Abstract
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal tumors of the gastrointestinal tract, usually arising in the stomach or in the small bowel. Most GISTs are diagnosed early due to the presence of symptoms (e.g., abdominal discomfort/pain, anemia, etc.); at times, diagnosis could be incidental (e.g., ultrasound or endoscopic examinations performed for other reasons, surgical intervention for a different disease, etc.). Diagnosis occurs when the tumor is already metastatic in 10-20% of cases. The most common metastatic sites are liver, peritoneum, and loco-regional lymph nodes. Here, we present the case of a male patient with an atypical presentation of disease: as a matter of fact, during his oncological history, he developed metastases in unlikely sites, such as penis, scrotum, myocardium, and soft tissues.
Keywords: GIST, metastases, exon 11 mutation, imatinib, case report
Introduction
Gastrointestinal Stromal Tumors (GISTs) are malignant mesenchymal tumors derived from interstitial cells of Cajal or their precursors with an annual incidence of 10-15 cases per million. The pathogenesis is associated in 95% of cases with mutations of tyrosine kinase receptor gene KIT (CD117) or Platelet Derived Growth Factor Receptor-A (PDGFR-A), but genomic alterations such as Neurotrophic Tyrosine Receptor Kinase (NTRK) rearrangements and B-Rapidly Accelerated Fibrosarcoma (BRAF) mutations could be rarely found.
GISTs belong to the soft tissue sarcoma family and are the most common ones in the gastrointestinal tract. The prevalent primary sites are stomach (60-70%) and small intestine (20-30%), whereas less frequent sites are colon and esophagus (1-5%) (1).
Detecting GISTs can sometimes be challenging, as they may be entirely asymptomatic or present with nonspecific signs and symptoms, such as vague abdominal discomfort or anemia caused by chronic bleeding that can also lead to positive fecal occult blood test. In other cases, GISTs may present acutely with massive bleeding and hemodynamic instability (2).
Tyrosine Kinase Inhibitors (TKIs) represents the backbone of GIST treatment in the metastatic setting (3); in particular, patients’ survival rates have improved significantly since the approval of imatinib (4, 5). Most of GISTs show sensitivity to imatinib, in particular when a mutation of the exon 11 of KIT is detected, and a dose of 400 mg/d is recommended. When a mutation of the exon 9 of the KIT gene occurs, a double dose of imatinib (800 mg/d) is indicated (6).
At progression, sunitinib (50 mg 4 weeks on/2 weeks off or 37.5 mg continuously) is the standard second line of therapy (7). At further failure, regorafenib represents another approved treatment (8). In May 2020, FDA approved ripretinib for patients with metastatic GISTs that had progressed to three or more TKIs, including imatinib (9).
Secondary KIT mutations represent the most frequent mechanism of acquired resistance to imatinib and other TKIs, leading to progressive disease and the need to change the therapeutic strategy (10).
In rare cases, NTRK rearrangements and BRAF mutations can be discovered, which could be sensitive respectively to NTRK inhibitors (such as entrectinib or larotrectinib) and BRAF inhibitors (11).
The most common sites of metastasizing are liver (50-60%), peritoneum (30-40%) and loco-regional lymph nodes (12). Nevertheless, in some cases GISTs can metastasize in less common sites like bone, bone marrow, intracranial area, skin and subcutaneous tissue, skeletal muscle, orbit, pancreas, spleen, heart, gonads, kidneys, and bladder.
Here, we present an unusual case of GIST, arising in a male patient, and presenting with a high burden of disease at baseline and the development of multiple metastases in uncommon sites.
Case report
In March 2019, a 57-year-old man in good clinical conditions (Eastern Cooperative Oncology Group Performance Status 0) and without significant comorbidities or familiarity for oncological diseases underwent ultrasound examination of the abdomen for abdominal discomfort and night sweating over the past three months; furthermore, palpable masses localized in the right hypochondrium and epigastrium had become evident. Ultrasound examination showed multiple expansive lesions in the right hepatic lobe (the biggest one measuring 14 cm in the longest diameter) and suspected peritoneal localizations. Computed Tomography (CT) scan confirmed both hepatic involvement (biggest lesion: 16x14 cm in S6) and peritoneal involvement (biggest lesion: 16x11cm, close to the descending colon). Eventually, a Positron Emission Tomography (PET) examination was performed, with uptake in the lesions highlighted at the CT scan. No primitive tumor was found.
Liver biopsy demonstrated liver metastasis of spindle cell GIST (IHC: CD117+, DOG1+, MDM2+, Ki67 20%). A mutation in exon 11 of the KIT gene was detected (p.Thr574_Asp579dup), whereas BRAF, PDGFR-A, PDGFR-B, SDHA genes were all wild-type and immunohistochemistry analysis for pan-TRK was negative.
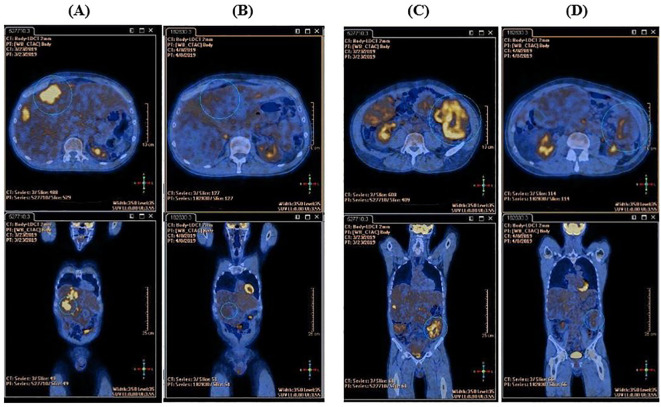
In March 2019, first-line therapy with imatinib (400 mg/d) was started. PET examination, performed two weeks after treatment start, demonstrated near-complete metabolic response with absence of FDG uptake in the known sites of disease ( Figure 1 ).
Figure 1.
Comparison between the basal FDG-PET and the FDG-PET performed after two weeks of imatinib therapy. (A) High tracker uptake in liver metastasis at baseline. (B) Complete metabolic response in liver metastasis after two weeks of imatinib therapy. (C) High tracker uptake in peritoneal metastasis at baseline. (D) Near-complete metabolic response in peritoneal metastasis after two weeks of imatinib therapy.
Best response at CT scan-based restaging was partial response (PR); treatment was well-tolerated.
Unfortunately, in August 2021, CT scan demonstrated hepatic and peritoneal disease progression (PD); imatinib’s dose was escalated to 800 mg/d, with further hepatic PD. Second-line therapy with sunitinib was then started in December 2021.
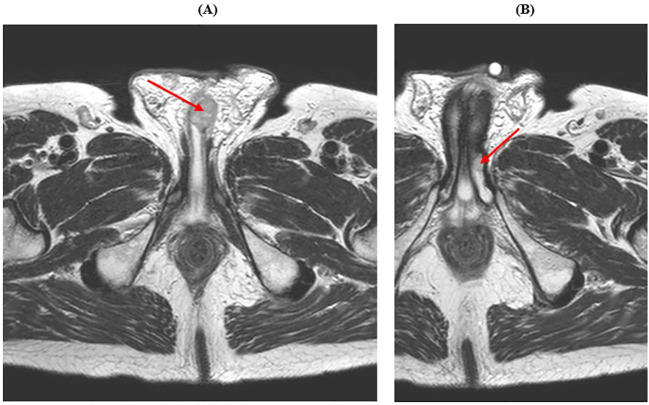
In January 2022, the patient reported urinary discomfort and swelling at the base of the penis; pelvic Magnetic Resonance Imaging (MRI) demonstrated a 16x26x13 mm nodule in the corpus spongiosum, which caused compression on the urethra “ab estrinseco”, and a 7x9x10 mm lesion in the corpus cavernosum ( Figure 2 ).
Figure 2.
Pelvic MRI showing male genital system metastases. (A) The red arrow indicates the lesion in the corpus spongiosum. (B) The red arrow indicates the lesion in the corpus cavernosum.
In February 2022, the patient was admitted to the Urology Department and the corpus spongiosum lesion was removed; histological examination was compatible with the diagnosis of GIST (IHC: CD117+, DOG1+) and the margins of resection were free of neoplasia (R0). The urinary problem was resolved and the sunitinib therapy was restarted.
CT scan performed in April 2022 showed further hepatic PD and third-line therapy with regorafenib was started. Best response was stable disease (SD), but the treatment was poorly tolerated, due to skin toxicity and hypertension.
In October 2022 MRI showed penile PD and appearance of a lymph node conglomerate in the right inguinal region; rechallenge with imatinib (400 mg/d) was started.
In February 2023 further penile PD, causing urinary discomfort, was documented by MRI together with the appearance of a lesion in the right gluteus medius and multiple scrotal nodules (biggest lesion: 38x32 mm); two scrotal nodules were removed in May 2023 and histological examination confirmed the diagnosis of GIST metastases, with again a R0 resection.
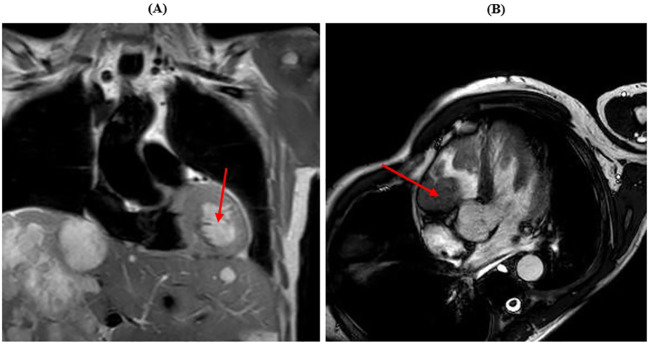
Shortly thereafter, following an episode of amaurosis fugax, echocardiogram was performed, with the evidence of two right and left ventricular masses, with conserved left ventricular function. Cardiac MRI confirmed a mass near the left ventricular apex (4 cm), a mass in the right ventricle under the tricuspid valve (5 cm) and another mass in the right atrium near the atrial septum (3 cm), with imaging strongly suggestive for GIST metastases ( Figure 3 ).
Figure 3.
Cardiac MRI showing myocardial metastases. (A) The red arrow shows the lesion in the left ventricle. (B) The red arrow shows the lesion in the right ventricle.
The patient eventually died, due to a cardiac arrest while sleeping, in July 2023.
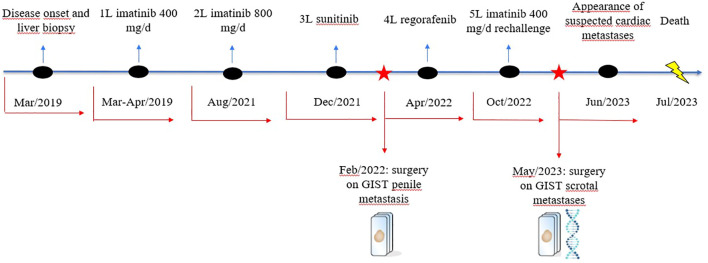
To sum up, in the ( Figure 4 ) is illustrated a schematic representation of the patient’s treatment history.
Figure 4.
Schematic representation of the patient’s treatment history.
Discussion
The unicity of the case presented here is related to the atypical disease behavior. GISTs most frequently metastasize to the liver and peritoneum and very infrequently outside the abdomen. A recent review regarding the rare metastatic localization of GIST showed that cardiac and soft tissue metastases are uncommon (1% of cases), but male genital system metastases are even more infrequent (< 1% of cases) (13). To the best of our knowledge, this is the first report of a patient with the concomitant presence of these three unusual localizations. While in this case the high disease burden at presentation may have favored widespread dissemination to unusual localizations, the reasons why sometimes GISTs metastasize to these rare sites remain unclear.
The specific mutation of the KIT gene (p.Thr574_Asp579dup) is an in-frame insertion resulting in a duplication of a stretch of 6 aminoacids located in the juxtamembrane domain of the protein. At present, it is not surveyed and functionally validated, but it is considered to be likely oncogenic and druggable by FDA- and EMA-approved imatinib (AMP tier 1, ESCAT tier 1) (14); only a few GISTs cases exhibiting this specific mutation have been reported in the literature (15). Although little is known on the biology deriving from this specific mutation, the clinical behavior observed in the case reported here is not routinely expected from GISTs harboring exon 11 mutations of the KIT gene: this subtype of tumors is frequently associated with an indolent course and better prognosis, while exon 9-mutated GISTs occur more frequently in extra-gastric sites, metastasize to unusual locations, and show a more aggressive and therapy-resistant course (16).
The duration of response to imatinib in this case is in line with expectations from registration studies (median time to treatment failure in responding patients: 122 weeks, 95% CI 106-147 weeks) (17), which is notable considering the “bulky” onset of disease, which is associated with a poorer prognosis. Early FDG-PET evaluation after treatment start showed rapid decreases of the Standardized Uptake Value (SUV), for the objective clinical response and satisfactory outcome subsequently observed, again in line with previous literature (18).
Molecular evolution of the disease was assessed by Next Generation Sequencing (NGS) analysis (Myriapod NGS Cancer Panel DNA-based, Illumina Platform, Diatech Pharmacogenetics) performed on one of the scrotal metastases removed in May 2023, at the time of the fourth progression (after imatinib, sunitinib, regorafenib, and imatinib rechallenge). Alongside with the same exon 11 mutation detected at baseline, we found another exon 17 KIT alteration (p.D820Y). As discussed above, secondary KIT mutations are a frequent mechanism of acquired resistance in patients pre-treated with either imatinib or other TKIs (10) and exon 17 mutations (including p.D820Y) seem to be the most frequent mechanisms of resistance to sunitinib (19).
Besides unusual presentation and uncommon sites of metastatic spread, another peculiar aspect may be of interest in this case: two of these unusual localizations (penile localizations to corpus spongiosum and scrotal lesions) were treated by surgical removal, based on the appearance of symptoms (in February 2022 and May 2023, respectively). In the metastatic setting, surgery is not routinely recommended for GISTs; however, in our case, surgery of progressing lesions has certainly improved patient’s quality of life and psychological balance and may have impacted on long-term outcome, for example by avoiding potential urinary obstruction and the need for more invasive interventions during second-line sunitinib.
Conclusion
This case report shows an unusual presentation and clinical behavior of GIST, only partially explained by the specific mutational landscape and molecular evolution; in that respect, extended molecular profiling at presentation and non-invasive molecular monitoring could be important tools to guarantee better treatments for patients. Moreover, multidisciplinary integration with surgery and/or local ablative treatments may be employed to treat oligoprogression and maintain better quality of life.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MR: Writing – original draft, Writing – review & editing. FS: Writing – original draft, Writing – review & editing. MZ: Writing – review & editing. AM: Writing – review & editing. MP: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing.
Conflict of interest
MM: Honoraria: AstraZeneca, MSD Oncology, Ipsen, Hippocrates Research, Viatris, Servier. Consulting of Advisory Role: Astrazeneca, MSD Oncology, Janssen Oncology. Research funding: Roche. Travel, Accommodation, Expenses: AstraZeneca. Other relationship: Novartis, Oncosil.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, et al. Gastrointestinal stromal tumours: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv68–78. doi: 10.1093/annonc/mdy095 [DOI] [PubMed] [Google Scholar]
- 2. Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, Constantinescu G, et al. Gastrointestinal stromal tumors-A mini review. J Pers Med. (2021) 11:694. doi: 10.3390/jpm11080694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. (2021) 14:2. doi: 10.1186/s13045-020-01026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay J-Y, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. (2004) 364:1127–34. doi: 10.1016/S0140-6736(04)17098-0 [DOI] [PubMed] [Google Scholar]
- 5. Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. (2008) 26:626–32. doi: 10.1200/JCO.2007.13.4452 [DOI] [PubMed] [Google Scholar]
- 6. The Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) . Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. J Clin Oncol. (2010) 28:1247–53. doi: 10.1200/JCO.2009.24.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. (2006) 368:1329–38. doi: 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 8. Demetri GD, Reichardt P, Kang Y-K, Blay J-Y, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. (2013) 381:295–302. doi: 10.1016/S0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Mehren M, Heinrich MC, George S, Zalcberg JR, Bauer S, Gelderblom H, et al. 1540P Ripretinib as ≥4th-line treatment in patients with advanced gastrointestinal stromal tumor: Long-term update from the phase III INVICTUS study. Ann Oncol. (2021) 32:S1120–1. doi: 10.1016/j.annonc.2021.08.870 [DOI] [Google Scholar]
- 10. Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. (2008) 216:64–74. doi: 10.1002/path.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falkenhorst J, Hamacher R, Bauer S. New therapeutic agents in gastrointestinal stromal tumours. Curr Opin Oncol. (2019) 31:322–8. doi: 10.1097/CCO.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 12. Menge F, Jakob J, Kasper B, Smakic A, Gaiser T, Hohenberger P. Clinical presentation of gastrointestinal stromal tumors. Visc Med. (2018) 34:335–40. doi: 10.1159/000494303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu X, Liang X, Wen K. Clinical characteristics and prognosis of gastrointestinal stromal tumors with rare site metastasis (Review). Oncol Lett. (2022) 24:453. doi: 10.3892/ol.2022.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Available online at: https://www.oncokb.org/gene/KIT/T574insTQLPYD#tab=FDA. (Accessed May 30, 2024).
- 15. Silva M, Veiga I, Ribeiro FR, Vieira J, Pinto C, Pinheiro M, et al. Chromosome copy number changes carry prognostic information independent of KIT/PDGFRA point mutations in gastrointestinal stromal tumors. BMC Med. (2010) 8:26. doi: 10.1186/1741-7015-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Napolitano A, Thway K, Smith MJ, Huang PH, Jones RL. KIT exon 9-mutated gastrointestinal stromal tumours: biology and treatment. Chemotherapy. (2022) 67:81–90. doi: 10.1159/000521751 [DOI] [PubMed] [Google Scholar]
- 17. Serrano C, George S. Recent advances in the treatment of gastrointestinal stromal tumors. Ther Adv Med Oncol. (2014) 6:115–27. doi: 10.1177/1758834014522491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weeda YA, Kalisvaart GM, van Velden FHP, Gelderblom H, van der Molen AJ, Bovee JVMG, et al. Early prediction and monitoring of treatment response in gastrointestinal stromal tumors by means of imaging: A systematic review. Diagnostics. (2022) 12:2722. doi: 10.3390/diagnostics12112722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W-L, Conley A, Reynoso D, Nolden L, Lazar AJ, George S, et al. Mechanisms of resistance to imatinib and sunitinib in gastrointestinal stromal tumor. Cancer Chemotherapy Pharmacol. (2011) 67:15–24. doi: 10.1007/s00280-010-1513-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.