Abstract
(E)-(S)-4-((S)-2-{3-[(5-methyl-isoxazole-3-carbonyl)-amino]-2-oxo-2H-pyridin-1-yl}-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester (Compound 1) is a novel, irreversible inhibitor of human rhinovirus (HRV) 3C protease {inactivation rate constant (Kobs/[I]) of 223,000 M−1s−1}. In cell-based assays, Compound 1 was active against all HRV serotypes (35 of 35), HRV clinical isolates (5 of 5), and related picornaviruses (8 of 8) tested with mean 50% effective concentration (EC50) values of 50 nM (range, 14 to 122 nM), 77 nM (range, 72 to 89 nM), and 75 nM (range, 7 to 249 nM), respectively. Compound 1 inhibited HRV 3C-mediated polyprotein processing in infected cells in a concentration-dependent manner, providing direct confirmation that the cell-based antiviral activity is due to inhibition of 3C protease. In vitro and in vivo nonclinical safety studies showed Compound 1 to be without adverse effects at maximum achievable doses. Single oral doses of Compound 1 up to 2,000 mg in healthy volunteers were found to be safe and well tolerated in a phase I-ascending, single-dose study. Compound 1 estimated free observed maximum concentration in plasma (Cmax) for 500-, 1,000-, and 2,000-mg doses were higher than the protein binding-corrected EC50 required to inhibit 80% of the HRV serotypes tested. Treatment of HRV 52-infected cells with one to five 2-h pulses of 150 nM Compound 1 (corresponding to the Cmax at the 500-mg dose) was sufficient to effect a significant reduction in viral replication. These experiments highlight Compound 1 as a potent, orally bioavailable, irreversible inhibitor of HRV 3C protease and provide data that suggest that Cmax rather than the Cmin might be the key variable predicting clinical efficacy.
The Picornaviridae comprise one of the largest families of known human and animal pathogens. Included in the picornavirus family are the human rhinoviruses (HRV) and human enteroviruses (HEV). Consisting of over 100 different serotypes, the HRV are the single most important etiological agents of the common cold (7, 23, 26). In most individuals, HRV infections are mild and self-limiting; however, in patients with underlying respiratory disorders, infections may result in exacerbations of asthma, cystic fibrosis, bronchitis, and chronic obstructive pulmonary disease (1, 2, 6, 12, 18, 25, 28). To date, there are no marketed antiviral therapies available for the prevention or treatment of HRV-related illnesses.
We have focused our drug discovery efforts on the HRV 3C protease, an enzyme that is absolutely required for the majority of the proteolytic cleavage events that occur during the viral life cycle (7, 17, 19, 23, 26). DNA sequence comparisons among numerous HRV serotypes and several related picornaviruses have demonstrated a significant degree of homology among amino acid residues involved in key 3C protease inhibitor-binding interactions, providing an additional rationale for targeting research activities (3, 20, 22). Early drug discovery efforts led to the identification of rupintrivir, an intranasally administered, irreversible inhibitor of HRV 3C protease that has demonstrated broad-spectrum, potent in vitro antiviral activity against multiple HRV serotypes, HRV clinical isolates, and related picornaviruses (8, 9, 14, 20, 24, 33, 34). Proof of concept for the mechanism of 3C protease inhibition was shown in a recent study in which rupintrivir moderated the severity of illness and reduced viral load in human subjects following experimental HRV infection (13). Parallel research efforts to discover an orally bioavailable inhibitor of HRV 3C protease have culminated in the identification of (E)-(S)-4-((S)-2-{3-[(5-methyl-isoxazole-3-carbonyl)-amino]-2-oxo-2H-pyridin-1-yl}-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester (Compound 1, formerly referred to as Compound 3) (10). Compound 1 is a novel, irreversible inhibitor of HRV 3C protease with demonstrated oral bioavailability in dogs and cynomolgus monkeys with 7-h plasma concentrations similar to or exceeding the in vitro antiviral activity against the seven HRV serotypes evaluated (10). In this study, we describe the in vitro antiviral activity of Compound 1 against a number of different HRV serotypes, clinical isolates, and related picornaviruses in cell-based systems. In addition, we have determined the pharmacokinetics, safety, and tolerability of single ascending doses of Compound 1 in healthy volunteers.
MATERIALS AND METHODS
Compounds.
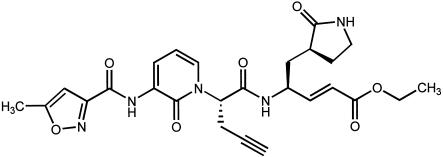
(E)-(S)-4-((S)-2-{3-[(5-Methyl-isoxazole-3-carbonyl)-amino]-2-oxo-2H-pyridin-1-yl}-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester (Fig. 1; Compound 1, formerly referred to as Compound 3) was synthesized at Pfizer Global Research and Development (formerly Agouron Pharmaceuticals, Inc.), San Diego, CA (10).
FIG. 1.
Chemical structure of (E)-(S)-4-((S)-2-{3-[(5-methyl-isoxazole-3-carbonyl)-amino]-2-oxo-2H-pyridin-1-yl}-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester (Compound 1).
Biochemical assay.
In vitro biochemical determinations were performed as described (10). Briefly, the inactivation constant (Kobs/[I]) was determined using a continuous fluorometric assay and various concentrations of inhibitor. The initial data were analyzed with the first order rate kinetic equation (in ENZFITTER), and the resulting rate constants were then plotted against inhibitor concentration as a linear regression (30, 31). Specificity assays against a variety of serine and cysteine proteases (except calpain) were done in a 96-well plate continuous spectrophotometric format under established conditions with enzyme and compound incubated for 10 min prior to starting the reaction. Calpain was done using a continuous fluorometric assay under established conditions with the same 10-min preincubation.
Cells and virus strains.
All numbered HRV serotypes as well as all related picornaviruses were purchased from American Type Culture Collection (ATCC; Rockville, MD). HRV Hanks was kindly provided by Ronald Turner from the Department of Pediatrics, University of Virginia Health Sciences Center, Charlottesville, VA. HRV serotypes and HEV (e.g., coxsackievirus A21, B2, B3, and B5) were propagated and antiviral assays were performed in H1-HeLa cells (ATCC) incubated at 34°C. Other HEV (e.g., echovirus 6, 9, 11, and enterovirus 70) were propagated and antiviral assays were performed in MRC-5 cells (ATCC) incubated at 37°C. Nasal lavage samples from patients infected with HRV 39, HRV Hanks, HRV 23, or HRV 16 were kindly provided by Fred Hayden from the University of Virginia, Charlottesville, VA, and Ronald Turner. Clinical viral isolates were isolated following incubation of nasal lavage samples with H1-HeLa cells. All HRV clinical isolates were propagated and antiviral assays were performed in H1-HeLa cells incubated at 34°C. Cells were grown in minimal essential medium (Life Technologies, Gaithersburg, MD) with 10% fetal bovine serum (HyClone, Logan, UT).
Cell protection assay.
The ability of Compound 1 to protect cells against HRV or HEV infection was measured in 96-well plates by the standard cytopathic effect (CPE) inhibition assay using the XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide; Sigma] dye reduction method (27, 32) or, for HRV clinical isolates, by a modified CPE inhibition assay (14) also using the XTT dye reduction endpoint. In the standard CPE assay, H1-HeLa and MRC-5 cells were initially resuspended at a final concentration of 2 × 105 and 5 × 104 cells per ml, respectively, and infected with virus (50 μl) at a constant multiplicity of infection (MOI) determined to yield ≥65% to ≤95% cell death for each individual virus (MOI range, 0.004 to 1) or mock infected with medium only. One to 5 days later, cultures were evaluated for CPE microscopically and XTT with phenazine methosulfate (PMS; Sigma) was added to the test plates and the amount of formazan produced was quantified spectrophotometrically at 450 and 650 nm. Data were expressed as the percent of formazan produced in compound-treated cells compared to formazan produced in wells of uninfected, compound-free cells. The 50 and 90% effective concentrations (EC50 and EC90) were calculated as the concentrations of compound that increased the percent of formazan production in infected, compound-treated cells to 50% and 90% of that produced by uninfected, compound-free cells. The 50% cytotoxicity concentration (CC50) was calculated as the concentration of compound that decreased the percentage of formazan produced in uninfected, compound-treated cells to 50% of that produced in uninfected, compound-free cells. The therapeutic index was calculated by dividing the cytotoxicity (CC50) by the antiviral activity (EC50). In some experiments, assays were performed in the presence or absence of either human α1-acid glycoprotein (AAG) or human serum albumin (HSA) (Sigma, St. Louis, MO) at a final concentration of 1 mg/ml or 45 mg/ml, respectively. Differences in EC50 values obtained in experiments performed in the presence or absence of either AAG or HSA were analyzed for significance by means of a two-tailed students t test where P = 0.05.
The modified CPE assay, which allows for the simultaneous determination of antiviral activity and optimal CPE, was utilized with HRV clinical isolates. Briefly, each clinical HRV isolate was inoculated at six serial 10-fold dilutions in quadruplicate for a 50% tissue culture infectious dose (TCID50) determination (backtitration) and at three 10-fold virus dilutions for compound titration. The EC50 values were determined utilizing the inoculum dilution for which the calculated TCID50/ml was between 32 and 320. XTT-PMS was added to the test plates and processed as above.
Cell protection assay, pulse experiments.
The ability of short duration exposures (“pulses”) of Compound 1 to protect cells against HRV infection was measured in 96-well plates by the standard CPE inhibition assay as described above. Briefly, H1-HeLa cells were resuspended at a concentration of 1.5 × 105 cells per ml and infected with HRV 52 at an MOI of 0.004 or mock infected with medium only. Two hours following infection, cells were washed and resuspended in 2 nM Compound 1, 150 nM Compound 1, or medium alone as controls. At various times after infection, cells that had been maintained in 2 nM Compound 1 were washed and treated with one (2 h), three (2, 9, and 24 h), four (2, 9, 24, and 31 h), or five (2, 9, 24, 31, and 51 h) 2-h pulses of 150 nM Compound 1. After each pulse, cells were washed and resuspended in 2 nM Compound 1. Fifty-three hours after initial viral inoculation, XTT-PMS was added to the test plates and the amount of formazan produced was quantified spectrophotometrically at 450 and 650 nm as previously described (27, 32). The data was expressed as percent inhibition (± standard error) utilizing the mock infected cell control and compound-free virus control as the 100% and 0% virus inhibition values, respectively. Differences in the percent inhibition obtained in experiments performed in the presence or absence of pulse treatments were analyzed for significance by means of a two-tailed students t test where P = 0.05.
Analysis of proteolytic processing.
The ability of Compound 1 to inhibit HRV 14 3C-mediated proteolytic processing was assessed by polyacrylamide gel electrophoresis (PAGE) of radiolabeled sodium dodecyl sulfate (SDS)-solubilized lysates of HRV 14-infected cells. Initially, H1-HeLa cells (8 × 105 cells/well) were infected with HRV 14 at an MOI of 50 in a total volume of 250 μl. Eight and one half hours after infection, the monolayers were washed with phosphate-buffered saline (PBS) and the medium was replaced with methionine/cysteine-deficient medium (Life Technologies, Grand Island, NY). At nine hours after infection, appropriate concentrations of compound were added. After 30 min of exposure to the compound, 50 μCi of [35S]Met/[35S]Cys (Expre35S 35S protein label; New England Nuclear, Boston, MA) were added. One hour later, the monolayers were washed twice with PBS (4°C) and lysed in 250 μl of lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS), sonicated, and stored at −80°C for subsequent analysis. Proteins present in the solubilized cell lysates were resolved by 12% PAGE. Following electrophoresis, gels were stained with Coomassie brilliant blue, destained, and treated with Amplify (Amersham, Arlington Heights, IL). The gels were then fixed in a solution of 40% methanol and 10% acetic acid. Gels were air dried overnight on 3 M paper and exposed to film at −80°C.
Nonclinical safety studies.
To support the administration of Compound 1 to healthy volunteers in phase 1 clinical trials, a traditional nonclinical safety program was undertaken to assess the safety and potential toxicity of the compound. This series of studies included genotoxicity evaluations using in vitro test systems (Ames assay using Salmonella enterica serovar Typhimurium and Escherichia coli up to 500 μg/plate; chromosomal aberrations with human lymphocytes up to 1,500 μg/ml), single-dose oral toxicity studies (mouse, 1,500 mg/kg; dog, up to 2,000 mg/kg; monkey, up to 2,000 mg/kg) and 14-day repeat-dose oral toxicity studies (rat, up to 1,500 mg/kg/day; dog, up to 1,500 mg/kg/day). All in vivo studies were performed with animals in the fed state. A full necropsy and histopathologic evaluation were performed on these in vivo studies. Additionally, full toxicokinetic profiles of Compound 1 were evaluated in the repeat-dose studies. Because of the potential for reactive binding of a Michael acceptor to glutathione, the ability of Compound 1 to deplete liver glutathione was also assessed in the dog after 14 days of dosing of Compound 1 up to 1,500 mg/kg/day. Safety pharmacology studies to identify undesirable pharmacodynamic properties of Compound 1 were also assessed both in vitro (human ether-a-go-go-gene [hERG] assay up to 30 μg/ml) and in single-dose studies in vivo (central nervous system safety in rat up to 1,500 mg/kg and cardiovascular safety in dog up to 1,500 mg/kg).
Phase I first-in-human study.
A phase I single-dose study was conducted to assess the safety and tolerability of ascending single oral doses of Compound 1 in healthy men and to determine the pharmacokinetics of Compound 1 under fasted and fed conditions. Healthy volunteers received the following single oral doses under fed conditions: 200 mg, 500 mg, 1,000 mg, and 2,000 mg. Subjects also received a 500-mg dose under fasting conditions for food-effect evaluation. All doses were separated by a washout period of at least 7 days. Serial pharmacokinetic blood samples were collected for up to 30 h after dosing. Plasma concentrations of Compound 1 were determined using high-performance liquid chromatography (HPLC) with tandem mass spectrometry. Pharmacokinetic parameter values were estimated by noncompartmental methods using WinNonlin (Version 3.1). The following single-dose pharmacokinetic parameters were estimated for Compound 1: observed maximum concentration in plasma (Cmax), time to maximum concentration in plasma (Tmax), area under the plasma concentration-time curve from time zero extrapolated to infinity (AUC0-∞), terminal elimination half-life (t1/2β), and apparent oral clearance (CL/F). Safety was assessed via physical examinations, electrocardiograms (ECG), vital sign measurements, clinical laboratory testing, and monitoring of adverse events.
RESULTS
Activity against 3C protease.
(E)-(S)-4-((S)-2-{3-[(5-methyl-isoxazole-3-carbonyl)-amino]-2-oxo-2H-pyridin-1-yl}-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester (Compound 1; Fig. 1) (molecular weight of 524) is a novel, 2-pyridone-containing peptidomimetic which incorporates an unsaturated ethyl ester Michael acceptor moiety that forms an irreversible covalent adduct with the active-site cysteine residue of the HRV 3C protease. Compound 1 has demonstrated potent inhibition of 3C protease with an inactivation rate constant (Kobs/[I]) of 223,000 M−1 s−1 (10). Compound 1 (10 μM) produced no significant inhibition against a variety of mammalian serine or cysteine proteases (e.g., human elastase, human thrombin, bovine trypsin, bovine chymotrypsin, human cathepsin B, and porcine calpain) demonstrating specificity for picornaviral 3C protease (data not shown).
In vitro antiviral activity against HRV serotypes, clinical isolates, and HEV.
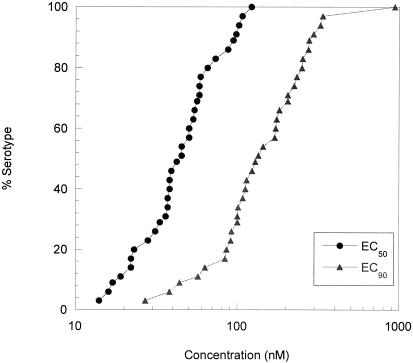
The in vitro antiviral activity of Compound 1 was evaluated against a panel of 35 different HRV serotypes representative of minor and major receptor groups (29) as well as antiviral groups A and B in a cell protection assay utilizing H1-HeLa cells (Fig. 2, Table 1). Compound 1 was active against all the HRV serotypes tested (35 of 35), with a mean EC50 value of 50 nM (range, 14 to 122 nM) and mean EC90 value of 179 nM (range, 27 to 941 nM). In parallel experiments, results indicated that the concentration of Compound 1 required for antiviral activity was significantly less than the 50% cytotoxic concentration (CC50; 572 μM), indicating a favorable therapeutic index of 4,689 to 40,857.
FIG. 2.
In vitro antiviral activity of Compound 1 against HRV serotypes. EC50 and EC90 values of Compound 1 for 35 serotypes were determined by measuring XTT dye reduction following 1 to 5 days of infection of H1-HeLa cells as described in Materials and Methods.
TABLE 1.
In vitro antiviral activity of Compound 1 against HRV serotypes, clinical isolates, and HEVa
| Virus | Antiviral activity
|
|
|---|---|---|
| EC50 (nM) | EC90 (nM) | |
| HRV serotype | ||
| 2ce | 13, 32 | 77, 95 |
| 3bd | 21, 53 | 63, 105 |
| 9cd | 79 ± 37 | 239 ± 73 |
| 10cd | 48, 42 | 89, 168 |
| 11cd | 33 ± 25 | 112 ± 49 |
| 12cd | 107 ± 49 | 325 ± 109 |
| 13bd | 60, 55 | 195, 268 |
| 14bd | 53 ± 37 | 204 ± 83 |
| 16cd | 38, 46 | 98, 171 |
| 17bd | 53, 23 | 97, 84 |
| 19cd | 50, 49 | 187, 316 |
| 23cd | 42, 32 | 135, 92 |
| 25cd | 98 ± 41 | 335 ± 52 |
| 28cd | 59 ± 32 | 175 ± 109 |
| 29ce | 39 ± 17 | 100 ± 21 |
| 31ce | 33, 11 | 97, 28 |
| 36cd | 52, 56 | 132, 211 |
| 38cd | 102 ± 58 | 277 ± 69 |
| 39cd | 43, 73 | 88, 200 |
| 45bd | 23, 20 | 94, 106 |
| 49ce | 17 ± 13 | 57 ± 38 |
| 52bd | 20, 7 | 37, 16 |
| 53cd | 36 ± 59 | 56, 20 |
| 59cd | 16 ± 12 | 44 ± 35 |
| 68cd | 76, 168 | 941 |
| 73cd | 59, 70 | 117, 245 |
| 78cd | 61, 50 | 232, 268 |
| 81cd | 130, 43 | 427, 120 |
| 84bd | 28 ± 22 | 204 ± 175 |
| 86bd | 71, 74 | 237, 211 |
| 87bf | 45 ± 21 | 90, 111 |
| 89cd | 26, 11 | 149, 35 |
| 95bd | 48, 51 | 108, 137 |
| 1Ace | 36, 40 | 78, 261 |
| Hankscd | 49, 13 | 149, 67 |
| HRV clinical isolateg | ||
| 119 | 83, 64 | 167, 149 |
| O | 76, 74 | 142, 152 |
| J | 91, 53 | 186, 187 |
| 15 | 88, 59 | 174, 141 |
| T | 99, 79 | 282, 145 |
| HEV | ||
| CVA 21 | 249 ± 150 | 1,003 ± 392 |
| CVB 2 | 57, 29 | 100, 97 |
| CVB 3 | 82 ± 80 | 204 ± 118 |
| CVB 5 | 122 ± 68 | 223 ± 110 |
| EV 70 | 7 ± 6 | 28 ± 1 |
| EV 6 | 43 ± 19 | 86 ± 12 |
| EV 9 | 12, 25 | 26, 76 |
| EV 11 | 32 ± 17 | 89, 59 |
Antiviral activity was determined by measuring XTT dye reduction following 1 to 6 days of infection of H1-HeLa or MRC-5 cells. Results represent the mean ± standard deviation (3 to 15 experiments) or individual values (1 or 2 experiments).
Antiviral group A (17).
Antiviral group B (17).
Major receptor group (29).
Minor receptor group (29).
Neither major nor minor receptor group (29).
Clinical isolates were derived from nasal lavage samples collected from subjects infected with HRV serotype 39 (denoted as 119, O), Hanks (denoted as J), 23 (denoted as 15), or 16 (denoted as T).
Compound 1 was also evaluated against 5 HRV clinical isolates derived from human subjects experimentally infected with challenge strains of HRV. Compound 1 demonstrated in vitro antiviral activity comparable to that observed against numbered HRV serotypes, with a mean EC50 value of 77 nM (range, 72 to 89 nM) and a mean EC90 of 173 nM (range, 147 to 214 nM) (Table 1). The in vitro antiviral activity of Compound 1 against a panel of 8 related picornaviruses in H1-HeLa or MRC-5 cell protection assays was likewise evaluated. Compound 1 was active against all eight HEV tested, with a mean EC50 value of 75 nM (range, 7 to 249 nM) and a mean EC90 value of 221 nM (range, 28 to 1,003 nM) (Table 1). Overall, Compound 1 demonstrated comparable antiviral potencies, with EC50 values varying only 36-fold among the different HRV serotypes, isolates, and HEV tested.
Inhibition of HRV 3C proteolytic processing.
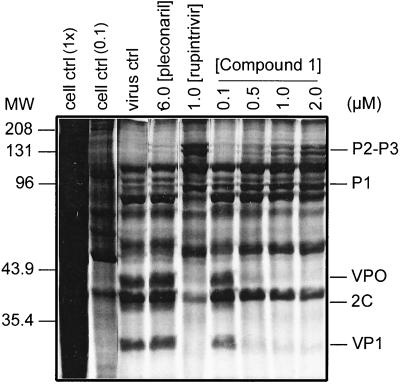
To confirm that the in vitro antiviral activity of Compound 1 observed in cell-based assays was derived from a direct inhibition of HRV 3C-mediated proteolytic processing, HRV 14-infected cells were treated with varying concentrations of Compound 1 (0.1 μM to 2.0 μM) and radiolabeled polyproteins resolved by SDS-PAGE as described in Materials and Methods. Rupintrivir (1 μM), an inhibitor of HRV 3C protease (EC50 value against HRV 14 of 13 nM), was included as a positive control (24). Pleconaril (6 μM), an inhibitor of virus capsid binding, was included as a negative control (11, 21). SDS-PAGE analysis (Fig. 3) indicated a concentration-dependent accumulation of large HRV 14 precursor polyproteins (e.g., P1 [97 kDa] and P2-P3 [146 kDa]) with a concomitant reduction of low molecular weight cleavage products (e.g., VP1 [33 kDa], VP0 [37 kDa], and 2C [38 kDa]). As expected, proteolytic processing was not affected by pleconaril but was inhibited by rupintrivir.
FIG. 3.
Inhibition of HRV 14 3C-mediated proteolytic processing by Compound 1. SDS solubilized lysates were prepared from uninfected H1-HeLa cells (cell ctrl) or infected cells treated with Compound 1, pleconaril, rupintrivir, or medium only (virus ctrl). Equal amounts of protein were analyzed by polyacrylamide gel electrophoresis (PAGE) as described in Materials and Methods except for cell control (0.1×), which represents 1/10th the amount of uninfected cell lysate protein analyzed. P2-P3, P1, VPO, 2C, and VP1 designate HRV-specific polypeptides.
Effect of added protein on the in vitro antiviral activity of Compound 1.
The effects of protein binding on the in vitro antiviral activity of Compound 1 were evaluated by the addition of physiologically relevant plasma concentrations of human serum albumin (HSA) and α1-acid glycoprotein (AAG) (Table 2). No statistically significant change was observed in the antiviral activity of Compound 1 in the presence of either 45 mg/ml HSA (P value = 0.118) or 1 mg/ml of AAG (P value = 0.894). The mean EC50 values for Compound 1 against HRV 14 in the absence of AAG and HSA were 28 and 81 nM, respectively, while in the presence of AAG and HSA, the mean EC50 values were 26 and 41 nM, respectively.
TABLE 2.
In vitro antiviral activity of Compound 1 in the presence of α1-acid glycoprotein or human serum albumina
| Protein treatmentb | Mean EC50 (nM) | Mean EC90 (nM) |
|---|---|---|
| - | 28 ± 16 | 109 ± 30 |
| AAG | 26 ± 13 | 139 ± 94 |
| - | 81 ± 31 | 217 ± 120 |
| HSA | 41 ± 16 | 73 ± 8 |
Antiviral activity was determined by measuring XTT dye reduction following 2 to 4 days of infection. Results represent the mean ± standard deviation of 3 experiments.
Experiments were conducted in the presence or absence (-) of either 1 mg/ml α1-acid glycoprotein (AAG) or 45 mg/ml human serum albumin (HSA).
Nonclinical safety studies.
At maximum achievable in vitro concentrations or in vivo doses, no adverse effects were identified, including no effects on hepatic glutathione concentrations, and margins of exposure in these studies exceeded the predicted clinical exposure (data not shown). Cross-species extrapolation suggests that the median dog exposure on day 14 at the no observed adverse effect level (NOAEL) would be approximately 350-fold and 35-fold higher than the predicted single-dose exposures in human at the starting dose of 200 mg and the highest planned dose of 2,000 mg, respectively.
Phase I first-in-human study.
In the clinical investigation, 14 white males who ranged in age from 18 to 55 years were enrolled and completed the study. Single doses of Compound 1 up to 2,000 mg were safe and well tolerated. There were no clinically significant changes in vital signs, ECG, and clinical lab parameters after single-dose administration of Compound 1. Treatment-emergent adverse events were all mild in severity, and few were considered related to study drug. Treatment-related adverse events included somnolence (n = 7), headache (n = 2), dizziness (n = 1), and skin irritation (n = 1).
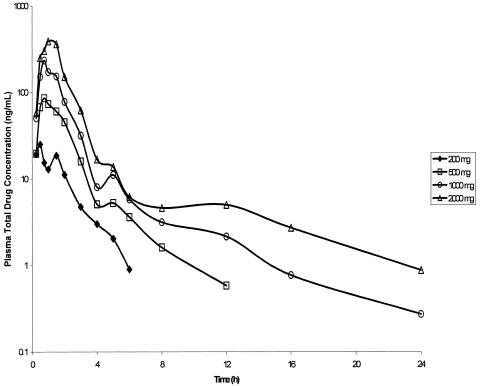
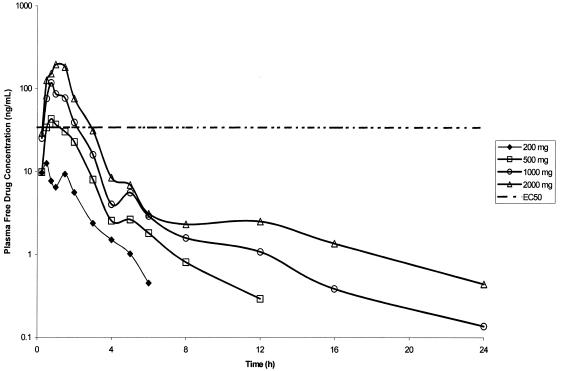
Food increased Compound 1 AUC0-∞ and Cmax by approximately 75% (data not shown). Compound 1 absorption after oral administration under fed conditions was rapid; Tmax occurred between 0.75 and 1.50 h (Table 3, Fig. 4). A secondary peak was observed at approximately 5 h after dosing (Fig. 4), suggesting enterohepatic recirculation. The single-dose pharmacokinetics of Compound 1 appear to be linear, such that AUC0-∞ and Cmax increased approximately in proportion to increasing dose (Table 3). The Compound 1 t1/2β was approximately 7 to 9 h (Table 3). The estimated free Compound 1 plasma concentration-time profile (corrected for 34% protein binding [10]) relative to the tissue culture binding-corrected EC50 value (corrected for 3.6% protein binding, unpublished data) required to inhibit 80% of HRV serotypes (34 ng/ml or 64 nM, Fig. 2) is shown in Fig. 5. Although plasma free drug levels of Compound 1 for all doses were below the EC50 value at 4 h postdose, the Compound 1 Cmax values for the 500-, 1,000-, and 2,000-mg doses were higher than the protein binding-corrected EC50 value (Fig. 5).
TABLE 3.
Summary of Compound 1 pharmacokinetic parameters following oral administration of Compound 1 single ascending doses under fed conditions (n = 6 per dose)a
| Dose (mg) | AUC0-∞ (ng · h/ml) | Cmax (ng/ml) | Tmax (h) | t1/2β (h) | CL/F (103 · liters/h) |
|---|---|---|---|---|---|
| 200 | 52.4 ± 14.4 | 41.2 ± 25.4 | 1.50 (0.25-2.00) | NDb | 4.05 ± 1.01 |
| 500 | 177 ± 59.2 | 125 ± 61.9 | 1.13 (0.50-2.00) | 6.60 ± 8.34 | 3.13 ± 1.11 |
| 1,000 | 398 ± 62.3 | 317 ± 108 | 0.75 (0.50-2.00) | 8.85 ± 6.15 | 2.57 ± 0.42 |
| 2,000 | 741 ± 125 | 522 ± 212 | 1.25 (0.75-1.50) | 7.06 ± 3.27 | 2.77 ± 0.50 |
Data are presented as mean±standard deviation, except for Tmax values, which are presented as median (range).
ND, not determined. The terminal elimination phase was not readily discernable due to many Compound 1 concentration values below the lower limit of quantitation.
FIG. 4.
Profiles of mean Compound 1 (total drug) concentrations in plasma after oral administration of ascending single doses under fed conditions (six subjects for each dose).
FIG. 5.
Profiles of mean Compound 1 (estimated free drug) concentrations in plasma after oral administration of ascending single doses under fed conditions (six subjects for each dose) relative to the tissue culture binding-corrected EC50 value required to inhibit 80% of HRV serotypes.
In vitro effect of treating infected cells with “pulses” of Compound 1.
The association between a specific pharmacokinetic (PK) parameter, in vitro antiviral activity, and clinical efficacy has not yet been established for anti-rhinoviral therapy. In this experiment, we evaluated whether treating HRV 52-infected cells that had been maintained in a concentration of Compound 1 known to incompletely suppress viral replication (e.g., 2 nM) with multiple short duration exposures (“pulses”) of Compound 1 at a concentration known to completely suppress viral replication (e.g., 150 nM) could effect a reduction in viral replication. Concentrations of 150 nM and 2 nM also represented concentrations corresponding to the total peak (Cmax) and trough (Cmin; concentration at 8 to 12 h after dosing, assuming the dosing frequency of every 8 h or 12 h) concentrations, respectively, that were measured following the administration of a 500-mg dose to healthy volunteers in the phase I clinical trial described above. The concentrations of 2 nM (Cmin) and 150 nM (Cmax) are approximately 7-fold lower and 11-fold higher, respectively, than the EC50 value for Compound 1 inhibition of HRV 52 (Table 1). When corrected for tissue-culture protein binding, these values are approximately 11-fold lower and 7-fold higher, respectively, than the EC50 value for Compound 1 inhibition of HRV 52 (Table 1).
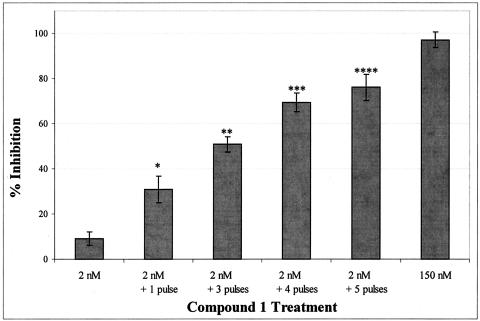
As results in Fig. 6 demonstrate, complete inhibition of virus replication (97.1% inhibition) was achieved by exposing cells to 150 nM Compound 1 throughout the incubation period. In contrast, incubating infected cells in 2 nM Compound 1 had little or no effect on virus replication (9.2% inhibition). Treatment of infected cells that had been maintained in 2 nM Compound 1 with one to five 2-h pulses of 150 nM was able to effect a significant reduction in viral replication when compared to treatment with 2 nM alone (P values range from 0.011 to 2.4 × 10−7). Increasing the number of times that infected cells were pulsed also increased the overall level of inhibition, with four and five pulses resulting in >50% inhibition of virus replication.
FIG. 6.
In vitro pulse experiments of Compound 1. Antiviral activity was determined by measuring XTT dye reduction following 53 h of infection with HRV 52 using the CPE inhibition assay as described in Materials and Methods. Infected cells were treated with 2 nM (n = 24), 150 nM Compound 1 (n = 24), or 2 nM Compound 1 and subsequently treated with one, three, four, or five 2-h pulses of 150 nM Compound 1 (n = 6). Data is expressed as percent inhibition (± standard error) utilizing the mock infected cell control and compound-free virus control as the 100% and 0% virus inhibition values, respectively. Significant reductions in viral replication due to treatment with pulses of 150 nM as compared to treatment with 2 nM alone were determined as described in Materials and Methods, with resulting P values of 0.011 (*), 2.4 × 10−7 (**), 1.5 × 10−7 (***), and 6.6 × 10−6 (****).
DISCUSSION
Although HRV has been the focus of significant drug discovery and development efforts, no antiviral therapy has been approved to date for the treatment and prevention of HRV infection. Our research efforts have focused on inhibition of the picornavirus 3C protease, an enzyme absolutely required for viral replication. Rupintrivir is a novel 3C protease inhibitor that was discovered by protein structure-based drug design methodologies and formulated for intranasal delivery. Although rupintrivir treatment was able to moderate severity of illness and reduce viral load in a human experimental HRV challenge trial (13), rupintrivir was not able to significantly effect virus reduction and moderate disease severity in subsequent natural infection studies in patients and thus was terminated for clinical development (data not shown). Parallel efforts to identify a 3C protease inhibitor that was orally bioavailable has led to the discovery of (E)-(S)-4-((S)-2-{3-[(5-methyl-isoxazole-3-carbonyl)-amino]-2-oxo-2H-pyridin-1-yl}-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester (Compound 1). Similar to rupintrivir, Compound 1 is an irreversible inhibitor incorporating a Michael acceptor moiety that forms a covalent bond with the 3C protease active site cysteine (10). In contrast to rupintrivir, however, Compound 1 was shown to be orally bioavailable in dogs and cynomolgus monkeys (10).
In this study, Compound 1 demonstrated antiviral activity against all HRV and related picornaviruses tested in in vitro cell-based assays. Moreover, Compound 1 demonstrated comparable antiviral activity against all picornaviruses tested (36-fold range in EC50 values). While this is similar to that reported for rupintrivir (3, 24), it is in contrast to that reported for inhibitors that target capsid-binding, e.g., pleconaril and pirodavir (24). In these latter studies, pleconaril and pirodavir demonstrated a significantly wider range in antiviral potency (1,590- to 2,707-fold range in EC50 values) and activity against most but not all HRV serotypes tested. The comparable range in antiviral activity of Compound 1 when tested against numerous different picornaviruses is consistent with DNA sequence analyses performed on 3C protease coding gene regions, which demonstrate a significant level of homology in substrate/inhibitor binding regions (3). In mechanism of action studies, Compound 1 was also shown to inhibit the HRV 14 3C-mediated proteolytic cleavage of viral precursor polyproteins into their processed viral products, confirming that the cell-based in vitro antiviral activity of Compound 1 is due to a direct inhibition of HRV 3C protease. Profiles of polyproteins predicted to accumulate or be reduced were consistent with the cleavage profile observed when certain amino acid substitutions are introduced into the 3C protease catalytic site (5, 15).
Although oral delivery of antirhinoviral compounds could theoretically increase the risk of side effects relative to nasal or topical delivery, oral delivery may allow compounds access to additional HRV replication sites that would otherwise not be available through nasal delivery. In addition, oral delivery may allow treatment of other picornaviral infections which are not localized in respiratory tract (e.g., meningitis). Previous studies have shown Compound 1 to be orally bioavailable in dogs and cynomolgus monkeys with 7-h plasma concentrations similar to or exceeding the in vitro antiviral activity against seven HRV serotypes evaluated (10). In this study, Compound 1 was evaluated in humans in an ascending, single-dose study. Single oral doses of Compound 1 up to 2,000 mg were safe and well tolerated. When administered with food, Compound 1 exposure increased approximately in proportion to increasing dose over the 200-mg to 2,000-mg dose range studied.
With some viral diseases, e.g., human immunodeficiency virus (HIV), the pharamocokinetic/pharmacodynamic (PK/PD) relationship for HIV protease inhibitors has been well studied (4, 16). In contrast, the relationship between drug exposure to appropriate PD measurements or biomarkers has not been evaluated for antirhinoviral therapy. Although for orally deliverable drugs one can assume that plasma PK may predict the intracellular concentration of compound in nasal epithelial cells, the critical PK parameter (e.g., Cmin, Cmax, etc.) that predicts clinical efficacy has not been determined. In our phase I trial, we found that doses at 500 mg or higher produced free Cmax levels that were above the protein-binding-corrected EC50 value required to inhibit 80% of the HRV serotypes tested. In contrast, trough drug concentrations (e.g., concentrations at 8 to 12 h after dosing) were below this value. With chronic diseases such as HIV, maintaining free drug levels at or above Cmin levels is critical for continual suppression of viral replication and delayed emergence of virus resistance (4). Similar to HIV, resistance to HRV can be generated in vitro following serial passage in the presence of increasing concentrations of protease inhibitors (data not shown). HRV differs from HIV, however, in that it is not capable of establishing persistent infections and the illness it induces is acute and is typically resolved immunologically. In addition, the irreversible nature of the interaction between Compound 1 and HRV 3C protease may also be expected to impact the PK/PD relationship. To better understand the relationship between drug exposure and in vitro antiviral activity, we evaluated whether treating infected cells maintained in a concentration of Compound 1 known to incompletely suppress viral replication and that corresponded to the total Cmin (2 nM), with “pulses” of Compound 1 at a concentration known to completely suppress viral replication and that corresponded to the total Cmax (150 nM) could effect a reduction in viral replication. Treatment of infected cells over a 53-h period with one to five 2-h pulses of 150 nM Compound 1 effected a significant reduction in viral replication when compared to infected cells maintained in 2 nM Compound 1. These results suggest that Cmax and not Cmin should be considered when evaluating clinical effective doses.
In this study, we describe the in vitro antiviral activity and single-dose pharmacokinetic evaluations of Compound 1, a novel, irreversible inhibitor of 3C protease. Although we are currently not progressing Compound 1 into clinical development, these results continue to support HRV 3C protease as an attractive target for antiviral drug discovery and development. In addition, our results provide initial data that explores the relationship between specific PK and PD parameters.
Acknowledgments
We thank Jennifer Hammond for her critical review of the manuscript and Jules Beardsley for help in preparation of the manuscript.
REFERENCES
- 1.Arruda, E., and F. G. Hayden. 1995. Clinical studies of antiviral agents for picornaviral infections, p. 321-355, In D. J. Jeffries and E. De Clerq (ed.), Antiviral chemotherapy. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 2.Atmar, R. L., E. Guy, K. K. Guntupalli, J. L. Zimmerman, V. D. Bandi, B. D. Baxter, and S. B. Greenberg. 1998. Respiratory tract viral infections in inner-city asthmatic adults. Arch. Intern. Med. 158:2453-2459. [DOI] [PubMed] [Google Scholar]
- 3.Binford, S. L., F. Maldonado, M. A. Brothers, P. T. Weady, L. S. Zalman, J. W. Meador III, D. A. Matthews, and A. K. Patick. Conservation of amino acids in human rhinovirus 3C protease correlates with broad spectrum antiviral activity of rupintrivir, a novel human rhinovirus 3C protease inhibitor. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 4.Brun, S., D. Kempf, K. Garren, A. Molla, M. King, B. Richards, T. Marsh, R. Bertz, A. Hsu, and E. Sun. 2001. The inhibitory quotient as a predictor of viral evolution following viral load rebound during Lopinavir/r therapy, poster no. 89. 5th International Workshop on HIV Drug Resistance and Treatment Strategies, Scottsdale, Ariz.
- 5.Cheah, K.-C., L. E.-C. Leong, and A. G. Porter. 1990. Site-directed mutagenesis suggests close functional relationship between a human rhinovirus 3C cysteine protease and cellular trypsin-like serine proteases. J. Biol. Chem. 265:7180-7187. [PubMed] [Google Scholar]
- 6.Collinson, J., K. G. Nicholson, E. Cancio, J. Ashman, D. C. Ireland, V. Hammersley, J. Kent, and C. O'Callaghan. 1996. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 51:1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch, R. B. 1990. Rhinoviruses, p. 607-629. In B. N. Fields and D. M. Knipe (ed.), Virology, Raven Press, New York, N.Y.
- 8.Dragovich, P. S., S. E. Webber, R. E. Babine, S. A. Fuhrman, A. K. Patick, D. A. Matthews, C. A. Lee, S. H. Reich, T. J. Prins, J. T. Marakovits, E. S. Littlefield, R. Zhou, J. Tikhe, C. E. Ford, M. Wallace, J. W. Meador III, R. A. Ferre, E. L. Brown, S. L. Binford, J. E. V. Harr, D. M. DeLisle, and S. T. Worland. 1998. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 1. Michael acceptor structure-activity studies. J. Med. Chem. 41:2806-2818. [DOI] [PubMed] [Google Scholar]
- 9.Dragovich, P. S., T. J. Prins, R. Zhou, T. O. Johnson, E. L. Brown, F. C. Maldonado, S. A. Fuhrman, L. S. Zalman, A. K. Patick, D. A. Matthews, X. Hou, J. W. Meador III, R. A. Ferre, and S. T. Worland. 2002. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. Part 7: structure-activity studies of bicyclic 2-pyridone-containing peptidomimetics. Bioorg. Med. Chem. Lett. 12:733-738. [DOI] [PubMed] [Google Scholar]
- 10.Dragovich, P. S., T. J. Prins, R. Zhou, T. O. Johnson, Y. Hua, H. T. Luu, S. K. Sakata, E. L. Brown, F. C. Maldonado, T. Tuntland, C. A. Lee, S. A. Fuhrman, L. S. Zalman, A. K. Patick, D. A. Matthews, E. Y. Wu, M. Guo, B. C. Borer, N. K. Nayyar, T. Moran, L. Chen, M. C. Guzman, E. Z. Dovalsantos, S. Lee, K. McGee, M. Mohajeri, A. Liese, J. Tao, M. B. Kosa, B. Liu, M. R. Batugo, J. R. Gleeson, Z. P. Wu, J. Liu, J. W. Meador III, and R. A. Ferre. 2003. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 8. Pharmacological optimization of orally bioavailable 2-pyridone-containing peptidomimetics. J. Med. Chem. 46:4572-4585. [DOI] [PubMed] [Google Scholar]
- 11.Fromtling, R. A., and J. Castaner. 1997. VP-63843. Drugs Future 22:40-44. [Google Scholar]
- 12.Gwaltney, J. M., Jr., B. Winther, J. T. Patrie, and J. O. Hendley. 2002. Combined antiviral-antimediator treatment for the common cold. J. Infect. Dis. 186:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden, F. G., R. B. Turner, J. M. Gwaltney, K. Chi-Burris, M. Gersten, P. Hsyu, A. K. Patick, G. J. Smith III, and L. S. Zalman. 2003. Phase II, randomized, double-blind, placebo-controlled studies of rupintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 47:3907-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser, L., C. E. Crump, and F. G. Hayden. 2000. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antiviral Res. 47:215-220. [DOI] [PubMed] [Google Scholar]
- 15.Kean, K. M., M. T. Howell, S. Grünert, M. Girard, and R. J. Jackson. 1993. Substitution mutations at the putative catalytic triad of the poliovirus 3C protease have differential effects on cleavage at different sites. Virology 194:360-364. [DOI] [PubMed] [Google Scholar]
- 16.Kempf, D., A. Hsu, J. Isaacson. 2001. Evaluation of the inhibitory quotient as a pharmacodynamic predictor of the virologic response to protease inhibitor therapy. 2nd International Workshop of Clinical Pharmacology of HIV Therapy, Noordwijk, the Netherlands. Abstract 7.1.
- 17.King, A. M. Q., F. Brown, P. Christian, T. Hovi, T. Hyypiä, N. J. Knowles, S. M. Lemon, P. D. Minor, A. C. Palmenberg, T. Skern, and G. Stanway. 2000. Picornaviridae. p. 657-678. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, C. H. Calisher, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. Wickner (ed.), Virus taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses, R.B. Academic Press, New York, N.Y.
- 18.Krilov, L., L. Pierik, E. Keller, K. Mahan, D. Watson, M. Hirsch, V. Hamparian, and K. McIntosh. 1986. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J. Med. Virol. 19:345-352. [DOI] [PubMed] [Google Scholar]
- 19.Kong, J.-S., S. Venkatraman, K. Furness, S. Nimkar, T. A. Shepherd, Q. M. Wang, J. Aube, and R. P. J. Hanzlik. 1998. Synthesis and evaluation of peptidyl Michael acceptors that inactivate human rhinovirus 3C protease and inhibit virus replication. J. Med. Chem. 41:2579-2587. [DOI] [PubMed] [Google Scholar]
- 20.Matthews, D. A., P. S. Dragovich, S. E. Webber, S. A. Fuhrman, A. K. Patick, L. S. Zalman, T. F. Hendrickson, R. A. Love, T. J. Prins, J. T. Marakovits, R. Zhou, J. Tikhe, C. E. Ford, J. W. Meador III, R. A. Ferre, E. L. Brown, S. L. Binford, M. A. Brothers, D. M. DeLisle, and S. T. Worland. 1999. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. USA 96:11000-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinlay, M. A., D. C. Pevear, M. G. Rossmann. 1992. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Annu. Rev. Microbiol. 46:635-654. [DOI] [PubMed] [Google Scholar]
- 22.Meador, J. W., III, H. Ngo, C. E. Ford, A. K. Patick, R. A. Ferre, D. A. Matthews, and S. T. Worland. 1998. PCR amplification and determination of the RNA sequences for the P3 coding region of human rhinoviral serotypes. Antiviral Res. 37:A72. [Google Scholar]
- 23.Melnick, J. L. 1990. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 549-605. In B. N. Fields and D. M. Knipe (ed.), Virology, Raven Press, New York, N.Y.
- 24.Patick, A. K., S. L. Binford, M. A. Brothers, R. L. Jackson, C. E. Ford, M. D. Diem, F. Maldonado, P. S. Dragovich, R. Zhou, T. J. Prins, S. A. Fuhrman, J. W. Meador III, L. S. Zalman, D. A. Matthews, and S. T. Worland. 1999. Antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 43:2444-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotbart, H. A., and A. D. Webster. 2001. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 32:228-235. [DOI] [PubMed] [Google Scholar]
- 26.Rueckert, R. R. 1990. Picornaviridae and their replication, p. 507-548. In B. N. Fields and D. M. Knipe (ed.), Virology, Raven Press, New York, N.Y.
- 27.Scudiero, D. A., R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, and M. R. Boyd. 1988. XTT evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48:4827-4833. [PubMed] [Google Scholar]
- 28.Seemungal, T. A. R., R. Harper-Owen, A. Bhowmik, D. J. Jeffries, and J. A. Wedzicha. 2000. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 16:677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 30.Webber, S., J. Tikhe, S. T. Worland, S. A. Fuhrman, T. F. Hendrickson, D. A. Matthews, R. A. Love, A. K. Patick, J. W. Meador III, R. A. Ferre, E. L. Brown, D. M. DeLisle, C. E. Ford, and S. L. Binford. 1996. Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J. Med. Chem. 39:5072-5082. [DOI] [PubMed] [Google Scholar]
- 31.Webber, S. E., K. Okano, T. L. Little, S. Reich, Y. Xin, S. T. Worland, S. A. Fuhrman, D. A. Matthews, T. F. Hendrickson, R. A. Love, A. K. Patick, J. W. Meador III, R. A. Ferre, E. L. Brown, C. E. Ford, and S. L. Binford. 1998. Tripeptide aldehyde inhibitors of human rhinovirus 3C protease: design, synthesis, biological evaluation, and cocrystal structure solution of P1 glutamine isosteric replacements. J. Med. Chem. 41:2786-2805. [DOI] [PubMed] [Google Scholar]
- 32.Weislow, O. S., R. Kiser, D. L. Fine, J. Bader, R. H. Shoemaker, and M. R. Boyd. 1989. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for aids-antiviral activity. J. Natl. Cancer Inst. 81:577-586. [DOI] [PubMed] [Google Scholar]
- 33.Witherell, G. 2000. AG-7088 Pfizer. Curr. Opin. Investig. Drugs 1:297-302. [PubMed] [Google Scholar]
- 34.Zalman, L. S., M. A. Brothers, P. S. Dragovich, R. Zhou, T. J. Prins, S. T. Worland, and A. K. Patick. 2000. Inhibition of human rhinovirus-induced cytokine production by AG7088, a human rhinovirus 3C protease inhibitor. Antimicrob. Agents Chemother. 44:1236-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]