Abstract
Aim
The current review aims to explore postoperative neurological complications in third molar extractive surgery.
Materials and Methods
The PRISMA protocols were followed when conducting this review. We found a total of 2,250 articles that matched our topic using the Boolean keywords, mandibular nerve complications AND oral surgery, from PubMed (1,083), Scopus (435), and Web of Science (732), with the filters of English language articles, time range January 1, 2003, to September 30, 2023, and human studies. After 762 duplicates were eliminated, there remained 1,488 articles. Eleven final articles were deemed of the highest relevance to our topic by eliminating articles in animals, non-English language, reviews, meta-analysis, and off-topic. A potential risk in the third molar extraction was temporary loss of sensibility often caused by mild compression or irritation of the mandibular nerve. This typically resolves within weeks or months, but in severe cases, recovery might take longer. Permanent loss of sensation can occur, indicating significant nerve damage and lasting effects on touch, temperature, or pain perception.
Conclusions
Various treatments exist for nerve damage, including low-level laser therapy, pain management medications, or physical therapy. While these therapies may improve neurosensory impairment, patients often report a decline in their quality of life.
1. Introduction
Lower molar disinclusion surgery is a common dental procedure that involves the extraction of third molars that are partially or completely included in the mandibular bone. Although generally considered safe, this operation can involve a number of neurological complications, which vary in severity and can significantly affect the patient's quality of life.
Postoperative neurological complications are a major concern for oral and maxillofacial surgeons.
Major complications include damage to the inferior alveolar nerve (IAN) and lingual nerve (LN) [1]. The IAN, which runs along the mandible, is particularly susceptible to injury during removal of the lower included molars [2, 3, 4]. Damage to this nerve can manifest with symptoms such as paresthesia, anesthesia, dysesthesia, and, in rare cases, neuropathic pain [3, 5, 6, 7, 8].
The main branches of the LN provide sensitivity to various areas, including the jaw [9]. The IAN is responsible for innervating the lower teeth, gums, oral mucosa, and part of the skin of the jaw itself [10, 11]. This branch transmits tactile, thermal, and pain sensitivity signals from the jaw [12]. Thanks to the LN, innervate the front part of the tongue and part of the floor of the mouth. Contributing to the sensitivity of the tongue and the associated oral mucosa [13], thanks to the buccal nerve, it provides sensitivity to the cheek mucosa and part of the skin of the cheek itself [14, 15, 16].
It is important to note that the mandibular nerve, as part of the TN, is involved in the transmission of sensory signals from the facial region to the brain, contributing to the perception of sensitivity and response to various stimuli coming from these areas of the face (Figure 1) [17].
Figure 1.
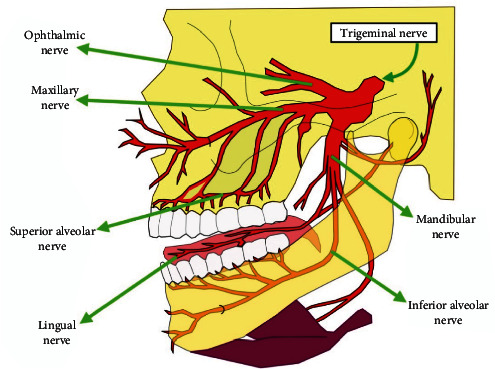
The ophthalmic nerve enters the superior orbital fissure, the maxillary nerve goes through the round hole, and the mandibular nerve escapes via the oval hole of the sphenoid bone.
This is the anatomy of the TN, which has three branches that exit the skull at separate sites [18, 19].
The IAN travels in an unusual way within the mandibular bone, but its structure can promote good recovery after damage, while the LN is more vulnerable, since if damaged, it has less chance of complete regeneration due to its more exposed position and structure (Figure 2) [20, 21].
Figure 2.
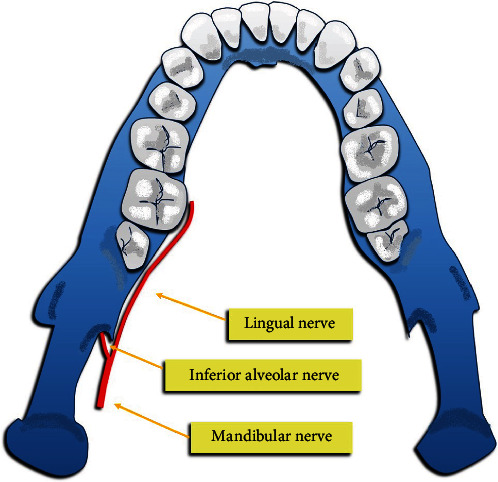
Course of the mandibular nerve with its branches, lingual and inferior alveolar nerve.
The anatomical relationship between the IAN and M3 can be assessed radiographically to predict the risk of damage (Figure 3). Some radiographic factors have been linked to IAN injury, but the presence of these factors does not always accurately predict actual damage [22, 23].
Figure 3.
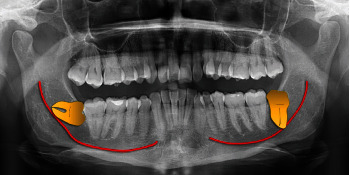
A radiographic example of the possible anatomical relationships between IAN and M3.
Injuries to the LN are varied, and their incidence varies widely. They may depend on the surgical technique, and raising and retracting a lingual mucoperiosteal flap have been associated with increased LN damage [24, 25, 26]. In general, the incidence of injury to the IAN, during dental procedures such as tooth extraction, varies between 0.2% and 5% [27, 28]. Regarding lesions in the LN, the incidence is generally reported to be lower than in the IAN, with estimates varying between 0.4% and 0.6% [28].
However, it is important to note that these figures can be influenced by numerous factors and may vary based on the specific nature of the surgery and individual patient characteristics [29, 30, 31]. Therefore, an accurate assessment of the risk of nerve injury during dental procedures requires a thorough case evaluation by the surgeon [32, 33, 34]. Other risk factors include the patient's age and tooth impaction [35, 36, 37, 38].
The buccal nerve, if damaged, can cause anesthesia of the cheek. Its structure and location close to the M3 make it vulnerable, especially during surgery involving the removal of the bone [39].
Complications associated with this nerve can emerge from several factors, including trauma, disease, or medical procedures. Some of these complications are [40, 41] as follows:
Mandibular nerve neuropathy: this condition involves damage or injury to the mandibular nerve, which can result from direct trauma, surgery to the jaw or surrounding structures, localized infections, or chronic compression of the nerve [42]. This can cause numbness, tingling, or pain in the area innervated by the nerve, affecting sensation in the jaw, cheek, and surrounding area [15, 43, 44].
Peripheral neuropathy: this can occur when the mandibular nerve suffers damage or injury near its peripheral branches. This can lead to conditions such as trigeminal neuralgia, in which the patient experiences intense, debilitating episodes of excruciating pain in the maxillary, mandibular, or facial region [41, 45]. Often, the pain is due to inflammation of the mandibular or maxillary nerve; more rarely, it is due to the ophthalmic nerve. In most cases, neuralgia is caused by compression of the nerve root by a blood vessel [46, 47]. Compression is responsible for the demyelination of nerve fibers. When myelin, a sheath that surrounds the nerve fiber, is missing, the nerve becomes hypersensitive to stimuli, and for this reason, neuralgia occurs [25, 48]. A noninvasive curative treatment is trigeminal neurolysis with targeted fluoroscopy, a minimally invasive therapy performed with a sedated patient. It is a safe, rapid, and often decisive treatment [49, 50].
Surgical complications: during surgery on the jaw, face, or surrounding structures, the mandibular nerve can be accidentally damaged [51, 52, 53]. This can cause postoperative symptoms such as persistent numbness or tingling in the affected area, with possible impairment of sensory and motor function [5, 54, 55]. Some dental conditions or dental procedures can also affect the mandibular nerve, such as the extraction of wisdom teeth in the lower jaw region which can pose a risk of injury to the mandibular nerve, leading to tingling, numbness, or pain in the surrounding area [56, 57, 58]. Implantology procedures [5] can lead also to nerve complications: insertion torque and sample size of implants should be adequately chosen [59, 60, 61].
Side effects from medical treatments, such as chemotherapy or radiation therapy, for tumors in the head/neck region. Radiation therapy, in particular, can cause damage to surrounding tissue, including the nerve, leading to neuropathy and associated symptoms [62, 63].
Synovitis of the temporomandibular joint (TMJ): inflammation of the TMJ can compress or irritate the mandibular nerve, causing pain, numbness, or dysfunction in the affected area [45, 64].
TN complications can range from mild to severe, affecting the patient's quality of life. Treatment depends on the underlying cause and may include drug therapies for pain, surgery to repair injury or damage, rehabilitation therapies, and symptom management to improve the patient's function and comfort [65, 66, 67].
It is important to avoid these injuries with adequate procedures [68] and properly manage patients with nerve injuries, including accurately diagnosing the type of injury, monitoring recovery, and treating appropriate cases [69, 70, 71].
Some authors have drawn up management protocols for lesions of the inferior alveolar and LN in the M3 extraction surgery.
For the IAN, they are as follows:
Compression and section during surgery, if the IAN is compressed or cut, management depends on the severity of the injury [72].
First degree (neurapraxia): mild compression of the nerve can cause a temporary block of nerve conduction. Usually, this leads to a complete recovery [73].
Second degree (axonotmesis): compresses or crushes the nerve causing degeneration of the axons beyond the site of injury. Recovery depends on the regeneration of damaged axons [74].
Third, fourth, and fifth degrees (more severe injuries): more serious injuries may require surgery to repair the nerve, especially if a complete section of the nerve occurs [35, 75].
For LN, they are as follows:
LN rupture: if the LN is damaged during extraction, treatment depends on the severity of the injury [76].
Minor injuries: in some cases, minor injuries may not require intervention, but monitoring is essential [10, 77].
More serious injuries: if sensory loss persists or complete anesthesia occurs, radiographic investigation may be necessary to evaluate the damage and possible surgical repair [78, 79, 80].
The treatment protocols are as follows:
Evaluation: postoperative evaluation is essential to monitor sensory recovery in patients [81].
Surgical treatment: for severe or persistent injuries, especially if diagnosed within a certain postoperative period, restorative surgeries may be considered to facilitate sensory recovery [50].
Repair surgery procedures: from neurolysis (freeing trapped nerves) to direct repair of the nerve with sutures [82, 83].
Monitoring patients, sensory testing, and observing recovery are key to determining whether surgery is needed and what type of surgery will be best if nerve damage occurs [84, 85]. Prevention of neurological complications begins with detailed preoperative planning. Advanced surgical techniques, such as the use of lasers and the piezoelectric approach, have been developed to reduce trauma to nerve tissue. In addition, rigorous postoperative follow-up is crucial for early diagnosis and timely management of complications [86].
In conclusion, while lower molar disinclusion surgery is a relatively common procedure, it requires careful consideration of potential neurological complications. The combination of accurate preoperative evaluation, modern surgical techniques, and effective postoperative management can help minimize risks and improve outcomes for patients [87].
The aim of this study is to investigate the neurological complications resulting from lower molar disinclusion surgery. In particular, we intend to evaluate the frequency, nature, and risk factors associated with lesions of the mandibular nerve and its branches during and after the surgical procedure [88]. This study also aims to compare the effectiveness of preoperative radiographic techniques, such as computed tomography (CT) and panoramic radiography, in preventing nerve damage. Furthermore, it aims to examine postoperative management strategies to minimize sensorineural dysfunction and improve patients' quality of life. Through a detailed analysis of injury mechanisms and predisposing factors, the study aims to provide clinical guidelines to reduce the risk of neurological complications in the future.
2. Materials and Methods
2.1. Protocol and Registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols were followed when conducting this review, and the protocol was registered at PROSPERO under the ID: 496565.
2.2. Search Processing
We searched PubMed, Scopus, and Web of Science with a constraint on English language papers from January 1, 2003, to September 30, 2023, that matched our topic. The following Boolean keywords were utilized in the search strategy: mandibular nerve complications AND oral surgery (Table 1). These terms were chosen because they best described the goal of our inquiry, which was to learn more about postsurgical neurological complications in extractive surgery of the lower molars.
Table 1.
Indicators for database searches.
| Article screening strategy |
|---|
| Keywords: A, mandibular nerve complications; B, oral surgery |
| Boolean indicators: A AND B |
| Timespan: 2003–2023 |
| Electronic databes: PubMed; Scopus; WOS |
2.3. Eligibility Criteria and Study Selection
Research focusing on the impact of nerve complications postsurgery were chosen for analysis. The selection process involved two phases: firstly, evaluating titles and abstracts, followed by a thorough examination of full-text articles. Articles meeting the following criteria were included: (a) human-based studies, (b) comparative analysis of treatments with other interventions, and (c) availability of full-text content in English. Reviews, meta-analyses, and studies involving animals were excluded from consideration. The titles and abstracts from the initial search were collected and reviewed for relevance. Full publications of pertinent research were acquired for further assessment. Two different reviewers appraised the gathered studies based on the specified criteria to determine their inclusion.
2.4. Data Processing
Two independent reviewers conducted a database search to identify pertinent studies using predetermined selection criteria. The quality assessment of the chosen articles was also independently performed by these reviewers. Selected articles were stored in Zotero (version 6.0.15). Any disparities between the reviewers were resolved by consulting a senior reviewer (F.I.). The selection procedure and a summary of the chosen records are presented in Figure 3 and Table 2, respectively.
Table 2.
PICOS criteria.
| Criteria | Application in the present study |
|---|---|
| Population | Adults |
| Intervention | Extractive surgery of the lower molar |
| Comparisons | Total extraction of the third molar compared with coronectomy of the third molars, analyzing the risk factors for IAN injury |
| Outcomes | Recovery periods of neuronal lesions caused by surgical procedures |
| Study design | Clinical trials and retrospective studies |
2.5. Quality Assessment
The quality of the included papers was assessed by two reviewers, E.d.R. and A.D.N., using the ROBINS which is a tool developed to assess risk of bias in the results of nonrandomized studies that compare health effects of two or more interventions. Seven points were evaluated, and each was assigned a degree of bias. A third reviewer (F.I.) was consulted in the event of a disagreement until an agreement was reached. The question in the domains evaluated in the ROBINS is the following:
Bias due to confounding
Bias arising from measurement of exposure
Bias in the selection of participants into the study
Bias due to postexposure intervention
Bias due to missing data
Bias arising from measurement of the outcome
Bias in the selection of the reported results.
2.6. PICOS Criteria
Table 2 illustrates the components of the PICOS framework (Population, Intervention, Comparison, Outcome, Study Design), outlining the criteria for population, intervention, comparison, outcomes, and research design utilized in this assessment.
3. Results
There were 2,250 entries altogether from the first search (1,083 from PubMed, 435 from Scopus, and 732 from WOS), and 1,488 articles remained after 762 duplicates were eliminated. However, 1,345 of them were eliminated because of 135 reviews and meta-analysis, seven animal-related articles, and 1,203 off-topic articles. The other 25 reports were eliminated because they were not retrieved. Eleven records were deemed eligible after an additional 107 publications were eliminated from the list due to insufficient interest in the presented data (Figure 4). Each study's findings were presented in Table 3.
Figure 4.
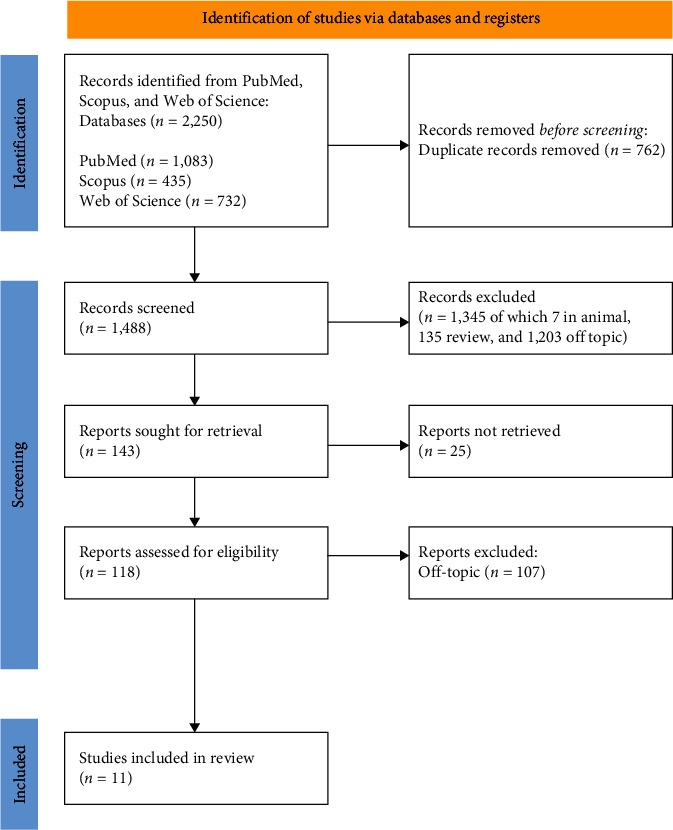
PRISMA flowchart.
Table 3.
A descriptive item selection summary.
| Authors (year) | Type of the study | Aim of the study | Materials | Results |
|---|---|---|---|---|
| Kung et al., 2017 [89] | Retrospective study | Using the modeling and visual interpretation of cone-beam CT, this retrospective study assessed the location, incidence, and dimensions of the mandibular lingual canal and the anterior loop in the Taiwanese population. | There were 215 patients in the sample group (105 men and 110 women, with a mean age of 57 years). Cone-beam CT and three-dimensional reconstructed images were used to identify and determine the anterior loop length, median lingual canal, and symphysis bone thickness. Unpaired statistical analysis was used to evaluate and examine the correlation of all the data for both men and women. |
Every patient had at least one median lingual canal in the symphysis, and the major branchʼs diameter varied between 0.21 and 1.48 mm (mean, 0.85 mm), with statistically significant gender differences (longer in males than in women). Neurovascular damage was shown to be 13.0% likely at a harvesting depth of 4 mm for the distance from the buccal bone to the terminal end of the median lingual canal; this risk was significantly higher in women (19.1%) than in males (6.7%). |
|
| ||||
| Hasegawa et al., 2013 [53] | Comparative study | Compare panoramic radiographs and computed tomography (CT) in investigating the relationships among the risk factors for IAN injury. | Univariate and multivariate analysis on 2,528 surgical avulsion of mandibular M3 of patients with a mean age of 36.2 ± 12.2 years performed by dentists at the Department of Oral and Maxillofacial Surgery. | CT findings may be able to predict the development of IAN injury more accurately than panoramic findings. |
|
| ||||
| Renton et al., 2004 [90] | Randomized controlled clinical trial | Randomization of 128 patient who needed M3 extractions and with radiological evidence of the M3 closeness to the IAN canal. |
The patients (128) were subjected to two different operations, extraction (102) and coronectomy (94), and coronectectomy in two soubgroups, successful coronectomy (n = 58) and failed coronectomy (n = 36). Follow up lasted an average of 25 (13) months. | Following extraction, 19 nerves (19%) were injured, none following a successful coronectomy and three after failed coronectomy. This is the first clinical trial of the efficacy of coronectomy in preserving the IAN. |
|
| ||||
| Ozen et al., 2006 [91] | Clinical study | Report of the effects of LLT in four patients with lomgstanding sensory after M3 extraction. | LLT was applied to four female patients who underwent M3 surgery and had chronic nerve damage. | There was a notable acceleration of the time course and degree of neurosensory return when the neurosensory assessment scores following LLL therapy treatment were compared with the baseline values before to treatment. |
|
| ||||
| Misch and Resnik, 2012 [24] | Clinical study | Establish a policy designed especially for general dentists to handle cases when a nerve has been damaged during bone grafting or implant surgery. | The procedure outlined in this article is broken down into five phases: (1) nerve damage is suspected during surgery; (2) nerve transection is known; (3) 1 week follows the procedure; and (4) 12 weeks. | It is recommended to follow the right course of therapy (pharmacology, monitoring, etc.) during each period, and when necessary, to refer the patient to a nerve expert. |
|
| ||||
| Schwartz-Arad et al., 2018 [92] | Retrospective study | Examine the relationship between risk variables and the incidence of problems after M3 extraction. | 463 individuals who had their mandibular M3 extracted between 2001 and 2011 (by one surgeon, DSA) were included in the research. 665 mandibular M3 were removed in total. The patients varied in age from 13 to 75 years old, with a median age of 26 years and an average age of 29 ± 11.30 years. Medical and general data were gathered from patient records. | Age, the degree of impaction, the side of the extraction, and cigarette smoking all raise the risk of complications following mandibular M3 extraction. |
|
| ||||
| Baqain et al., 2008 [93] | Prospective cohort study | This study sought to determine the risk factors and approximate the incidence of postoperative problems following surgery on the mandibular M3. | Three groups of predictive factors were identified: anatomical, surgical, and patient-specific. Postoperative complications, recorded as present or absent, were considered as outcome variables. After bivariate analysis, a multivariable logistic regression model was used to identify independent predictors of the most frequent postoperative problems. | Longer operations, deeper impaction, M3 side dissimilarity from the operator's handedness, and advanced age all contribute to increased postoperative morbidity. |
|
| ||||
| Akadiri et al., 2009 [94] | Clinical study | The study aimed to record the incidence and duration of inferior alveolar, lingual, and buccal nerve damage after extraction of wisdom molars, identifying associated radiographic and operative risk factors. | 79 patients undergoing surgical extraction of unilateral lower judgment molars received sensorineural testing before and after surgery to evaluate the incidence and duration of complicated nerve lesions. Risk factors for nerve injury were identified among radiographic variables and documented operative events. | It is possible to predict nerve damage during M3 surgery based on some radiographic risk markers and some unanticipated intraoperative occurrences. The majority of injuries are just temporary in nature. |
|
| ||||
| Cheung et al., 2010 [95] | Prospective study | To study the frequency of future neurosensory impairment resulting from damage to the LN and IAN, investigate potential risk factors, and characterize the healing process. | 3,595 patients of different ages and genders underwent extraction of lower wisdom molars. Of a total of 4,338 extractions, 0.35% developed IAN deficiency and 0.69% LN deficiency. It was noted that distoangular impaction significantly increased the risk of LN deficiency, while the depth of impaction was related to the risk of IAN deficiency. College students more frequently caused LN deficits. | 67% of IAN deficits and 72% of LN deficits were fully healed at the end of the follow-up period. |
|
| ||||
| Pogrel et al., 2011 [96] | Clinical study | To analyze effects of permanent involvement of the inferior or lingual alveolar nerve due to dental treatments. | The study involved patients reviewed between 3 and 9 years after injury. Of 727 patients eligible for the study, 145 (95 women and 50 men) completed the telephone survey. Overall, patients experienced improvement over time, often using different coping mechanisms. Men showed a greater decrease in symptoms than women, while patients over 40 reported more long-term pain than younger patients. |
Symptoms of the LN improved more than those of the IAN, but for most treated patients, there was improvement in symptoms over time. |
|
| ||||
| Gallas-Torreira et al., 2003 [97] | Clinical study | To implement a therapeutic protocol for an endodontic complication that led to paresthesia of the IAN. | 45-year-old woman who has had pain in the left half of her jaw and numbness in her left lip for 15 days. After endodontic treatment on a lower left molar, a portion of filling material was found beyond the apex of the tooth root, requiring tooth extraction. Despite the extraction, the pain and numbness did not decrease. The pain disappeared 15 days after the gutta-percha residue was removed, but the lip numbness persisted for about a month before resolving. | There are three potential pathways for nerve injury: heat, chemical, and mechanical. Compression, stretching, partial or complete resection, and laceration are examples of mechanical injuries. Axonotmesis, or discontinuity of the nerve due to Wallerian degeneration of the covering's distal and integrated fibers, or neurotmesis, or complete sectioning of the nerve, are the two possible outcomes of the injury. |
3.1. Quality Assessment and Risk of Bias of Included Articles
The risk of bias in the included studies is reported in Figure 5. Regarding the bias due to confounding, most studies have a high risk. The bias arising from measurement is a parameter with low risk of bias. Many studies have a low risk of bias due to bias in the selection of participants. Bias due to postexposure cannot be calculated due to high heterogeneity. The bias due to missing data is low in many studies. Bias arising from measurement of the outcome is low. Bias in the selection of the reported results is high in most studies. The final results show that five studies have high risk of bias, two have a very high risk of bias, and three have low risk of bias.
Figure 5.
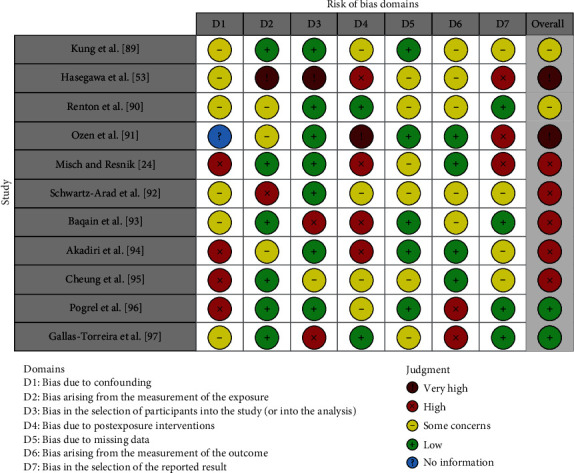
Bias assessment evaluated by ROBINS.
4. Discussion
Knowing the anatomy and histology of nerves provides a more detailed understanding of the correlation between the structure of the nerves themselves and the possible impacts of injury on nerve tissue. One of the cranial nerves most subject to trauma is the mandibular nerve, a branch of the TN, the largest of the cranial nerves [98, 99]. It is responsible for carrying sensory sensations from the lower part of the lip, chin, lower teeth, and associated soft tissues, as well as from the bone tissue of the jaw and some areas of the external ear. Anatomically, the mandibular nerve is composed of connective tissue and neural components [100]. The smallest functional units in a TN are the nerve fibers, which can be myelinated or unmyelinated. The more abundant myelinated nerve fibers consist of a single axis enclosed by a Schwann cell. These fibers and Schwann cells are surrounded by a protective layer of endoneurial connective tissue, composed of basal lamina, collagen fibers, and endoneurial capillaries. These nerve fibers are grouped into bundles, surrounded by a thin, dense layer of connective tissue called the perineurium, which maintains intrafascicular pressure and acts as a diffusion barrier to protect the individual fibers. Injury to these extraneural tissues can impair nerve transmission of individual fibers, leading to sensory disturbances. The extent of damage to each tissue type can determine the degree of sensorineural impairment. A primary concern related to injuries to the IAN is alteration of sensory functions such as touch, pressure, temperature, or pain. Loss of sensation in the cheek or lower lip can cause soft tissue damage when chewing and affect the ability to drink. Furthermore, the lack of sensation can cause significant pain, becoming debilitating for the patient [24]. One of the most frequent side effects of surgical removal of M3 is IAN injury which supplied sensation to the oral and facial regions. IAN injuries occur with an incidence ranging from 0.26% to 8.4% of cases, with most of these injuries being temporary and resolving within 6 months. In comparison, lesions to the LN have an incidence ranging from 0.1% to 22% of cases, with a good percentage of these that are also temporary, but with more cases that can become permanent than IAN. Most injuries to both nerves resolve within a few weeks or months, but a small number of patients may experience permanent sensory deficit [91]. Preoperative radiographical examination can prevent the occurrence of injury to the peripheral branches of the TN during surgical procedure [101]. Hasegawa et al. compared CT with panoramic radiographs on 2,528 teeth extracted of patients with a mean age of 36.2 ± 12.2 years using univariate and multivariate analyses. According to this comparative study, CT is able to prevent risk of nervous damage more accurately than orthopantomography through the presence of radiographic findings such as darkening of the root where it crosses the inferior alveolar canal, interruption or obliteration of cortical lines which delimit the inferior alveolar canal, and diversion or bending of the inferior alveolar canal in contiguity with the root apices [53, 102]. Schwartz-Arad et al. [92] focused, in 2018, on the complications that occur after the extraction of M3 in the jaw. During a 10-year period (2001–2011), 1,038 extractions were performed on 500 patients, mainly mandibular molars. Approximately 16.9% of cases presented postoperative complications, mainly in the form of dry abscesses (11.6%) and other symptoms such as inflammation, pain, hematoma, and temporary loss of sensation in some areas of the face. The severity of the complications is related to the degree of impaction of the tooth: completely or partially impacted molars show a higher incidence of complications than completely erupted ones. Additionally, smokers have a higher risk of complications, especially dry abscesses, than nonsmokers. Age, gender, side of extraction, and use of contraceptives do not appear to be factors significantly related to postoperative complications [95]. In conclusion, it is highlighted that smoking, the degree of impaction of the tooth, and the age of the patient can influence complications after the extraction of mandibular M3. It also recommends the use of specific antibiotics to reduce the risk of dry abscesses, especially in extractions of partially erupted teeth [92]. Baqain's [93] study conducted in 2008 also focuses on the frequency and risk factors of postoperative complications after surgical extraction of mandibular M3. The work examined several predictive variables and associated them with the complications encountered, including alveolar osteitis, trismus, and postoperative pain. Key findings and conclusions include alveolar osteitis with higher incidence in older individuals, when the side of the M3 differs from the operator's dexterity and with lingual retraction during surgery. Lingual retraction has been identified as an independent risk factor, associated with greater trauma of the operation. The greater risk in deeper impacts, especially totally bony ones, is trismus. Longer operative time was associated with a greater risk of prolonged pain. Advanced age has been linked to increased postoperative complications, especially alveolar osteitis. The correlation between the side of the M3 and operator dexterity suggests that removal of the M3 on the operator's nondominant side may be more complex. As final thoughts, alveolar osteitis has been identified as a common complication impacting productivity. The use of multivariable regression models allowed us to better identify risk factors. In summary, identifying these risk factors can guide the management of postoperative complications in the removal of mandibular M3, offering useful information for patients and healthcare professionals [93]. Other authors, such as Akadiri et al. [94], in 2009, they discussed sensorineural complications related to the surgical extraction of mandibular M3. Seventy-nine patients were evaluated for IAN, LN, and buccal (BN) nerve injuries through pre- and postoperative testing to determine incidence and duration. We sought to identify radiographic and operative risk factors associated with these injuries. The reported incidence of lesions was 6.6% for IAN, 2.6% for LN, and 4.0% for LBN, with most lesions resolving within 2 weeks. Depth of impaction and linguoversion emerged as significant risk factors for IAN and LN injuries, respectively, while no risk factors were identified for BN injuries. Most lesions were transient and resolved within a few weeks. The study also highlights the limitations of 2D imaging techniques for evaluating impacted M3. Some significant intra- and postoperative events were related to the observed nerve lesions. For example, patients with prolonged intra-alveolar bleeding have experienced injury to the IAN. Injuries to the BN have been traced to possible trauma during the surgical procedure [94]. For the prospective study by Cheung et al. [95] in 2010, conducted on extractions of the lower M3, it involved 3,595 patients subjected to 4,338 extractions, detecting a development of 0.35% of deficit in the IAN and 0.69% in the LN. Distoangular impaction emerged as a significant risk factor for LN deficit, while depth of impaction was associated with the risk of IAN deficit. Operator experience influenced the results, with dental students accounting for more LN deficits. Factors such as gender, age, lingual flap elevation, protection of the LN with a retractor, removal of the distolingual cortex, sectioning of the tooth, and difficulty in elevating the tooth were not significantly related to IAN or LN lesions [95]. Recovery was more significant at 3 months postsurgery for the IAN deficit and at 6 months for the LN deficit. At the end of the follow-up period, 66.7% of IAN deficits and 72.0% of LN deficits fully recovered. The study highlighted the importance of distoangular impaction and operator experience as significant risk factors for postoperative neurosensory deficits. Overall incidences of deficits were low, 0.35% for IAN and 0.69% for LN, with no significant differences related to age or sex. Furthermore, the recovery of deficits was monitored over time, finding that the majority of patients achieved complete recovery within the first 6 months postsurgery. The percentages of permanent sensorineural deficits were estimated to be 0.12% for the IAN and 0.16% for the LN, low values compared to other studies [95]. A study was conducted to avoid or reduce the risk of complications resulting from lower M3 extraction caused by injury to the lower alveolar nerve, which occurs in up to 3.6% of cases. The following were selected for this randomized clinical trial: 128 patients who required extraction of the mandibular M3 and who had radiological evidence of the proximity of the M3 to the IAN canal. Of these, 102 underwent extraction, while 94 underwent failed or successful coronectomy. During the planned coronectomy, some roots became loose and were removed, creating two groups of failed (36) or successful (58) coronectomy. The follow-up period lasted only about 2 years. Nineteen patients' nerves sustained injury following extraction, none following a successful coronectomy and three following a successful coronectomy. The study conducted therefore examines the effectiveness of coronectomy in maintaining, and therefore reducing damage, the IAN (Figure 6) [90].
Figure 6.
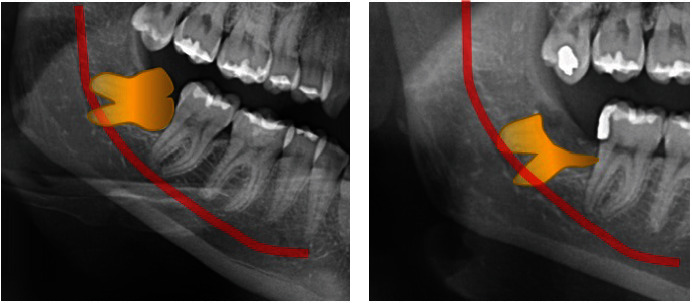
Clinical case in which a M3 coronectomy was performed, due to the very close relationships between M3 roots and IAN: (a) radiographic image before surgery and (b) postsurgery radiographic image.
The text by Gallas-Torreira et al. [97] of 2003 discusses paresthesias of the inferior dental nerve, which can result from various factors such as trauma, tumors, connective tissue diseases, infections, or idiopathic lesions. The most common cause is traumatic neuropathy of the IAN, often linked to extraction of the lower M3. Nerve injury even after endodontic treatments is a rare but possible complication as highlighted in a case of a 45-year-old woman who complained of pain and numbness after endodontic treatment on a lower left molar. After discovering that a gutta-percha tip had penetrated the dental nerve, surgical removal of it was performed. Although the pain disappeared after removal, the numbness persisted but then gradually resolved. Direct or indirect mechanical trauma, nerve compression due to hematoma or root canal filling materials, and chemical damage due to intraductal toxic substances are some of the mechanisms of nerve injury during endodontic treatment. Finally, we emphasize the importance of timely elimination of the cause of the nerve injury and control of inflammation to manage paresthesia [24]. The risk of damage to the nerves of the face can occur not only during extraction surgery of the lower molars, but also during other surgical procedures [35]. Misch and Resnik [24], in 2012, for example, examined potential complications related to implant surgery involving the sensory nerves connected to the TN. The most commonly affected nerves during implant surgery are the IAN and its mental branch, as well as others such as the LN, buccal nerve, and infraorbital nerve, due to their anatomical location. Nerve injuries can occur during different phases of surgery, from anesthesia administration to implant placement and soft tissue swelling after surgery. The incidence of these lesions can vary greatly, from 0 up to 44%. When nerve damage occurs, it is important for the provider to recognize the type and extent of the damage to provide the best postoperative care. Traumatic or iatrogenic complications may involve total or partial nerve resection, pinching, stretching, or entrapment of the nerves, resulting in sensory deficits ranging from mild loss of sensation to permanent painful dysfunction [24]. The paper conducted by Pogrel et al. [103] in 2011 examined patients who suffered permanent damage to the inferior alveolar or LN due to dental treatments. The study interviewed patients 3–9 years after the accident from the Oral and Maxillofacial Surgery Clinic at the University of California, San Francisco. Of the 727 eligible patients, 145 completed surveys [103]. Many have sought both conventional and alternative treatments, and some have undergone surgery elsewhere. The results showed significant impacts on the daily lives of patients, including employment, relationship problems, depression, speaking, and eating difficulties. However, over time, most patients experienced improvement, often adopting various coping mechanisms. Men tended to report greater reduction in symptoms than women, while patients over 40 reported more long-term pain than younger ones. The LN showed greater improvement than the alveolar nerve, although complete recovery was rare. Although long-term problems persisted, most patients showed modest improvement over time, often attributable to adaptation rather than actual medical improvements [96]. Further research could explore less common complications, such as infections and sensorineural deficits [93]. The text of Ozen et al. [91], in 2006, discusses a study that focuses on the use of low-level laser (LLL) therapy in patients who have experienced nerve injuries following the extraction of mandibular M3. These injuries are commonly associated with the roots of impacted mandibular molars and the IAN. While these issues occur in a frequency range of 0.4%–8.4%, most cases are temporary, but some can result in long-term and even permanent incapacity if they persist beyond 6 months. The study presents the results of employing low-level laser therapy (LLLT) to address these nerve injuries. Specifically, four female patients with paresthesia and dysesthesia in various areas (lip, chin, gingiva, and buccal areas) underwent clinical neurosensory tests before and after the therapy. These tests included assessments like brush stroke directional discrimination, two-point discrimination, and subjective evaluations using a virtual analog scale. The results were then tracked over time. The study observed a significant improvement in the speed and extent of neurosensory recovery when comparing the scores from neurosensory assessments after LLL therapy with the initial values before treatment. This suggests that low-intensity laser therapy could expedite the return of neurosensory function following M3 surgery. In conclusion, the study indicates that LLLT shows promise in reducing chronic sensory nerve impairment resulting from M3 surgery. However, it also highlights the need for further research to better understand and optimize the therapeutic potential of this treatment option [91]. The text by Gallas-Torreira et al. [97] of 2003 discusses paresthesias of the inferior dental nerve, which can result from various factors such as trauma, tumors, connective tissue diseases, infections, or idiopathic lesions. The most common cause is traumatic neuropathy of the IAN, often linked to extraction of the lower M3 [104]. Nerve injury even after endodontic treatments is a rare but possible complication as highlighted in a case of a 45-year-old woman who complained of pain and numbness after endodontic treatment on a lower left molar. After discovering that a guttapercha tip had penetrated the dental nerve, surgical removal of it was performed. Although the pain disappeared after removal, the numbness persisted but then gradually resolved. Direct or indirect mechanical trauma, nerve compression due to hematoma or root canal filling materials, and chemical damage due to intraductal toxic substances are some of the mechanisms of nerve injury during endodontic treatment [105]. Finally, we emphasize the importance of timely elimination of the cause of the nerve injury and control of inflammation to manage paresthesia [97]. Injuries to the IAN during implant surgery usually occur after the nerve enters the lingula of the mandibular ramus and along its path into the body and/or as it exits the mental foramen. Injuries to these tissues can affect the transmission of sensations and lead to neurosensory disorders: changes in touch, pressure, temperature, and pain. The terminology used to describe sensory alterations is paresthesia, dysesthesia, and anesthesia, with the aim of standardizing the description of neurosensory deficits:
Paresthesia is a sensory alteration that is not necessarily painful. Typically, paresthesia involves abnormal sensations such as tingling, numbness, or a “pins and needles” sensation. Although it is a change in sensitivity, it does not necessarily cause discomfort or pain [106].
Dysesthesia includes abnormal or unpleasant sensory sensations, often described as painful, uncomfortable, or unpleasant. It may include burning, stinging, aching, or numbness sensations that may be constant or intermittent [107].
Anesthesia means complete loss of sensitivity or sensation. In this case, the affected area completely loses the ability to perceive touch, pressure, temperature, or pain. Anesthesia results in a total lack of sensation [108].
Many factors, including the type of injury and the patient's age and gender, influence the neurological response to an injury [109]. Pharmacological therapy with corticosteroids and nonsteroidal anti-inflammatories is recommended to reduce inflammation and minimize neurological complications [110]. For other possible pharmacological treatments, such as antidepressants, anticonvulsants, and topical anti-inflammatory agents, we emphasize the need for caution in the use of such drugs and management by clinicians experienced in nerve injury. Carrying out a timely and accurate assessment of these injuries and sending them to specialists in nerve injuries based on the severity and type of injury allows the resolution, in most cases, of postsurgical nerve pathologies [24]. In order to reduce complications during surgical procedures with symphysis block, the retrospective study by Kung et al. [89], in 2017, evaluated the location, incidence, and dimensions of the mandibular lingual canal and anterior loop using CT cone beam. Three-dimensional images are of enormous help in planning surgical interventions, particularly in the mandibular area. This allows for safer and more predictable procedures. Three-dimensional images of 215 patients (105 men and 110 women) were used to examine the median lingual canal, symphysis bone thickness, and anterior loop length. Each patient had at least one median lingual canal in the symphysis, and the diameter of the major ramus ranged between 0.21 and 1.48 mm (mean 0.85). The study brought to light statistically significant gender differences in the lingual canal: longer in males than in females. The finding implies that, due to the numerous and complex anatomical differences, routine CT scans are required before any surgical treatment in the symphysis region [89].
4.1. Limitations of the Study
Many data come from limited and specific samples, reducing the generalizability of the findings. Short-term follow-up in some studies does not allow full evaluation of long-term complications. The use of various methods to evaluate complications may introduce inconsistencies in the results. Some studies do not adequately consider all confounding factors, such as pre-existing medical conditions or operator experience. Two-dimensional images may be insufficient compared to three-dimensional ones for precise risk assessment. There is a risk of bias in observational studies, affecting the reliability of the results [111]. The low diversity in the samples can limit the representativeness of the results. Less common complications may be underreported due to the rarity of events. The prevalence of observational and retrospective studies limits the ability to reliably determine causality. These limitations highlight the need for further research with larger samples, longer follow-ups, and more rigorous study designs to improve the understanding and management of postoperative neurological complications [112].
5. Conclusions
Complications related to the mandibular nerve, the largest branch of the fifth (V) cranial nerve, can arise from various sources such as surgical procedures, diseases, or trauma. Procedures like impacted third molar (M3) extraction, endodontic treatments, implant placements, postinfection or trauma neuropathy, synovitis of the temporomandibular joint, or medical interventions may lead to involvement or damage of the mandibular nerve, encompassing the IAN and lingual branches. In assessing the risk and progression of nerve damage, CT scans might offer more precise predictions compared to panoramic imaging. A comprehensive preoperative evaluation of potential risks associated with surgical procedures aids in planning microsurgical interventions to minimize neurosensory damage risks to the mandibular nerve. Nonetheless, patients should be fully informed preoperatively about potential postoperative complications as part of the informed consent process. Should hypesthesia or dysesthesia of the mandibular nerve occur as a surgical complication, continuous monitoring via neurosensory tests is essential, with possible referral to neurosurgery. Various treatments exist for nerve damage, including LLLT, pain management medications, or physical therapy. While these therapies may improve neurosensory impairment, patients often report a decline in their quality of life. Surgeons should prioritize preventing such occurrences through thorough preoperative investigations. Conservative therapies can be attempted to ameliorate this medical condition. This manuscript emphasizes the importance of detailed understanding of nerve anatomy to prevent and manage postsurgical neurological complications associated with lower molar disinclusion. It provides an overview of frequent mandibular and LN injuries during lower third molar extraction and proposes preventive and therapeutic measures based on recent clinical studies.
This research highlights specific risk factors for postsurgical neurological complications. The use of LLLT for the treatment of nerve injuries helps to improve clinical practice and reduce postoperative complications.
Dental professionals can apply the knowledge gained from this manuscript in their clinical practice through more accurate surgical planning and the adoption of advanced surgical techniques to minimize the risk of nerve injury. In addition, the implementation of more detailed radiographic examinations, such as CT, can improve the prediction and prevention of complications. Awareness of potential complications and their management enables clinicians to adequately inform patients and provide targeted postoperative follow-up.
Abbreviations
- CT:
Computed tomography
- IAN:
Inferior alveolar nerve
- IVRO:
Intraoral verticosagittal ramus osteotomy
- LN:
Lingual nerve
- LLL:
Low level laser
- M3:
Mandibular third molar
- TMJ:
Temporomandibular joint
- TN:
Trigeminal nerve.
Contributor Information
Francesco Inchingolo, Email: francesco.inchingolo@uniba.it.
Gianna Dipalma, Email: giannadipalma@tiscali.it.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Alessio Danilo Inchingolo, Francesco Inchingolo, Angelo Michele Inchingolo, Antonio Mancini, and Gianna Dipalma contributed to conceptualization; Angela Di Noia, Elisabetta de Ruvo, Laura Ferrante, and Andrea Palermo contributed to methodology; Francesco Inchingolo, Gianna Dipalma, Angela Di Noia, Elisabetta de Ruvo, Andrea Palermo, Laura Ferrante, and Gaetano Del Vecchio contributed to software;, Angelo Michele Inchingolo, Gianna Dipalma, Marco Di Blasio, and Andrea Palermo contributed to validation; Angela Di Noia, Gaetano Del Vecchio, Laura Ferrante, Elisabetta de Ruvo, and Francesco Inchingolo contributed to formal analysis; Angelo Michele Inchingolo, Francesco Inchingolo, and Laura Ferrante contributed to investigation; Angelo Michele Inchingolo, Andrea Palermo, Alessio Danilo Inchingolo, Francesco Inchingolo, Gaetano Del Vecchio, and Angela Di Noia contributed to resources; Gianna Dipalma, Francesco Inchingolo, Andrea Palermo, and Laura Ferrante contributed to data curation; Alessio Danilo Inchingolo, Antonio Mancini, Angelo Michele Inchingolo, Gianna Dipalma, and Elisabetta de Ruvo contributed to writing, original draft preparation; Francesco Inchingolo, Antonio Mancini, Laura Ferrante, Alessio Danilo Inchingolo, Gaetano Del Vecchio, Angela Di Noia, and Gianna Dipalma contributed to writing, review and editing; Elisabetta de Ruvo, Laura Ferrante, Angela Di Noia, Andrea Palermo, and Alessio Danilo Inchingolo contributed to visualization; Francesco Inchingolo, Alessio Danilo Inchingolo, Angela Di Noia, Antonio Mancini, Marco Di Blasio, and Laura Ferrante contributed to supervision; Gianna Dipalma, Andrea Palermo, Angelo Michele Inchingolo, and Francesco Inchingolo contributed to project administration. All authors have read and agreed to the published version of the manuscript. Antonio Mancini and Angelo Michele Inchingolo contributed equally to this work as first authors. Alessio Danilo Inchingolo and Gianna Dipalma contributed equally to this work as last authors.
References
- 1.Loescher A. R., Smith K. G., Robinson P. P. Nerve damage and third molar removal. Dental Update . 2003;30(7):375–382. doi: 10.12968/denu.2003.30.7.375. [DOI] [PubMed] [Google Scholar]
- 2.Shankar Kikkeri N., Nagalli S. StatPearls . Treasure Island (FL): StatPearls Publishing; 2024. Trigeminal neuralgia. [PubMed] [Google Scholar]
- 3.Erman A., Kejner A., Hogikyan N., Feldman E. Disorders of cranial nerves IX and X. Seminars in Neurology . 2009;29(1):85–92. doi: 10.1055/s-0028-1124027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inchingolo A. M., Malcangi G., Ferrante L., et al. Surface coatings of dental implants: a review. Journal of Functional Biomaterials . 2023;14(5) doi: 10.3390/jfb14050287.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inchingolo F., Ballini A., Cagiano R., et al. Immediately loaded dental implants bioactivated with platelet-rich plasma (PRP) placed in maxillary and mandibular region. La Clinica terapeutica . 2015;166(3):e146–152. doi: 10.7417/CT.2015.1845. [DOI] [PubMed] [Google Scholar]
- 6.Ramadorai A., Tay A. B. G., Vasanthakumar G., Lye W. K. Nerve injury after surgical excision of mandibular third molars under local anesthesia: an audit. Journal of Maxillofacial and Oral Surgery . 2019;18(2):307–313. doi: 10.1007/s12663-018-1151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H. J., Jo Y. J., Choi J. S., Kim H. J., Kim J., Moon S. Y. Anatomical risk factors of inferior alveolar nerve injury association with surgical extraction of mandibular third molar in Korean population. Applied Sciences . 2021;11(2) doi: 10.3390/app11020816.816 [DOI] [Google Scholar]
- 8.Pippi R., Spota A., Santoro M. Journal of oral and maxillofacial surgery. Journal of Oral and Maxillofacial Surgery . 2017;75(5) doi: 10.1016/S0278-2391(17)30275-6.A1 [DOI] [PubMed] [Google Scholar]
- 9.Avenanti A., Bolognini N., Maravita A., Aglioti S. M. Somatic and motor components of action simulation. Current Biology . 2007;17(24):2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Baldini A., Nota A., Santariello C., Assi V., Ballanti F., Cozza P. Influence of activation protocol on perceived pain during rapid maxillary expansion. The Angle Orthodontist . 2015;85(6):1015–1020. doi: 10.2319/112114-833.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermesan D., Prejbeanu R., Poenaru D. V., et al. Do intramedullary implants improve survival in elderly patients with trochanteric fractures? A retrospective study. La Clinica Terapeutica . 2015;166(3):e140–e145. doi: 10.7417/T.2015.1844. [DOI] [PubMed] [Google Scholar]
- 12.Pharma P. Aphthas and stomatitis | mouth, teeth and gums. 2023. Accessed on 23 December, https://www.prodecopharma.com/en/symptoms-and-relief/mouth-teeth-and-gums/aphthas-and-stomatitis.
- 13.Jain P., Rathee M. StatPearls . Treasure Island (FL): StatPearls Publishing; 2023. Embryology, tongue. [PubMed] [Google Scholar]
- 14.Scarano A., Inchingolo F., Lorusso F. Facial skin temperature and discomfort when wearing protective face masks: thermal infrared imaging evaluation and hands moving the mask. International Journal of Environmental Research and Public Health . 2020;17(13) doi: 10.3390/ijerph17134624.4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanteri V., Cavagnetto D., Abate A., et al. Buccal bone changes around first permanent molars and second primary molars after maxillary expansion with a low compliance Ni–Ti leaf spring expander. International Journal of Environmental Research and Public Health . 2020;17(23) doi: 10.3390/ijerph17239104.9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suazo Galdames I. C., Cantín López M. G., Zavando Matamala D. A. Inferior alveolar nerve block anesthesia via the retromolar triangle, an alternative for patients with blood dyscrasias. Medicina Oral, Patologia Oral, Cirugia Bucal . 2008;13:E43–47. [PubMed] [Google Scholar]
- 17.Haggard P., de Boer L. Oral somatosensory awareness. Neuroscience & Biobehavioral Reviews . 2014;47:469–484. doi: 10.1016/j.neubiorev.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Huff T., Weisbrod L. J., Daly D. T. StatPearls . Treasure Island (FL): StatPearls Publishing; 2023. Neuroanatomy, cranial nerve 5 (trigeminal) [PubMed] [Google Scholar]
- 19.Inchingolo F., Ballini A., Mura S. A., et al. Use of platelet rich fibrin and bio-OSS/SINT-Oss for implant-prosthetic rehabilitation in maxillary atrophy with sinus pathology: a 48-month follow-up. European Journal of Inflammation . 2015;13(1):58–65. doi: 10.1177/1721727X15578346. [DOI] [Google Scholar]
- 20.Khoury J., Townsend G. Neural blockade anaesthesia of the mandibular nerve and its terminal branches: rationale for different anaesthetic techniques including their advantages and disadvantages. Anesthesiology Research and Practice . 2011;2011:7. doi: 10.1155/2011/307423.307423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy T. R., Grundy E. M. The inferior alveolar neurovascular bundle at the mandibular foramen. The Dental practitioner and dental record . 1969;20(2):41–48. [PubMed] [Google Scholar]
- 22.Bhardwaj P., Bhardwaj Y., Ram R., Parmar M., Ghezta N., Sinha A. Radiographic factors associated with inferior alveolar nerve exposure during mandibular third molar surgery and their influence on neurosensory deficit: a prospective study. Journal of Oral Biology and Craniofacial Research . 2022;12(6):818–822. doi: 10.1016/j.jobcr.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaeser B. F., August M. A., Donoff R. B., Kaban L. B., Dodson T. B. Panoramic radiographic risk factors for inferior alveolar nerve injury after third molar extraction. Journal of Oral and Maxillofacial Surgery . 2003;61(4):417–421. doi: 10.1053/joms.2003.50088. [DOI] [PubMed] [Google Scholar]
- 24.Misch C. E., Resnik R. Mandibular nerve neurosensory impairment after dental implant surgery: management and protocol. Implant Dentistry . 2010;19(5):378–386. doi: 10.1097/ID.0b013e3181effa92. [DOI] [PubMed] [Google Scholar]
- 25.Boyle J. O., Reid V. Complications of surgery of the oral cavity. In: Eisele D. W., Smith R. V., editors. Complications in Head and Neck Surgery . 2nd. Philadelphia: Mosby; 2009. pp. 257–266. [Google Scholar]
- 26.Farronato M., Farronato D., Giannì A. B., et al. Effects on muscular activity after surgically assisted rapid palatal expansion: a prospective observational study. Bioengineering . 2022;9(8) doi: 10.3390/bioengineering9080361.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haug R. H., Perrott D. H., Gonzalez M. L., Talwar R. M. The american association of oral and maxillofacial surgeons age-related third molar study. Journal of Oral and Maxillofacial Surgery . 2005;63(8):1106–1114. doi: 10.1016/j.joms.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Mason D. A. Lingual nerve damage following lower third molar surgery. International Journal of Oral and Maxillofacial Surgery . 1988;17(5):290–294. doi: 10.1016/S0901-5027(88)80005-5. [DOI] [PubMed] [Google Scholar]
- 29.Huff T., Weisbrod L. J., Daly D. T. StatPearls . Treasure Island (FL): StatPearls Publishing; 2024. Neuroanatomy, cranial nerve 5 (trigeminal) [PubMed] [Google Scholar]
- 30.Shafique S., M Das J. StatPearls . Treasure Island (FL): StatPearls Publishing; 2023. Anatomy, head and neck, maxillary nerve. [PubMed] [Google Scholar]
- 31.Inchingolo A. D., Patano A., Coloccia G., et al. Genetic pattern, orthodontic and surgical management of multiple supplementary impacted teeth in a rare, cleidocranial dysplasia patient: a case report. Medicina . 2021;57(12) doi: 10.3390/medicina57121350.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takezawa K., Ghabriel M., Townsend G. The course and distribution of the buccal nerve: clinical relevance in dentistry. Australian Dental Journal . 2018;63(1):66–71. doi: 10.1111/adj.12543. [DOI] [PubMed] [Google Scholar]
- 33.Rodella L. F., Buffoli B., Labanca M., Rezzani R. A review of the mandibular and maxillary nerve supplies and their clinical relevance. Archives of Oral Biology . 2012;57(4):323–334. doi: 10.1016/j.archoralbio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Cossellu G., Ugolini A., Beretta M., et al. Three-dimensional evaluation of slow maxillary expansion with leaf expander vs. rapid maxillary expansion in a sample of growing patients: direct effects on maxillary arch and spontaneous mandibular response. Applied Sciences . 2020;10(13) doi: 10.3390/app10134512.4512 [DOI] [Google Scholar]
- 35.La Monaca G., Vozza I., Giardino R., Annibali S., Pranno N., Cristalli M. P. Prevention of neurological injuries during mandibular third molar surgery: technical notes. Annali di Stomatologia . 2017;8(2):45–52. doi: 10.11138/ads/2017.8.2.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Israr M., Asim M. A., Israr A. R. Lingual nerve injury during extraction of mandibular third molars: are we extracting correctly? Journal of Shifa Tameer-e-Millat University . 2022;5(2):92–98. doi: 10.32593/jstmu/Vol5.Iss2.209. [DOI] [Google Scholar]
- 37.Long H., Zhou Y., Liao L., Pyakurel U., Wang Y., Lai W. Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews . Centre for Reviews and Dissemination (UK); 2012. Coronectomy vs total removal for third molar extraction: a systematic review. [DOI] [PubMed] [Google Scholar]
- 38.Inchingolo A. D., Malcangi G., Inchingolo A. M., et al. Benefits and implications of resveratrol supplementation on microbiota modulations: a systematic review of the literature. International Journal of Molecular Sciences . 2022;23(7) doi: 10.3390/ijms23074027.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loescher A. R., Smith K. G., Robinson P. P. Nerve damage and third molar removal. Dental Update . 2023;30(7) doi: 10.12968/denu.2003.30.7.375. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed S., Venigalla H., Mekala H. M., Dar S., Hassan M., Ayub S. Traumatic brain injury and neuropsychiatric complications. Indian Journal of Psychological Medicine . 2017;39(2):114–121. doi: 10.4103/0253-7176.203129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben Aziz M., Cascella M. StatPearls . Treasure Island (FL): StatPearls Publishing; 2023. Peripheral neurolytic blocks. [PubMed] [Google Scholar]
- 42.Schiavone M., Ziccardi V. B. Trigeminal nerve injuries in oral and maxillofacial surgery: a literature review. Frontiers of Oral and Maxillofacial Medicine . 2021;3 doi: 10.21037/fomm. [DOI] [Google Scholar]
- 43.Hillerup S., Jensen R. Nerve injury caused by mandibular block analgesia. International Journal of Oral and Maxillofacial Surgery . 2006;35(5):437–443. doi: 10.1016/j.ijom.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Pogrel M. A. Permanent nerve damage from inferior alveolar nerve blocks: a current update. Journal of the California Dental Association . 2012;40(10):795–797. doi: 10.1080/19424396.2012.12220955. [DOI] [PubMed] [Google Scholar]
- 45.Balzanelli M. G., Distratis P., Lazzaro R., et al. Analysis of gene single nucleotide polymorphisms in COVID-19 disease highlighting the susceptibility and the severity towards the infection. Diagnostics . 2022;12 doi: 10.3390/diagnostics12112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox K., Purcell J., Basso J., et al. Trigeminal neuralgia: a case study. Physiopedia . 2023 [Google Scholar]
- 47.Inchingolo A. D., Dipalma G., Inchingolo A. M., et al. The 15-months clinical experience of SARS-CoV-2: a literature review of therapies and adjuvants. Antioxidants . 2021;10(6) doi: 10.3390/antiox10060881.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isacco C. G., Ballini A., De Vito D., et al. Prebiotics and Probiotics—Potential Benefits in Nutrition and Health . Intechopen; 2020. Probiotics in health and immunity: a first step toward understanding the importance of microbiota system in translational medicine. [DOI] [Google Scholar]
- 49.Bašić Kes V., Zadro Matovina L. Accommodation to diagnosis of trigeminal neuralgia. Acta Clinica Croatica . 2017;56:157–161. doi: 10.20471/acc.2017.56.01.21. [DOI] [PubMed] [Google Scholar]
- 50.Radoš I. Treatment options for trigeminal neuralgia. Acta Clinica Croatica . 2022;61:96–102. doi: 10.20471/acc.2022.61.s2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarano A., Assenza B., Inchingolo F., Mastrangelo F., Lorusso F. New implant design with midcrestal and apical wing thread for increased implant stability in single postextraction maxillary implant. Case Reports in Dentistry . 2019;2019:4. doi: 10.1155/2019/9529248.9529248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bataineh A. B. Sensory nerve impairment following mandibular third molar surgery. Journal of Oral and Maxillofacial Surgery . 2001;59(9):1012–1017. doi: 10.1053/joms.2001.25827. [DOI] [PubMed] [Google Scholar]
- 53.Hasegawa T., Ri S., Shigeta T., et al. Risk factors associated with inferior alveolar nerve injury after extraction of the mandibular third molar—a comparative study of preoperative images by panoramic radiography and computed tomography. International Journal of Oral and Maxillofacial Surgery . 2013;42(7):843–851. doi: 10.1016/j.ijom.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 54.Agbaje J. O., Van de Casteele E., Hiel M., Verbaanderd C., Lambrichts I., Politis C. Neuropathy of trigeminal nerve branches after oral and maxillofacial treatment. Journal of Maxillofacial and Oral Surgery . 2016;15(3):321–327. doi: 10.1007/s12663-015-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romsa B., Ruggiero S. L. Diagnosis and management of lingual nerve injuries. Oral and Maxillofacial Surgery Clinics of North America . 2021;33(2):239–248. doi: 10.1016/j.coms.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Delcanho R. E. Neuropathic implications of prosthodontic treatment. The Journal of Prosthetic Dentistry . 1995;73(2):146–152. doi: 10.1016/S0022-3913(05)80154-4. [DOI] [PubMed] [Google Scholar]
- 57.Juodzbalys G., Kubilius M. Clinical and radiological classification of the jawbone anatomy in endosseous dental implant treatment. Journal of Oral and Maxillofacial Research . 2013;4 doi: 10.5037/jomr.2013.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juodzbalys G., Wang H.-L., Sabalys G. Injury of the inferior alveolar nerve during implant placement: a literature review. Journal of Oral and Maxillofacial Research . 2011;2 doi: 10.5037/jomr.2011.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Comuzzi L., Tumedei M., Romasco T., et al. Insertion torque, removal torque, and resonance frequency analysis values of ultrashort, short, and standard dental implants: an in vitro study on polyurethane foam sheets. Journal of Functional Biomaterials . 2023;14(1) doi: 10.3390/jfb14010010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doh R.-M., Shin S., You T. M. Delayed paresthesia of inferior alveolar nerve after dental surgery: case report and related pathophysiology. Journal of Dental Anesthesia and Pain Medicine . 2018;18(3):177–182. doi: 10.17245/jdapm.2018.18.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagheri S. C., Meyer R. A. When to refer a patient with a nerve injury to a specialist. The Journal of the American Dental Association . 2014;145(8):859–861. doi: 10.14219/jada.2014.45. [DOI] [PubMed] [Google Scholar]
- 62.Azzam P., Mroueh M., Francis M., Abou Daher A., Zeidan Y. H. Radiation-induced neuropathies in head and neck cancer: prevention and treatment modalities. ecancermedicalscience . 2020;14 doi: 10.3332/ecancer.2020.1133.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inchingolo A. D., Pezzolla C., Patano A., et al. Experimental analysis of the use of cranial electromyography in athletes and clinical implications. International Journal of Environmental Research and Public Health . 2022;19(13) doi: 10.3390/ijerph19137975.7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibi M. Inflammation and temporomandibular joint derangement. Biological and Pharmaceutical Bulletin . 2019;42(4):538–542. doi: 10.1248/bpb.b18-00442. [DOI] [PubMed] [Google Scholar]
- 65.Dydyk A. M., Sizemore D. C., Trachsel L. A., Dulebohn S. C., Porter B. R. StatPearls . Treasure Island (FL): StatPearls Publishing; 2023. Tennessee controlled substance prescribing for acute and chronic pain. [PubMed] [Google Scholar]
- 66.Chen R.-P., Wang Q.-H., Li M.-Y., et al. Progress in diagnosis and treatment of acute injury to the anterior talofibular ligament. World Journal of Clinical Cases . 2023;11(15):3395–3407. doi: 10.12998/wjcc.v11.i15.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malcangi G., Patano A., Palmieri G., et al. Oral piercing: a pretty risk-a scoping review of local and systemic complications of this current widespread fashion. International Journal of Environmental Research and Public Health . 2023;20(9) doi: 10.3390/ijerph20095744.5744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inchingolo F., Dipalma G., Paduanelli G., et al. Computer-based quantification of an atraumatic sinus augmentation technique using CBCT. Journal of Biological Regulators and Homeostatic Agents . 2019;33(6 Suppl. 2):31–39. [PubMed] [Google Scholar]
- 69.Sonawane K., Dixit H., Thota N., Jayaraj A., Balavenkatasubramanian J. Knowing it before blocking it, the ABCD of the peripheral nerves: part D (approach to the patient with nerve injuries) Cureus . 2023;15 doi: 10.7759/cureus.41782.e41782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bage T., Power D. M. Iatrogenic peripheral nerve injury: a guide to management for the orthopaedic limb surgeon. EFORT Open Reviews . 2021;6(8):607–617. doi: 10.1302/2058-5241.6.200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palermo A., Giannotti L., Di Chiara Stanca B., et al. Use of CGF in oral and implant surgery: from laboratory evidence to clinical evaluation. International Journal of Molecular Sciences . 2022;23(23) doi: 10.3390/ijms232315164.15164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barakat K., Hafez A., Zaki M. A new surgical technique for treatment of neuropathic pain conceived by compressed inferior alveolar nerve. Egyptian Journal of Oral and Maxillofacial Surgery . 2021;12(3):216–221. doi: 10.21608/omx.2021.70052.1117. [DOI] [Google Scholar]
- 73.Carballo Cuello C. M., De Jesus O. StatPearls . Treasure Island (FL): StatPearls Publishing; 2023. Neurapraxia. [PubMed] [Google Scholar]
- 74.Hussain G., Wang J., Rasul A., et al. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. International Journal of Biological Sciences . 2020;16(1):116–134. doi: 10.7150/ijbs.35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ziccardi V. B., Steinberg M. J. Timing of trigeminal nerve microsurgery: a review of the literature. Journal of Oral and Maxillofacial Surgery . 2007;65(7):1341–1345. doi: 10.1016/j.joms.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 76.Guerrero M. E., Nackaerts O., Beinsberger J., Horner K., Schoenaers J., Jacobs R. Inferior alveolar nerve sensory disturbance after impacted mandibular third molar evaluation using cone beam computed tomography and panoramic radiography: a pilot study. Journal of Oral and Maxillofacial Surgery . 2012;70(10):2264–2270. doi: 10.1016/j.joms.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 77.Stawicki S. P. A. Trends in nonoperative management of traumatic injuries—a synopsis. International Journal of Critical Illness and Injury Science . 2017;7(1):38–57. doi: 10.4103/IJCIIS.IJCIIS_7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrington M. J., Snyder G. L. Neurologic complications of regional anesthesia. Current Opinion in Anaesthesiology . 2011;24(5):554–560. doi: 10.1097/ACO.0b013e32834ae1f7. [DOI] [PubMed] [Google Scholar]
- 79.Kim H. J., Park S. H., Shin H. Y., Choi Y. S. Brachial plexus injury as a complication after nerve block or vessel puncture. The Korean Journal of Pain . 2014;27(3):210–218. doi: 10.3344/kjp.2014.27.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pozza D. H., Tavares I., Cruz C. D., Fonseca S. Spinal cord injury and complications related to neuraxial anaesthesia procedures: a systematic review. International Journal of Molecular Sciences . 2023;24(5) doi: 10.3390/ijms24054665.4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avidan M. S., Maybrier H. R., Abdallah A. B., et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. The Lancet . 2017;390(10091):267–275. doi: 10.1016/S0140-6736(17)31467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakellariou V. I., Badilas N. K., Stavropoulos N. A., et al. Treatment options for brachial plexus injuries. ISRN Orthopedics . 2014;2014:10. doi: 10.1155/2014/314137.314137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwaiger K., Abed S., Russe E., et al. Management of radial nerve lesions after trauma or iatrogenic nerve injury: autologous grafts and neurolysis. Journal of Clinical Medicine . 2020;9(12) doi: 10.3390/jcm9123823.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MacKay B. J., Cox C. T., Valerio I. L., et al. Evidence-based approach to timing of nerve surgery. Annals of Plastic Surgery . 2021;87(3):e1–e21. doi: 10.1097/SAP.0000000000002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.John A. A., Rossettie S., Rafael J., Cox C. T., Ducic I., Mackay B. J. Clinical assessment of pain and sensory function in peripheral nerve injury and recovery: a systematic review of literature. Archives of Plastic Surgery . 2022;49:427–439. doi: 10.1055/s-0042-1748658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mashour G. A., Woodrum D. T., Avidan M. S. Neurological complications of surgery and anaesthesia. British Journal of Anaesthesia . 2015;114(2):194–203. doi: 10.1093/bja/aeu296. [DOI] [PubMed] [Google Scholar]
- 87.Mossie A., Regasa T., Neme D., Aweke Z., Zemedkun A., Hailu S. Evidence-based guideline on management of postoperative delirium in older people for low resource setting: systematic review article. International Journal of General Medicine . 2022;15:4053–4065. doi: 10.2147/IJGM.S349232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prabhakar N. K., Chadwick A. L., Nwaneshiudu C., et al. Management of postoperative pain in patients following spine surgery: a narrative review. International Journal of General Medicine . 2022;15:4535–4549. doi: 10.2147/IJGM.S292698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kung C.-Y., Wang Y.-M., Chan C.-P., Ju Y.-R., Pan W.-L. Evaluation of the mandibular lingual canal and anterior loop length to minimize complications associated with anterior mandibular surgeries: a cone-beam computed tomography study. Journal of Oral and Maxillofacial Surgery . 2017;75(10):2116.e1–2116.e13. doi: 10.1016/j.joms.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 90.Renton T., Hankins M., Sproate C., McGurk M. A randomised controlled clinical trial to compare the incidence of injury to the inferior alveolar nerve as a result of coronectomy and removal of mandibular third molars. British Journal of Oral and Maxillofacial Surgery . 2005;43(1):7–12. doi: 10.1016/j.bjoms.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Ozen T., Orhan K., Gorur I., Ozturk A. Efficacy of low level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head & Face Medicine . 2006;2(1) doi: 10.1186/1746-160X-2-3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwartz-Arad D., Lipovsky A., Pardo M., Adut O., Dolev E. Interpretations of complications following third molar extraction. Quintessence International . 2017;49:33–39. doi: 10.3290/j.qi.a39334. [DOI] [PubMed] [Google Scholar]
- 93.Baqain Z. H., Karaky A. A., Sawair F., Khaisat A., Duaibis R., Rajab L. D. Frequency estimates and risk factors for postoperative morbidity after third molar removal: a prospective cohort study. Journal of Oral and Maxillofacial Surgery . 2008;66(11):2276–2283. doi: 10.1016/j.joms.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 94.Akadiri O. A., Fasola A. O., Arotiba J. T. Incidence and risk factors for nerve injuries in mandibular third molar surgery. Nigerian Journal of Medicine . 2010;18(4):402–408. doi: 10.4314/njm.v18i4.51252. [DOI] [PubMed] [Google Scholar]
- 95.Cheung L. K., Leung Y. Y., Chow L. K., Wong M. C. M., Chan E. K. K., Fok Y. H. Incidence of neurosensory deficits and recovery after lower third molar surgery: a prospective clinical study of 4338 cases. International Journal of Oral and Maxillofacial Surgery . 2010;39(4):320–326. doi: 10.1016/j.ijom.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 96.Pogrel M. A., Jergensen R., Burgon E., Hulme D. Long-term outcome of trigeminal nerve injuries related to dental treatment. Journal of Oral and Maxillofacial Surgery . 2011;69(9):2284–2288. doi: 10.1016/j.joms.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 97.Gallas-Torreira M. M., Reboiras-López M. D., García-García A., Gándara-Rey J. Mandibular nerve paresthesia caused by endodontic treatment. Medicina Oral : Organo Oficial de la Sociedad Espanola de Medicina Oral y de la Academia Iberoamericana de Patologia y Medicina Bucal . 2003;8:299–303. [PubMed] [Google Scholar]
- 98.Wu Y., Lan Y., Mao J., Shen J., Kang T., Xie Z. The interaction between the nervous system and the stomatognathic system: from development to diseases. International Journal of Oral Science . 2023;15(1) doi: 10.1038/s41368-023-00241-4.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rink-Notzon S., Reuscher J., Nohroudi K., Manthou M., Gordon T., Angelov D. N. Trigeminal sensory supply is essential for motor recovery after facial nerve injury. International Journal of Molecular Sciences . 2022;23(23) doi: 10.3390/ijms232315101.15101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muntean A., Mzoughi S. M., Pacurar M., et al. Silver diamine fluoride in pediatric dentistry: effectiveness in preventing and arresting dental caries—a systematic review. Children . 2024;11(4) doi: 10.3390/children11040499.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarikov R., Juodzbalys G. Inferior alveolar nerve injury after mandibular third molar extraction: a literature review. Journal of Oral and Maxillofacial Research . 2014;5 doi: 10.5037/jomr.2029.283X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanmartí-Garcia G., Valmaseda-Castellón E., Gay-Escoda C. Does computed tomography prevent inferior alveolar nerve injuries caused by lower third molar removal? Journal of Oral and Maxillofacial Surgery . 2012;70(1):5–11. doi: 10.1016/j.joms.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 103.Pogrel M. A. The results of microneurosurgery of the inferior alveolar and lingual nerve. Journal of Oral and Maxillofacial Surgery . 2002;60(5):485–489. doi: 10.1053/joms.2002.31841. [DOI] [PubMed] [Google Scholar]
- 104.Righesso L. A. R., Gil L. F., Pantoja D. S. M. C., Marin C., Granato R., Gil J. N. Evaluation of neurosensory disturbances of the inferior alveolar nerve after intraoral verticosagittal ramus osteotomy. Journal of Stomatology, Oral and Maxillofacial Surgery . 2018;119(3):192–195. doi: 10.1016/j.jormas.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 105.Shadmehr E., Shekarchizade N. Endodontic periapical lesion-induced mental nerve paresthesia. Journal of Dental Research . 2015;12:192–196. [PMC free article] [PubMed] [Google Scholar]
- 106.Bryce T. N., Budh C. N., Cardenas D. D., et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. The Journal of Spinal Cord Medicine . 2007;30(5):421–440. doi: 10.1080/10790268.2007.11753405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Imhoff B., Ahlers M. O., Hugger A., et al. Occlusal dysesthesia—a clinical guideline. Journal of Oral Rehabilitation . 2020;47(5):651–658. doi: 10.1111/joor.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bigley G. K. Sensation. In: Walker H. K., Hall W. D., Hurst J. W., editors. Clinical Methods: The History, Physical, and Laboratory Examinations . Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 109.Gupte R. P., Brooks W. M., Vukas R. R., Pierce J. D., Harris J. L. Sex differences in traumatic brain injury: what we know and what we should know. Journal of Neurotrauma . 2019;36(22):3063–3091. doi: 10.1089/neu.2018.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Magni A., Agostoni P., Bonezzi C., et al. Management of osteoarthritis: expert opinion on NSAIDs. Pain and Therapy . 2021;10(2):783–808. doi: 10.1007/s40122-021-00260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bellocchio L., Bordea I. R., Ballini A., et al. Environmental issues and neurological manifestations associated with COVID-19 pandemic: new aspects of the disease? International Journal of Environmental Research and Public Health . 2020;17(21) doi: 10.3390/ijerph17218049.8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tofthagen C. Threats to validity in retrospective studies. Journal of the Advanced Practitioner in Oncology . 2012;3:181–183. [PMC free article] [PubMed] [Google Scholar]


