Abstract
The ocular disposition of levofloxacin in patients receiving two 500-mg oral doses 10 h apart before cataract surgery was assessed with the intent of defining drug ocular exposure over time. The mean aqueous humor concentrations persisted above 1.5 mg/liter between 1.5 and 6.0 h after the second dose, with average aqueous-to-plasma ratios ranging between 0.33 and 0.57. This favorable ocular disposition provides support for trials of systemic levofloxacin for prophylaxis of postoperative endophthalmitis in selected patients or as adjunctive therapy for the treatment of this potentially devastating infective complication.
Endophthalmitis represents a rare but dramatic major infective complication occurring after elective cataract surgery. In a number of large-scale international studies, the incidence of postoperative endophthalmitis was estimated between 0.072 and 0.10% (9, 11, 21, 22, 44), but in other studies even higher rates were documented (13, 36, 39). Among the various bacterial etiological agents, Staphylococcus epidermidis and other coagulase-negative staphylococci predominate (1, 4, 16, 24, 41), whereas other gram-positive or gram-negative microorganisms are less commonly involved. Although the primary sources of infection are the patient's own eyelids and/or conjunctiva (4, 40) and the frequency of preoperative bacterial colonization in these sites may be high (5, 43), currently only the use of preoperative povidone-iodine antisepsis has a recognized role for bacterial endophthalmitis prophylaxis (6). On the contrary, the role of antimicrobial agents in cataract surgery is still a matter of continuous debate, since to date no sufficiently powerful study has been carried out (28). In spite of this, antimicrobial prophylaxis may be considered beneficial in cataract surgery (26, 27, 33). Accordingly, in a survey of ophthalmologists performing cataract surgery in 2000 in the United States, 79% reported use of a preoperative topical antibiotic (25).
Fluoroquinolones should be considered among the most interesting agents for antimicrobial prophylaxis in cataract surgery, since they have a comprehensive spectrum of activity covering most of the ocular pathogens and possess an excellent pharmacokinetic profile enabling penetration through several anatomical barriers (35). As far as ocular penetration of fluoroquinolones is concerned, levofloxacin was shown to present appropriate diffusion in aqueous humor after either topical and/or oral administration (3, 12, 14, 23, 45, 46), achieving concentrations higher than ciprofloxacin (8, 15, 30) or ofloxacin (10, 17, 19). Since no study appropriately addressed the issue of ocular disposition of levofloxacin after systemic administration with the intent of defining drug ocular exposure over time, ocular disposition and exposure to levofloxacin after oral administration was assessed in patients undergoing cataract surgery with the intent of defining how long throughout the interventional period optimal exposure in aqueous humor against potential contaminating pathogens may be ensured.
The study involved 101 patients (32 males and 69 females) who received two 500-mg oral doses of levofloxacin 10 hours apart before undergoing cataract surgery (9:00 the previous evening and 7:00 in the morning of surgical intervention). Median values (ranges) for the 101 patients follow: age, 76 years (45 to 90); weight, 70 kg (45 to 110); serum creatinine, 0.91 mg/dl (0.44 to 1.50); and estimated creatinine clearance, 0.93 ml/min/kg of body weight (0.40 to 1.68) (7). The study was approved by an internal review board, and informed consent was obtained from each patient. Exclusion criteria were as follows: serum creatinine of >1.5 mg/dl, age of <18 years, and treatment with antacids and/or sucralfate. In order to ensure maximum intraocular exposure throughout the vulnerable period, surgical intervention was performed in an interval ranging between 1.5 to 6.0 h after the second oral administration of levofloxacin. Single aqueous humor and blood samples for quantification of levofloxacin were simultaneously collected from each of the subjects, who were randomly assigned to one of the 30-min intervals after the second oral dose of levofloxacin (1.5 to 2.0, 2.0 to 2.5, 2.5 to 3.0, 3.0 to 3.5, 3.5 to 4.0, 4.0 to 4.5, 4.5 to 5.0, 5.0 to 5.5, and 5.5 to 6.0 h) according to the scheduled operation time. Approximately 100 μl of aqueous fluid was aspirated by paracentesis with a 26-gauge needle. Levofloxacin in plasma and in aqueous humor was quantified by means of a validated high-performance liquid chromatography method, as previously described (34). Data are expressed as means ± standard deviations (SD) and/or medians and ranges.
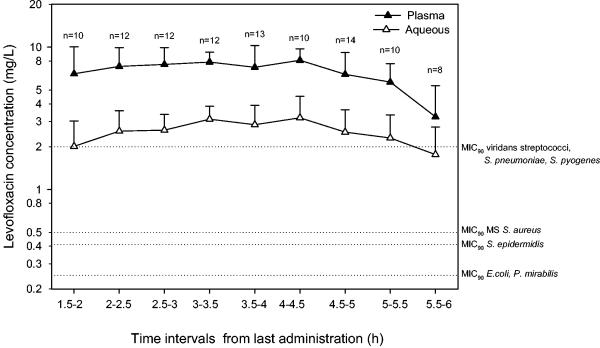
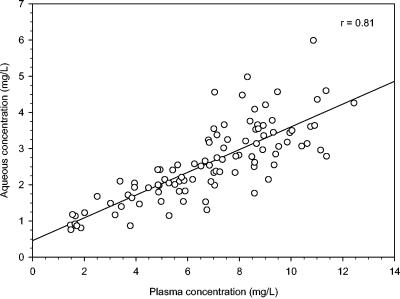
Levofloxacin concentration-versus-time profiles in plasma and aqueous humor samples are depicted in Fig. 1. The mean ± SD aqueous concentrations after the second oral levofloxacin dose were 2.01 ± 1.02 mg/liter at 1.79 ± 0.13 h, 2.57 ± 1.00 mg/liter at 2.23 ± 0.15 h, 2.61 ± 0.76 mg/liter at 2.76 ± 0.11 h, 3.12 ± 0.72 mg/liter at 3.31 ± 0.13 h, 2.85 ± 1.06 mg/liter at 3.83 ± 0.15 h, 3.19 ± 1.33 mg/liter at 4.30 ± 0.12 h, 2.53 ± 1.10 mg/liter at 4.77 ± 0.15 h, 2.30 ± 1.04 mg/liter at 5.34 ± 0.14 h, and 1.76 ± 0.99 mg/liter at 5.87 ± 0.34 h. The median values and ranges of levofloxacin concentrations in aqueous humor are listed in Table 1. The average aqueous-to-plasma ratios at different time intervals ranged between 0.33 and 0.57. A very good linear relationship between plasma and aqueous humor levels of levofloxacin was observed (r = 0.81 [Fig. 2 ]).
FIG. 1.
Levofloxacin concentrations in plasma and aqueous humor samples from patients undergoing cataract surgery (n = 101). Mean values ± SD (error bars) are shown. Reference lines show the MIC90s for levofloxacin (2) of the principal etiological agents of endophthalmitis (Streptococcus pneumoniae, Streptococcus pyogenes, methicillin-sensitive [MS] Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Proteus mirabilis).
TABLE 1.
Median values and ranges of aqueous humor levofloxacin concentrations
| Time interval (h)a | Median (range) (mg/liter) |
|---|---|
| 1.5-2 | 1.79 (0.81-4.26) |
| 2-2.5 | 2.59 (0.87-3.78) |
| 2.5-3 | 2.67 (1.54-4.36) |
| 3-3.5 | 3.05 (2.38-4.98) |
| 3.5-4 | 2.97 (1.14-4.60) |
| 4-4.5 | 3.29 (1.31-5.99) |
| 4.5-5 | 2.50 (0.88-4.56) |
| 5-5.5 | 2.08 (0.76-4.48) |
| 5.5-6 | 1.62 (0.87-3.66) |
Time interval after the second dose of levofloxacin.
FIG. 2.
Relationship between aqueous humor and plasma concentrations of levofloxacin in patients undergoing cataract surgery (n = 101).
Our results showing that levofloxacin may adequately penetrate the aqueous humor after oral administration are in agreement with other authors' findings. Fiscella et al. (12) first documented that levofloxacin concentrations in aqueous humor samples of patients undergoing vitrectomy were 0.33 ± 0.50 mg/liter at 1.35 ± 0.37 h and 0.85 ± 0.29 mg/liter at 3.47 ± 0.97 h after a single 500-mg oral dose and 1.98 ± 1.02 mg/liter at 4.18 ± 1.88 h after two 500-mg oral doses 12 h apart before surgery. Likewise, similar results were obtained by other authors (15). Of note, the assessment of levofloxacin disposition over time after the two oral doses enables our study not only to confirm that therapeutically active levofloxacin concentrations may be achieved in aqueous humor but also to highlight that these levels may persist for a long period of time above the MIC90s of most ocular pathogens (2). Since it has been suggested that for effective treatment of endophthalmitis, a concentration 2 to 10 times the MIC90 of the pathogen should be achieved (32, 38), the long-lasting exposure ensured by this oral regimen may be beneficial not only against S. epidermidis and the other coagulase-negative staphylococci, namely, the most frequent pathogens of postoperative endophthalmitis which have an MIC90 of 0.4 to 0.5 mg/liter, but also against almost all of the sensitive pathogens showing an MIC90 of <1 to 2 mg/liter. The optimal linear relationship between aqueous and plasma concentrations suggests that levofloxacin, with its low molecular weight, moderate lipophilicity, and low plasma protein binding, may consistently freely penetrate by passive diffusion in uninflamed eyes.
On these bases, some major considerations about the potential role of systemic levofloxacin in antimicrobial ocular prophylaxis and therapy may be proposed. First, the regimen studied may be an optimal choice for antimicrobial prophylaxis in selected patients undergoing ocular interventions, as this approach has some advantages over the use of topical antimicrobial agents. First, it may ensure more predictable concentrations during the vulnerable period versus topical application which, on the contrary, may be more dependent on the patient's compliance, timing and frequency of administration, or the variable degree of corneal absorption. Several authors assessed transcorneal penetration of topically applied 0.5% levofloxacin into the aqueous humor, highlighting large interindividual variability according to different schedule regimens (3, 19, 23, 45, 46). Additionally, the importance of early and frequent administration to maximize the benefit of preoperative topical prophylaxis has been recently strengthened (42). Accordingly, by enabling optimal exposure over time, the proposed oral regimen could improve the impact of antimicrobial prophylaxis since the administration of the drug at two predefined times 10 h apart before surgery (9:00 the evening before and 7:00 the morning of the operation) could simultaneously allow both an effective preoperative decontamination of the operating field and the possibility of performing an intervention in the presence of adequate antimicrobial covering any time included between 1.5 to 6 h after the morning dose. Obviously, antibiotic oral prophylaxis with levofloxacin should not be administered to all of the patients undergoing ocular surgery, since this would have a great impact on pharmaceutical expenditures and would probably cause a spread of resistant strains. It should be reserved for those patients at higher risk of postoperative endophthalmitis, namely, those with predisposing systemic or local factors such as diabetes, immunodeficiency, use of systemic steroids, skin diseases, or occlusion of the lachrymal system (29, 31, 36, 37, 47).
The long-lasting persistence of therapeutic levels above the MIC90s of most ocular pathogens in aqueous humor, coupled with the evidence of therapeutic level achievement in vitreous humor after oral administration (12, 20), might justify the use of 500-mg twice-daily systemic levofloxacin as adjunctive therapy in the treatment of endophthalmitis (1, 12), even if gatifloxacin and moxifloxacin might also be considered suitable for this purpose (18). Additionally, since inflammation may increase the permeability of blood-ocular barriers, even higher ocular drug penetration could be anticipated in the presence of endophthalmitis.
In conclusion, the favorable ocular disposition over time ensuring long-lasting optimal exposure against pathogens with an MIC90 of <1 to 2 mg/liter in the aqueous humor of patients with uninflamed eyes provides support for trials of systemic levofloxacin for prophylaxis of postoperative endophthalmitis in selected patients or as adjunctive therapy for the treatment of this potentially devastating infective complication.
Acknowledgments
This research was carried out thanks to departmental funds (DPMSC, University of Udine).
The technical assistance of Eliana Di Terlizzi is gratefully acknowledged.
REFERENCES
- 1.Benz, M. S., I. U. Scott, H. W. Flynn, Jr., N. Unonius, and D. Miller. 2004. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am. J. Ophthalmol. 137:38-42. [DOI] [PubMed] [Google Scholar]
- 2.Blondeau, J. M. 1999. A review of the comparative in-vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolones. J. Antimicrob. Chemother. 43(Suppl. B):1-11. [DOI] [PubMed] [Google Scholar]
- 3.Bucci, F. A., Jr. 2004. An in vivo study comparing the ocular absorption of levofloxacin and ciprofloxacin prior to phacoemulsification. Am. J. Ophthalmol. 137:308-312. [DOI] [PubMed] [Google Scholar]
- 4.Callegan, M. C., M. Engelbert, D. W. Parke II, B. D. Jett, and M. S. Gilmore. 2002. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 15:111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisari, G., G. Cavallaro, M. Reibaldi, and S. Biondi. 2004. Presurgical antimicrobial prophylaxis: effect on ocular flora in healthy patients. Int. J. Clin. Pharmacol. Ther. 42:35-38. [DOI] [PubMed] [Google Scholar]
- 6.Ciulla, T. A., M. B. Starr, and S. Masket. 2002. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology 109:13-24. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 8.Colin, J., S. Simonpoli, K. Geldsetzer, and A. Ropo. 2003. Corneal penetration of levofloxacin into the human aqueous humour: a comparison with ciprofloxacin. Acta Ophthalmol. Scand. 81:611-613. [DOI] [PubMed] [Google Scholar]
- 9.Colleaux, K. M., and W. K. Hamilton. 2000. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can. J. Ophthalmol. 35:373-378. [DOI] [PubMed] [Google Scholar]
- 10.Donnenfeld, E. D., H. D. Perry, R. W. Snyder, R. Moadel, M. Elsky, and H. Jones. 1997. Intracorneal, aqueous humor, and vitreous humor penetration of topical and oral ofloxacin. Arch. Ophthalmol. 115:173-176. [DOI] [PubMed] [Google Scholar]
- 11.Eifrig, C. W., I. U. Scott, H. W. Flynn, Jr., and D. Miller. 2003. Endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology 110:1714-1717. [DOI] [PubMed] [Google Scholar]
- 12.Fiscella, R. G., T. K. Nguyen, M. J. Cwik, B. A. Phillpotts, S. M. Friedlander, D. C. Alter, M. J. Shapiro, N. P. Blair, and J. P. Gieser. 1999. Aqueous and vitreous penetration of levofloxacin after oral administration. Ophthalmology 106:2286-2290. [DOI] [PubMed] [Google Scholar]
- 13.Fisch, A., A. Salvanet, T. Prazuck, F. Forestier, L. Gerbaud, G. Coscas, C. Lafaix, and The French Collaborative Study Group on Endophthalmitis. 1991. Epidemiology of infective endophthalmitis in France. Lancet 338:1373-1376. [PubMed] [Google Scholar]
- 14.Fukuda, M., A. Inoue, K. Sasaki, and N. Takahashi. 2004. The effect of the corneal epithelium on the intraocular penetration of fluoroquinolone ophthalmic solution. Jpn. J. Ophthalmol. 48:93-96. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Saenz, M. C., A. Arias-Puente, M. J. Fresnadillo-Martinez, and C. Carrasco-Font. 2001. Human aqueous humor levels of oral ciprofloxacin, levofloxacin, and moxifloxacin. J. Cataract Refract. Surg. 27:1969-1974. [DOI] [PubMed] [Google Scholar]
- 16.Han, D. P., S. R. Wisniewski, L. A. Wilson, M. Barza, A. K. Vine, B. H. Doft, and S. F. Kelsey. 1996. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 122:1-17. [DOI] [PubMed] [Google Scholar]
- 17.Hanioglu-Kargi, S., N. Basci, H. Soysal, A. Bozkurt, E. Gursel, and O. Kayaalp. 1998. The penetration of ofloxacin into human aqueous humor given by various routes. Eur. J. Ophthalmol. 8:33-36. [DOI] [PubMed] [Google Scholar]
- 18.Hanscom, T. A. 2004. Postoperative endophthalmitis. Clin. Infect. Dis. 38:542-546. [DOI] [PubMed] [Google Scholar]
- 19.Healy, D. P., E. J. Holland, M. L. Nordlund, S. Dunn, C. Chow, R. L. Lindstrom, D. Hardten, and E. Davis. 2004. Concentrations of levofloxacin, ofloxacin, and ciprofloxacin in human corneal stromal tissue and aqueous humor after topical administration. Cornea 23:255-263. [DOI] [PubMed] [Google Scholar]
- 20.Herbert, E. N., I. A. Pearce, J. McGalliard, D. Wong, and C. Groenewald. 2002. Vitreous penetration of levofloxacin in the uninflamed phakic human eye. Br. J. Ophthalmol. 86:387-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javitt, J. C., D. A. Street, J. M. Tielsch, Q. Wang, M. M. Kolb, O. Schien, A. Sommer, M. Bergner, E. P. Steinberg, and the Cataract Patient Outcomes Research Team. 1994. National outcomes of cataract extraction. Retinal detachment and endophthalmitis after outpatient cataract surgery. Ophthalmology 101:100-106. [DOI] [PubMed] [Google Scholar]
- 22.Kattan, H. M., H. W. Flynn, Jr., S. C. Pflugfelder, C. Robertson, and R. K. Forster. 1991. Nosocomial endophthalmitis survey. Current incidence of infection after intraocular surgery. Ophthalmology 98:227-238. [PubMed] [Google Scholar]
- 23.Kobayakawa, S., T. Tochikubo, and A. Tsuji. 2003. Penetration of levofloxacin into human aqueous humor. Ophthalmic Res. 35:97-101. [DOI] [PubMed] [Google Scholar]
- 24.Kunimoto, D. Y., T. Das, S. Sharma, S. Jalali, A. B. Majji, U. Gopinathan, S. Athmanathan, T. N. Rao, and the Endophthalmitis Research Group. 1999. Microbiologic spectrum and susceptibility of isolates: Part I. Postoperative endophthalmitis. Am. J. Ophthalmol. 128:240-242. [DOI] [PubMed] [Google Scholar]
- 25.Leaming, D. V., for the American Society of Cataract and Refractive Surgery. 2001. Practice styles and preferences of ASCRS members—2000 survey. J. Cataract Refract. Surg. 27:948-955. [DOI] [PubMed] [Google Scholar]
- 26.Liesegang, T. J. 1997. Prophylactic antibiotics in cataract operations. Mayo Clin. Proc. 72:149-159. [DOI] [PubMed] [Google Scholar]
- 27.Liesegang, T. J. 2001. Use of antimicrobials to prevent postoperative infection in patients with cataracts. Curr. Opin. Ophthalmol. 12:68-74. [DOI] [PubMed] [Google Scholar]
- 28.Mino de Kaspar, H., E. M. Shriver, E. V. Nguyen, P. R. Egbert, K. Singh, M. S. Blumenkranz, and C. N. Ta. 2003. Risk factors for antibiotic-resistant conjunctival bacterial flora in patients undergoing intraocular surgery. Graefes Arch. Clin. Exp. Ophthalmol. 241:730-733. [DOI] [PubMed] [Google Scholar]
- 29.Montan, P. G., G. Koranyi, H. E. Setterquist, A. Stridh, B. T. Philipson, and K. Wiklund. 1998. Endophthalmitis after cataract surgery: risk factors relating to technique and events of the operation and patient history: a retrospective case-control study. Ophthalmology 105:2171-2177. [DOI] [PubMed] [Google Scholar]
- 30.Morlet, N., G. G. Graham, B. Gatus, A. J. McLachlan, C. Salonikas, D. Naidoo, I. Goldberg, and C. M. Lam. 2000. Pharmacokinetics of ciprofloxacin in the human eye: a clinical study and population pharmacokinetic analysis. Antimicrob. Agents Chemother. 44:1674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norregaard, J. C., H. Thoning, P. Bernth-Petersen, T. F. Andersen, J. C. Javitt, and G. F. Anderson. 1997. Risk of endophthalmitis after cataract extraction: results from the International Cataract Surgery Outcomes study. Br. J. Ophthalmol. 81:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Day, D. M., D. B. Jones, J. Patrinely, and J. H. Elliott. 1982. Staphylococcus epidermidis endophthalmitis. Visual outcome following noninvasive therapy. Ophthalmology 89:354-360. [PubMed] [Google Scholar]
- 33.Olson, R. J. 2004. Reducing the risk of postoperative endophthalmitis. Surv. Ophthalmol. 49(Suppl. 2):S55-S61. [DOI] [PubMed] [Google Scholar]
- 34.Pea, F., E. Di Qual, A. Cusenza, L. Brollo, M. Baldassarre, and M. Furlanut. 2003. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin. Pharmacokinet. 42:589-598. [DOI] [PubMed] [Google Scholar]
- 35.Pea, F., F. Pavan, E. Nascimben, C. Benetton, P. G. Scotton, A. Vaglia, and M. Furlanut. 2003. Levofloxacin disposition in cerebrospinal fluid in patients with external ventriculostomy. Antimicrob. Agents Chemother. 47:3104-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz, S., H. B. Dick, F. Krummenauer, and N. Pfeiffer. 1999. Endophthalmitis in cataract surgery: results of a German survey. Ophthalmology 106:1869-1877. [DOI] [PubMed] [Google Scholar]
- 37.Scott, I. U., H. W. Flynn, Jr., and W. Feuer. 1995. Endophthalmitis after secondary intraocular lens implantation. A case-report study. Ophthalmology 102:1925-1931. [DOI] [PubMed] [Google Scholar]
- 38.Smith, A., P. M. Pennefather, S. B. Kaye, and C. A. Hart. 2001. Fluoroquinolones: place in ocular therapy. Drugs 61:747-761. [DOI] [PubMed] [Google Scholar]
- 39.Somani, S., A. Grinbaum, and A. R. Slomovic. 1997. Postoperative endophthalmitis: incidence, predisposing surgery, clinical course and outcome. Can. J. Ophthalmol. 32:303-310. [PubMed] [Google Scholar]
- 40.Speaker, M. G., F. A. Milch, M. K. Shah, W. Eisner, and B. N. Kreiswirth. 1991. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 98:639-650. [DOI] [PubMed] [Google Scholar]
- 41.Ta, C. N., R. T. Chang, K. Singh, P. R. Egbert, E. M. Shriver, M. S. Blumenkranz, and H. Mino de Kaspar. 2003. Antibiotic resistance patterns of ocular bacterial flora: a prospective study of patients undergoing anterior segment surgery. Ophthalmology 110:1946-1951. [DOI] [PubMed] [Google Scholar]
- 42.Ta, C. N., P. R. Egbert, K. Singh, E. M. Shriver, M. S. Blumenkranz, and H. Mino De Kaspar. 2002. Prospective randomized comparison of 3-day versus 1-hour preoperative ofloxacin prophylaxis for cataract surgery. Ophthalmology 109:2036-2041. [DOI] [PubMed] [Google Scholar]
- 43.Tervo, T., P. Ljungberg, T. Kautiainen, P. Puska, I. Lehto, I. Raivio, E. Jarvinen, P. Kuusela, and A. Tarkkanen. 1999. Prospective evaluation of external ocular microbial growth and aqueous humor contamination during cataract surgery. J. Cataract Refract. Surg. 25:65-71. [DOI] [PubMed] [Google Scholar]
- 44.Versteegh, M. F., J. M. Hooymans, V. W. De Lavalette, and G. Van Rij. 2000. Acute bacterial endophthalmitis after cataract extraction: results of treatment. Doc. Ophthalmol. 100:7-15. [DOI] [PubMed] [Google Scholar]
- 45.Yamada, M., H. Mochizuki, K. Yamada, M. Kawai, and Y. Mashima. 2002. Aqueous humor levels of topically applied levofloxacin in human eyes. Curr. Eye Res. 24:403-406. [DOI] [PubMed] [Google Scholar]
- 46.Yamada, M., H. Mochizuki, K. Yamada, M. Kawai, and Y. Mashima. 2003. Aqueous humor levels of topically applied levofloxacin, norfloxacin, and lomefloxacin in the same human eyes. J. Cataract Refract. Surg. 29:1771-1775. [DOI] [PubMed] [Google Scholar]
- 47.Zell, K., K. Engelmann, A. A. Bialasiewicz, and G. Richard. 2000. Endophthalmitis after cataract surgery: predisposing factors, infectious agents and therapy. Ophthalmologe 97:257-263. [DOI] [PubMed] [Google Scholar]