxacin | ||||
| 1 | ND | −1.5 | −2.4 | −2.7 |
Log10 of ratio of experimental condition at day 3 divided by bacterial concentration at day 1, before drug addition. A positive value denotes growth and a negative value growth inhibition or killing. Mean values of triplicate determinations are shown.
ND, not done.
Both a bioassay and chemical assay of LBM415 in tissue culture medium showed retained activity for up to 48 h. The LBM415 MICs (24-h incubation) of S. aureus were identical when using either Mueller-Hinton broth or tissue culture medium M199 with 20% fetal calf serum. A chemical assay of LBM415 concentration showed no significant changes in drug concentration (4 μg/ml) when the drug was incubated in M199-20% fetal calf serum (37°C, 5% CO2) for up to 48 h. Measured mean drug concentrations at 0, 5, and 48 h were 4.0 (95% confidence interval = 3.4 to 4.5), 3.9 (3.7 to 4.1), and 3.8 (3.5 to 4.0) μg/ml, respectively (P > 0.2 for comparison of 0- and 48-h concentrations, unpaired Student t test; n = 4 in each group).
Macrophages incubated with the highest LBM415 concentrations tested had no evidence of toxicity based on microscopic observations.
Because LBM415 was dissolved in ethanol, the intracellular activity of ethanol versus L. pneumophila was measured. The concentration of ethanol (0.5%) present in the wells containing LBM415 (8 μg/ml) had no effect on intracellular L. pneumophila growth (data not shown). However, a higher ethanol concentration (2%), the amount present in wells containing LBM415 (32 μg/ml), reduced intracellular L. pneumophila concentrations by 0.4 log10 CFU/ml in comparison to control wells (P = 0.007, nonpaired Student t test); in contrast, LBM415 (32 μg/ml) reduced bacterial concentrations by log10 3.5 CFU/ml in comparison to the no-drug controls.
These data indicate that LBM415 reversibly inhibits, but does not kill, intracellular L. pneumophila. The marked strain difference detected in intracellular susceptibility to LBM415 was not detected by extracellular MIC studies. Whether LBM415 will be potentially effective for the treatment of Legionnaires' disease will be unknown until more studies can be performed against a broad range of intracellular Legionella bacteria and complemented by experimental animal studies. Because of its easily reversible and solely inhibitory activity, LBM415 will be unlikely to be a treatment of choice for Legionnaires' disease, although it could possibly halt disease progression, depending on individual infecting strain susceptibility and drug pharmacokinetics.
Acknowledgments
The work was supported by a grant from Novartis Pharmaceuticals.
Timothy Bedman performed the drug assay, and Jason Ho provided technical assistance.
REFERENCES
- 1.Edelstein, P. H., and M. A. C. Edelstein. 1989. WIN 57273 is bactericidal for Legionella pneumophila grown in alveolar macrophages. Antimicrob. Agents Chemother. 33:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelstein, P. H., and M. A. C. Edelstein. 1991. In vitro activity of azithromycin against clinical isolates of Legionella species. Antimicrob. Agents Chemother. 35:180-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelstein, P. H., and M. A. C. Edelstein. 1993. Comparison of three buffers used in the formulation of buffered charcoal yeast extract medium. J. Clin. Microbiol. 31:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelstein, P. H., M. A. C. Edelstein, K. H. Lehr, and J. Ren. 1996. In-vitro activity of levofloxacin against clinical isolates of Legionella spp., its pharmacokinetics in guinea pigs, and use in experimental Legionella pneumophila pneumonia. J. Antimicrob. Chemother. 37:117-126. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein, P. H., W. J. Weiss, and M. A. Edelstein. 2003. Activities of tigecycline (GAR-936) against Legionella pneumophila in vitro and in guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 47:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Investig. 66:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, R. N., G. J. Moet, H. S. Sader, and T. R. Fritsche. 2004. Potential utility of a peptide deformylase inhibitor (NVP PDF-713) against oxazolidinone-resistant or streptogramin-resistant gram-positive organism isolates. J. Antimicrob. Chemother. 53:804-807. [DOI] [PubMed] [Google Scholar]