Abstract
In the present paper, we report the antimicrobial efficacy of three monoterpenes [linalyl acetate, (+)menthol, and thymol] against the gram-positive bacterium Staphylococcus aureus and the gram-negative bacterium Escherichia coli. For a better understanding of their mechanisms of action, the capability of these three monoterpenes to damage biomembranes was evaluated by monitoring the release, following exposure to the compounds under study, of the water-soluble fluorescent marker carboxyfluorescein from unilamellar vesicles with different lipidic compositions (phosphatidylcholine, phosphatidylcholine/phosphatidylserine [9:1], phosphatidylcholine/stearylamine [9:1], and phosphatidylglycerol/cardiolipin [9:1]). Furthermore, the interaction of the terpenes tested with dimyristoylphosphatidylcholine multilamellar vesicles as model membranes was monitored by means of differential scanning calorimetry. Finally, the results were related to the relative lipophilicity and water solubility of the compounds examined. Taken together, our findings lead us to speculate that the antimicrobial effect of (+)menthol, thymol, and linalyl acetate may result, at least partially, from a perturbation of the lipid fraction of microorganism plasma membrane, resulting in alterations of membrane permeability and in leakage of intracellular materials. Besides being related to physicochemical characteristics of the drugs (such as lipophilicity and water solubility), this effect seems to be dependent on lipid composition and net surface charge of microbial membranes. Furthermore, the drugs might cross the cell membranes, penetrating into the interior of the cell and interacting with intracellular sites critical for antibacterial activity.
Essential oils are mixtures of compounds characterized by their capacity to generate flavor or aroma and are generally obtained from spices, aromatic herbs, fruits, and flowers (12, 14). Analysis of essential oils shows that of the different constituent compounds, terpenoids are the most abundant and are present as either hemiterpenes, monoterpenes, or sesquiterpens and as their derivatives. High concentrations of monoterpenes in plant may repel many potential predators but may also attract other animals; furthermore, some monoterpenes are involved in plant-plant interactions.
Several previous reports were focused on the antimicrobial properties of essential oils and of the main monoterpenes found in them. In fact, many terpenes are known to be active against a wide variety of microorganisms, including gram-positive and gram-negative bacteria and fungi (3, 17). The antibacterial properties of essential oils and their components are exploited in such diverse commercial products as dental root canal sealers, antiseptics, food preservatives, and feed supplements (3, 17).
Toxic effects on membrane structure and function have been generally used to explain the antimicrobial action of essential oils and their monoterpenoid components. In fact, as a result of their lipophilic character, monoterpenes will preferentially partition from an aqueous phase into membrane structures (22, 23). This results in membrane expansion, increased membrane fluidity and permeability, disturbance of membrane-embedded proteins, inhibition of respiration, and alteration of ion transport processes. Helander et al. (9) have described the effects of selected essential oil components on outer membrane permeability in gram-negative bacteria, evidencing that monoterpene uptake is also determined by the permeability of the outer envelope of the target microorganism. However, specific mechanisms involved in the antimicrobial action of monoterpenes remain poorly characterized.
In the present paper, we report on the antimicrobial efficacy of three monoterpenes [linalyl acetate, (+)menthol, and thymol] against the gram-positive bacterium Staphylococcus aureus and the gram-negative bacterium Escherichia coli.
For a better understanding of their mechanisms of action, the capability of these three monoterpenes to damage biomembranes was evaluated by monitoring the release, following exposure to the compounds under study, of the water-soluble fluorescent marker carboxyfluorescein (CF) from large unilamellar vesicles (LUVs) with different lipid compositions. Lipid constituents of cell membrane are really important for its function; in fact, they provide the membrane with its barrier function and play a role in a variety of processes in the bacterial cell. However, the world of bacteria is large, and the diversity of lipids, especially in gram-positive bacteria, is immense (10, 13). LUVs employed in our study were constituted of phosphatidylcholine (PC), phosphatidylcholine/phosphatidylserine (PS) (9:1), phosphatidylcholine/stearylamine (SA) (9:1), or phosphatidylglycerol (PG)/cardiolipin (CL) (9:1) to determine whether the net surface charge of the target membrane (neutral for PC or dimyristoylphosphatidylcholine [DMPC] LUVs, positive for PC/SA LUVs, and negative for PC/PS LUVs) or a particular membrane composition (as well as carrying a negative charge at physiological pH, PG and CL are acidic phospholipids of bacterial membrane) may influence the susceptibility to the compounds tested.
Furthermore, since a knowledge of drug interaction with lipid model membranes can help to understand the mode of action of terpenoids on the membrane microenvironment, the interaction of the three terpenes under study with model membranes was monitored by means of the differential scanning calorimetry (DSC) technique. For this aim, DSC was employed to evaluate melting temperature depression of pure DMPC liposomes caused by the dissolution of the terpenes tested in the membrane as well as their migration through aqueous medium and the interaction with empty liposomes.
Finally, the results were also related to the relative lipophilicity and water solubility of the compounds examined.
MATERIALS AND METHODS
Drugs used.
Linalyl acetate (97%), (+)menthol (99%), thymol (>99.5%), l-α-phosphatidylcholine, phosphatidylserine, stearylamine, phosphatidylglycerol, cardiolipin, and dimyristoylphosphatidylcholine were purchased from Fluka (Milan, Italy). Crude carboxyfluorescein (Fluka) was purified by a method described previously by Mercadal et al. (15). All other chemicals were of reagent grade. Herein, the findings concerning (+)menthol are described; however, similar results were obtained by using (−)menthol and are not reported in this paper.
Microbiological studies.
Two American Type Culture Collection (ATCC) standard strains were used: S. aureus ATCC 6538P and E. coli ATCC 15221.
The antimicrobial activity was evaluated by determining the MIC according to the M7-A5 guidelines established by the National Committee for Clinical Laboratory Standards (18). The MICs of substances for the bacterial strains were determined on 96-well culture plates by a microdilution method using Mueller-Hinton broth (Becton-Dickinson, Milan, Italy). Eight twofold dilutions of the samples were carried out, starting from the concentration of 0.5% (wt/vol) (5% Tween 20). All preparations were sterilized with a 0.22-μm filter. The wells were inoculated with a microorganism suspension at a density of 105 cells/ml. The plates were incubated at 37°C for 24 h. After incubation, the plates were observed in order to determine the MICs. Proper blanks were prepared simultaneously. Samples were tested in triplicate.
Carboxyfluorescein studies.
In the present study, we monitored the release of CF trapped in LUVs with different lipid composition (PC, PC/PS [9:1], PC/SA [9:1], and PG/CL [9:1]) following exposure to the terpenes under investigation (9, 24). Briefly, multilamellar liposome vesicles (MLVs) were obtained by freshly prepared chloroform/methanol concentrated lipid solutions. The solvent was removed under nitrogen in a rotoevaporator, and the resulting film was kept overnight under a vacuum to remove the residual solvent. The dried film was then suspended in buffer (150 mM NaCl-5 mM HEPES, pH 7.4) containing 20 mM CF, and the dispersion was vortexed intermittently for 20 min at room temperature. The LUVs were prepared by submitting the previously prepared MLV dispersion (approximately 1 mg ml−1) to extrusion through 100-nm polycarbonate membranes (Avestin Inc., Ottawa, Canada) in an extruder system (LiposoFast Basic; Avestin Inc.). Free CF was removed by passage of the dispersion through a column (1 by 30 cm) of Sephadex G-50, where the vesicles eluted with the void volume. Aliquots of eluted liposomes were used to determine the amount of phospholipid by the phosphorous assay (20).
Aliquots of the liposomal stock preparations (diluted to about 20 μg/ml) were incubated with various concentrations of the terpene to be tested at room temperature, and fluorescence was recorded continuously for 15 min (λex [wavelength of excitation], 490 nm; λem [wavelength of emission], 520 nm). At the end of each experiment, total CF was determined after the liposomes were lysed with 10% Triton X-100 (150 μl), after which the mixture was heated at 100°C for 5 min and mixed vigorously for 2 min at room temperature. All experiments were carried out in triplicate.
The rates of CF leakage are expressed as a percentage of total trapped CF released:
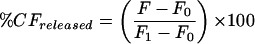 |
where F is the fluorescence intensity measured at a specified time, F0 is the fluorescence intensity measured at time zero, and Ft is the total fluorescence measured after Triton disruption. Ft is corrected for the dilution introduced by the addition of Triton. Incubation of liposomes with higher concentrations of Triton did not affect the value of Ft, indicating that the procedure resulted in a complete release of dye from the liposomes.
Calorimetric measurements.
DMPC MLVs were prepared in the absence and presence of increasing molar fractions of the terpenes under study by the following procedure previously described by Saija et al. (21). A chloroform-methanol (1:1, vol/vol) stock solution of DMPC was delivered in glass vials to contain the same amount of phospholipids (10 mg). Solvents were removed under a nitrogen flow in a rotary evaporator, and the resulting film was freeze dried under a vacuum to remove residual solvent. An exact terpene amount corresponding to the desired molar fraction was added to the phospholipid film. Liposomes were obtained by hydration with 50 mM Tris buffer (pH 7.4) and then heating at a temperature (37°C) above that of the DMPC gel-liquid crystalline phase transition and vortexing three times for 1 min.
Aliquots of 120 μl of lipid aqueous dispersion (5 mg of lipid), either pure or containing the terpenes under study, were transferred to a 160-μl DSC aluminium pan (Mettler Toledo Group, Greifensee, Switzerland), hermetically sealed, and submitted to DSC analysis. DSC was performed using a Mettler TC15 system equipped with a DSC-30 calorimetric cell and a TA STARe Mettler program (Mettler Toledo Group, Greifensee, Switzerland). The scan rate employed was 2°C/min, and the temperature range was 5 to 37°C. The sensitivity was automatically chosen as the maximum possible, and the reference pan was filled with Tris buffer solution. Enthalpies were evaluated from the peak area using the integration program of the TA processor, permitting the choice of different baselines and ranges of integration. After calorimetric scanning, all samples were extracted from the pan, and aliquots were used to quantify phospholipids using the phosphorous assay (20).
To study the capacity of the three examined terpenes to cross the model membrane, a kinetic experiment was carried out by putting a suspension of empty DMPC MLVs in contact with a fixed amount of pure terpene (0.09 molar fraction) placed at the bottom of a DSC crucible (19, 24). The crucible was gently shaken for 10 s and submitted to the following process: (i) a first scan between 5 and 37°C to detect the interaction between the compounds and model membrane, (ii) an isothermal period of 1 h at 37°C to permit the drug to permeate the lipid layer(s) in a disordered state at a temperature above the lipid transitional temperature, and (iii) a subsequent cooling scan between 37 and 5°C to restart the heating program. The whole procedure was performed at lest five times with increasing isothermal times until a near-constant drug-MLV interaction was observed.
Relative lipophilicity determination.
The relative lipophilicity (Rm) of the terpenes under investigation was measured by reversed-phase high-performance thin-layer chromatography (2). Briefly, Whatman KC 18F plates were used as the nonpolar stationary phase. The plates were dried at 105°C for 1 h before use. The mobile phase was a 2:1 (vol/vol) mixture of acetone and water. Each compound was dissolved in absolute ethanol (100 mg/ml), and 2 μl of solution was applied to the plate. The experiments were repeated five times with different disposition of the compounds on the plate. The retardation factor (Rf) values were expressed as the means of the five determinations. The Rm values were calculated from the experimental Rf values according to the formula Rm = log[(1/Rf) − 1]. Higher Rm values indicate higher lipophilicity.
RESULTS
The present paper reports the antimicrobial activity of three monoterpenes, linalyl acetate, (+)menthol, and thymol, against gram-positive and gram-negative bacteria (S. aureus and E. coli, respectively). Table 1 shows that each of the monoterpenes inhibits the growth of both of the microbial strains, although the gram-positive S. aureus appears more sensitive than the gram-negative E. coli toward all three compounds. The MICs demonstrate that thymol and, at a slightly lower degree, (+)menthol are considerably more toxic against S. aureus than linalyl acetate, whereas (+)menthol is the most toxic against E. coli.
TABLE 1.
Antimicrobial activity of thymol, (+)menthol, and linalyl acetate on two ATCC microbial strains
| Drug | MIC (mg/ml)
|
|
|---|---|---|
| S. aureus ATCC 6538P | E. coli ATCC 15221 | |
| Thymol | 0.31 | 5.00 |
| (+)Menthol | 0.62 | 2.50 |
| Linalyl acetate | 1.25 | 5.00 |
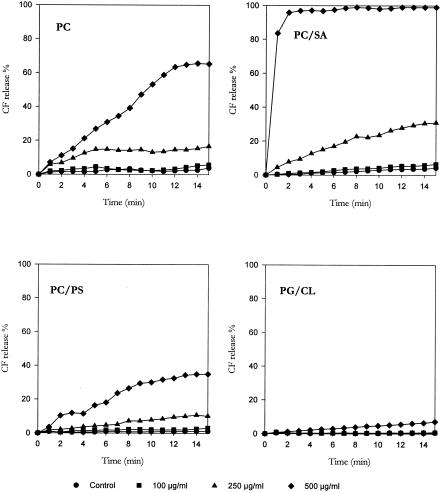
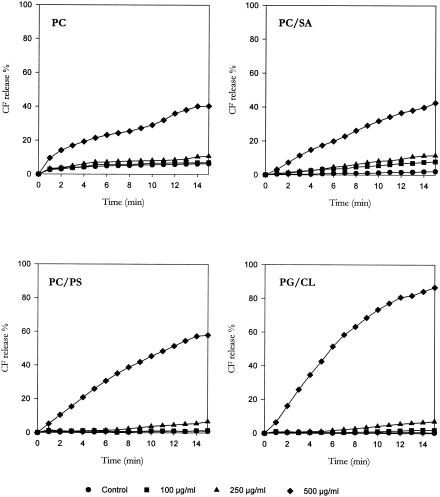
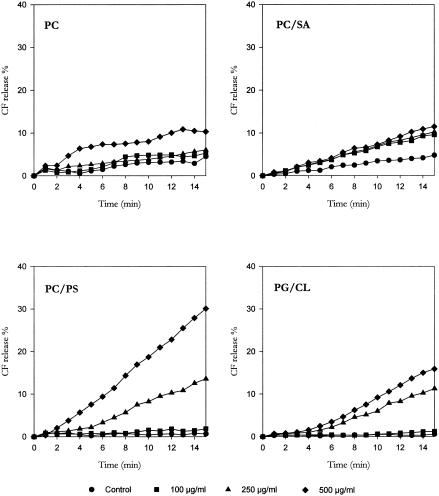
To characterize the mechanisms underlying these effects, the three monoterpenes were tested for their membrane-damaging activity by determining the release of CF entrapped in the intraliposomal space. Curves reported in Fig. 1, 2, and 3 illustrate the effects of monoterpene exposure on CF release from LUVs with different lipid compositions; the results, expressed as a percentage of the total trapped CF released in 15 min, are shown in Table 2.
FIG. 1.
Effect of thymol challenge on CF release from CF-encapsulated large unilamellar vesicles with different lipid compositions.
FIG. 2.
Effect of (+)menthol challenge on CF release from CF-encapsulated large unilamellar vesicles with different lipid compositions.
FIG. 3.
Effect of linalyl acetate challenge on CF release from CF-encapsulated large unilamellar vesicles with different lipid compositions.
TABLE 2.
CF release from liposomes with different lipid compositions following exposure to thymol, (+)menthol, and linalyl acetate
| Drug | Exposure concn (μg/ml) | CF releasea for lipid composition
|
|||
|---|---|---|---|---|---|
| PC | PC/SA (9:1) | PC/PS (9:1) | PG/CL (9:1) | ||
| (+)Menthol | 100 | 0.75 | 5.7 | 0.50 | 1.56 |
| 250 | 4.50 | 9.40 | 5.80 | 6.70 | |
| 500 | 34.10 | 40.42 | 57.04 | 86.29 | |
| Thymol | 100 | 1.88 | 2.17 | 1.98 | 0.18 |
| 250 | 12.78 | 26.67 | 9.05 | 0.39 | |
| 500 | 56.39 | 95.62 | 33.89 | 6.58 | |
| Linalyl acetate | 100 | 0.77 | 4.80 | 1.18 | 0.66 |
| 250 | 6.00 | 10.25 | 13.50 | 11.25 | |
| 500 | 5.79 | 6.70 | 16.35 | 15.31 | |
Data are expressed as the percent increase versus control of the total CF released in a 15-min period.
The basal rate of CF leakage from liposomes ranged from 2% to 5% for PC and PC/SA LUVs and from 0% to 3% for PC/PS and PG/CL LUVs. (+)Menthol and thymol, tested at the same concentrations, caused a concentration-dependent CF leakage from all types of LUVs employed, with the extent of dye release slowly increasing during the 15-min observation period. Conversely, only a slight CF release was observed from LUVs challenged with linalyl acetate. The present results lead us to speculate that the antimicrobial activity of monoterpenes may be the result of the perturbation of the lipid fraction of the plasma membrane. Similarly, the antimicrobial activity of other phytochemicals, such as catechins (11), Melaleuca alternifolia essential oil (4), and α,β-unsaturated aldehydes (1, 24), has been shown to result from modifications of cell membrane integrity.
When PC LUVs were employed, the rank order of membrane damage was as follows: thymol ≫ (+)menthol ≫ linalyl acetate. The same order was observed when PC/SA LUVs were used; however, thymol induced a much more marked CF leakage from PC/SA liposomes than from PC vesicles. Conversely, a different rank order was registered for PC/PS LUVs [(+)menthol > thymol > linalyl acetate] and PG/CL LUVs [(+)menthol ≫ linalyl acetate > thymol]. In particular, (+)menthol challenge evoked a CF leakage from PC/PS and, especially, PG/CL vesicles much greater than that induced from PC and PC/SA LUVs; furthermore, thymol was more effective on PC and PC/SA LUVS than on PC/PS vesicles and almost ineffective on PG/CL liposomes.
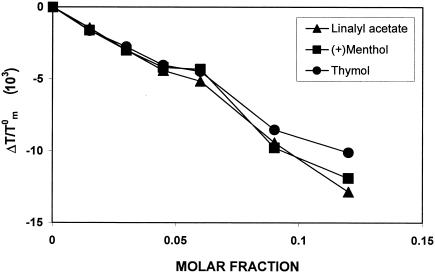
To evaluate the capability of the three terpenes under study to interact with the microenvironment of lipid membranes, we studied the thermotropic behavior of model phospholipidic membranes by means of DSC. In Fig. 4, the effect of MLV-encapsulated drug on the thermotropic behavior of DMPC has been expressed as ΔTm/T0m (ΔTm = Tm − T0m, where T0m and Tm are the transition temperatures of pure DMPC and drug-containing DMPC liposomes) and plotted versus increasing drug molar fractions. The calorimetric measurements indicate that the terpenes investigated are able to depress DMPC transitional temperature and shift the DMPC calorimetric peak toward lower values in a concentration-dependent way; however, no significant differences in their behavior were observed among the three monoterpenes employed. These results demonstrate that the monoterpenes investigated are able to interact with phospholipidic membranes, which may be largely explained in terms of a fluidifying effect attributable to the introduction of lipophilic molecules acting as interstitial impurities into the ordered structure of the lipid bilayer.
FIG. 4.
Effect of (+)menthol, thymol, and linalyl acetate, at different molar fractions, on the transitional temperature of dimyristoylphosphatidylcholine multilamellar liposomes.
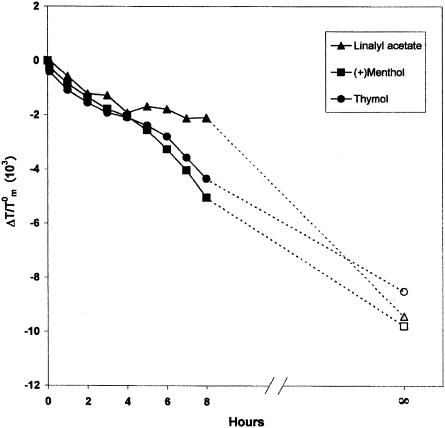
Figure 5 shows the data (expressed as ΔTm/T0m) obtained by the permeation experiments; these results are due to a succession of states consisting of the drug passing from the solid state to the solution state, crossing the aqueous medium, and then reaching, penetrating into, and remaining in the lipid membrane. A 0.09 molar fraction of drug was chosen to follow the process. The maximum exerted effect, corresponding to the maximum amount of drug transferable from pure drug to liposomes, is visualized in the figure as the point at t∞ (corresponding to the result obtained from the preparation of MLVs in the presence of a 0.09 molar fraction of terpene). From Fig. 5, one can see that these three monoterpenes interact with the lipid membranes, but their behavior is slightly different; in fact, the transfer of linalyl acetate molecules from the medium to void MLVs is much slower than that of thymol and (+)menthol. These findings are consistent with the relative lipophilicity and water solubility of the three monoterpenes examined; in fact, as demonstrated by results reported in Table 3, the lipophilicity order was (+)menthol > thymol ≫ linalyl acetate, and, unlike thymol and (+)menthol, linalyl acetate is absolutely insoluble in water (16).
FIG. 5.
Effect of (+)menthol, thymol, and linalyl acetate (X = 0.09) on Tm values of dimyristoylphosphatidylcholine MLVs evaluated by differential scanning calorimetry (full signs). Empty signs at t∞ refer to samples prepared by direct interaction between the examined compounds and MLVs, at the same organic fraction, by organic solvent dissolution and MLV preparation.
TABLE 3.
Relative lipophilicity (Rm) value and water solubility of thymol, (+)menthol, and linalyl acetate
| Drug | Rm | Water solubilitya |
|---|---|---|
| Linalyl acetate | −1.079 | Insoluble |
| (+)Menthol | 0.200 | Slightly solubleb |
| Thymol | 0.111 | Slightly solubleb |
See reference 16.
Equals 100 to 1,000 milliliters of solvent per gram of solute.
Taken together, the results obtained allow us to speculate that the antimicrobial activity of the terpenes under investigation may be caused, at least partially, by their penetration into the lipid assemblies and by a consequent perturbation of the lipid fraction of the plasma membranes; however, it is significantly influenced by their physicochemical characteristics and by the composition of bacterial membranes. More particularly, four issues need to be pointed out.
First, among the terpenes tested, thymol and, to a slightly lesser extent, (+)menthol showed a stronger toxic activity against S. aureus than linalyl acetate; furthermore, they showed the highest capacity to increase the permeability of PC LUVs and are better able to migrate across an aqueous medium and to interact with phospholipidic membranes. Since these two terpenes possess the best lipophilic characteristics and, unlike linalyl acetate, a detectable water solubility, we can speculate that their antimicrobial activity is well correlated to the capability to migrate across the aqueous extracellular medium and to interact with and damage lipid membranes.
Second, the exterior of the gram-negative outer membrane presents a strong negative charge conferred by lipopolysaccharide (11). In our experiments, (+)menthol was more effective than thymol against E. coli, while thymol was more toxic for S. aureus. These findings are in accordance with the observed increased capacity of (+)menthol and thymol to elicit CF leakage, respectively, from negatively charged PC/PS and PG/CL LUVs and from positively charged PC/SA LUVs.
However, the capability of a drug to affect membrane permeability is influenced not only by the net surface charge of the membrane but also by its lipid composition. In fact, the presence in the bilayer of PG and CL (typical of bacterial membranes, while PC is typical of eukaryotic membranes) strongly affects the permeabilizing capability of both (+)menthol and thymol.
Third, the outer layer of the gram-negative outer membrane is composed primarily of lipopolysaccharide molecules and forms a hydrophilic permeability barrier providing protection against the effects of highly hydrophobic drugs (7, 8). This may explain the low sensitivity of E. coli to the cytotoxic effect of the three lipophilic monoterpenes tested.
Finally, it is evident that some interaction with other targets of the microbial cell might play a key role in the observed antimicrobial effect of the monoterpenes under study. Similarly, Glover and coworkers (6), Denyer and Stewart (5), and Trombetta and coworkers (24) have demonstrated that perturbing the permeability of the cytoplasmatic membrane may not be immediately responsible for the biocidal efficiency of several surfactants. The results of permeation experiments and DSC measurements suggest a real transfer of monoterpenes through lipid bilayers; thus, these drugs might be able to permeate through cellular membrane and interact with intracellular components. This hypothesis may help to explain the good antimicrobial activity of linalyl acetate that is endowed with a low capability for disrupting lipid membranes.
In conclusion, the present data lead us to speculate that the antimicrobial effect of (+)menthol, thymol, and linalyl acetate may be due, at least partially, to a perturbation of the lipid fraction of bacterial plasma membranes, resulting in alterations of membrane permeability and in leakage of intracellular materials. Besides being related to physicochemical characteristics of the drugs (such as lipophilicity and water solubility), this effect appears to be dependent on the lipid composition and net surface charge of the bacterial membranes. Furthermore, the drugs might cross the cell membranes, penetrating the interior of the cell and interacting with intracellular sites critical for antibacterial activity.
REFERENCES
- 1.Bisignano, G., M. G. Laganà, D. Trombetta, S. Arena, A. Nostro, N. Uccella, G. Mazzanti, and A. Saija. 2001. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 198:9-13. [DOI] [PubMed] [Google Scholar]
- 2.Boyce, C. B. C., and B. V. Milborrow. 1965. A simple assessment of partition data for correlating structure and biological activity using thin-layer chromatography. Nature 208:537-539. [Google Scholar]
- 3.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox, S. D., C. M. Mann, J. L. Markham, H. C. Bell, J. E. Gustafson, J. R. Warmington, and S. G. Wyllie. 2000. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88:170-175. [DOI] [PubMed] [Google Scholar]
- 5.Denyer, S. P., and G. S. A. B. Stewart. 1998. Mechanisms of action of disinfectants. Int. Biodeter. Biodegr. 41:261-268. [Google Scholar]
- 6.Glover, R. E., R. R. Smith, M. V. Jones, S. K. Jackson, and C. C. Rowlands. 1999. An EPR investigation of surfactant action on bacterial membranes. FEMS Microbiol. Lett. 177:57-62. [DOI] [PubMed] [Google Scholar]
- 7.Hancock, R. E. W. 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5:37-42. [DOI] [PubMed] [Google Scholar]
- 8.Helander, I. M., H.-L. Alakomi, K. Latva-Kala, T. Mattila-Sandholm, I. Pol, E. J. Smid, L. G. M. Gorris, and A. von Wright. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 46:3590-3595. [Google Scholar]
- 9.Hirakura, Y., J. Alvarez-Bravo, S. Kurata, S. Natori, and Y. Kirino. 1996. Selective interaction of synthetic antimicrobial peptides derived from sapecin B with lipid bilayers. J. Biochem. 120:1130-1140. [DOI] [PubMed] [Google Scholar]
- 10.Huijbregts, R. P. H., A. I. P. M. de Kroon, and B. de Kruijff. 2000. Topology and transport of membrane lipids in bacteria. Biochim. Biophys. Acta 1469:43-61. [DOI] [PubMed] [Google Scholar]
- 11.Ikigai, H., T. Nakae, Y. Hara, and T. Shimamura. 1993. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1147:132-136. [DOI] [PubMed] [Google Scholar]
- 12.Kohlert, C., I. van Rensen, R. März, G. Schindler, E. U. Graefe, and M. Veit. 2000. Biovailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 66:495-505. [DOI] [PubMed] [Google Scholar]
- 13.Lohner, K., A. Latal, G. Degovics, and P. Garidel. 2001. Packing characteristics of a model system mimicking cytoplasmatic bacterial membranes. Chem. Phys. Lipids 111:177-192. [DOI] [PubMed] [Google Scholar]
- 14.Loza-Tavera, H. 1999. Monoterpenes in essential oils. Biosynthesis and properties. Adv. Exp. Med. Biol. 464:49-62. [DOI] [PubMed] [Google Scholar]
- 15.Mercadal, M., J. C. Domingo, M. Bermudez, M. Mora, and M. A. De Madariaga. 1995. N-Palmitoylphosphatidylethanolamine stabilizes liposomes in the presence of human serum: effect of lipidic composition and system characterization. Biochim. Biophys. Acta 1235:281-288. [DOI] [PubMed] [Google Scholar]
- 16.Merck & Co., Inc. 2001. The Merck index, 13th ed. Merck & Co., Inc., Whitehouse Station, N.J.
- 17.Nakatani, N. 1994. Antioxidative and antimicrobial constituents of herbs and spices, p. 251-271. In G. Charalambous (ed.), Spices, herbs and edible fungi. Elsevier Science BV, Amsterdam, The Netherlands.
- 18.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 19.Raudino, A., and F. Castelli. 1998. Modelling specific heat transient anomalies during permeation of liposomes by water-soluble substance. J. Colloid Interf. Sci. 200:52-58. [Google Scholar]
- 20.Rouser, G., S. Fkeischer, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 21.Saija, A., D. Trombetta, A. Tomaino, R. Lo Cascio, P. Princi, N. Uccella, F. Bonina, and F. Castelli. 1998. In-vitro evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int. J. Pharm. 166:123-133. [Google Scholar]
- 22.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1994. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269:8022-8028. [PubMed] [Google Scholar]
- 23.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trombetta, D., A. Saija, G. Bisignano, S. Arena, S. Caruso, G. Mazzanti, N. Uccella, and F. Castelli. 2002. Study on the mechanisms of the antibacterial action of some plant α,β-unsaturated aldehydes. Lett. Appl. Microbiol. 35:285-290. [DOI] [PubMed] [Google Scholar]