Abstract
The in vitro activity of citropin 1.1 against gram-positive cocci was measured by MIC, minimal bactericidal concentration, time-kill studies, and a checkerboard titration method. Streptococci and staphylococci were inhibited at concentrations between 1 and 16 mg/liter, respectively. Enterococci showed less susceptibility. Synergy was demonstrated when citropin 1.1 was combined with clarithromycin and doxycycline.
Staphylococcus aureus and Streptococcus pyogenes are the major organisms responsible for skin and soft-tissue infections (1, 3, 6, 9, 13). Recently, infections caused by multidrug-resistant pathogens have been frequently diagnosed (5, 14, 17). For this reason, it has become critical to identify agents to treat multidrug-resistant gram-positive infections with novel mechanisms of activity (16).
Antimicrobial cationic peptides are a new class of antibiotic, widely distributed in nature as a part of the innate immune system that has been found in many different organisms, including animal, plant, and bacterial species (4, 11, 12). The dual hydrophobic and hydrophilic nature of these molecules, due to a high content of basic amino acid residues and a large distribution of hydrophobic and hydrophilic residues, is important for their interaction with the bacterial membranes that lead to disruption of the ionic homeostasis of the bacteria (11, 12). Amphibians have rich chemical arsenal of antimicrobial peptides (2). Citropin 1.1 is the major antimicrobial peptide produced by both the dorsal and submental glands of the green tree frog Litoria citropa. It is one of the simplest, wide-spectrum amphibian antimicrobial peptides reported to this time (7, 19).
The aim of the present study was to evaluate the in vitro activity of citropin 1.1 against a number of gram-positive cocci causing skin and soft tissue infections as well as to investigate its in vitro interaction with five clinically used antibiotics.
Organisms.
The quality control strains were methicillin-susceptible (MR) S. aureus ATCC 29213, methicillin-resistant (MR) S. aureus ATCC 43300, vancomycin-susceptible (VS) Enterococcus faecalis ATCC 29212, vancomycin-resistant (VR) E. faecalis ATCC 51299, and S. pyogenes ATCC 19615. Twenty nosocomial isolates of each species were tested, with the exception of VR E. faecalis (12 strains). The isolates were obtained from distinct patients coming from central Italy with unrelated sources of infection and admitted to the Hospital Umberto I, Ancona, Italy, from January 2000 to December 2003.
Antimicrobial agents.
Citropin 1.1 (GLFDVIKKVASVIGGLNH2) was synthesized by 9-fluorenylmethoxycarbonyl (Fmoc) solid-phase chemistry by the Faculty of Pharmacy, Medical University of Gdańsk, Poland (10). The peptide was purified by high-pressure liquid chromatography (HPLC) on a Knauer K501 two-pump system and analyzed by chemical analysis and matrix-assisted laser-desorption ionization-time of flight mass spectrometry (MALDI-TOF).
Vancomycin and doxycycline (Sigma-Aldrich, Milan, Italy), amoxicillin-clavulanate (GlaxoSmithKline, Verona, Italy), clarithromycin (Abbott, Rome, Italy) and linezolid (Pharmacia & Upjohn, Kalamazoo, Michigan) were tested as control agents.
MIC and minimal bactericidal concentration (MBC) determinations.
Solutions of drugs were made fresh on the day of assay. The concentration range assayed for citropin 1.1 was 0.125 to 64 mg/liter and 0.250 to 256 mg/liter for the other antimicrobial agents.
MICs were assayed according to the procedures outlined by the National Committee for Clinical Laboratory Standards (15). Polypropylene 96-well plates (Sigma-Aldrich) were incubated for 18 h at 37°C in air. MIC was taken as the lowest drug concentration at which observable growth was inhibited. MBC was taken as the lowest concentration of each drug that resulted in more than 99.9% reduction of the initial inoculum. Experiments were performed in triplicate.
Bacterial killing assay.
The ATCC control strains were used for the bacterial killing assay. Aliquots of exponentially growing bacteria were resuspended in fresh Mueller-Hinton (MH) broth at approximately 107 cells/ml and exposed to citropin 1.1 at 2× MIC until 24 h at 37°C. Samples were serially diluted in 10 mM of sodium HEPES buffer (pH 7.2) to minimize the carryover effect and plated onto MH agar plates to obtain viable colonies.
Synergy studies.
Six strains of MS S. aureus, six of VS E. faecalis, and six of S. pyogenes were used to test the antibiotic combinations by a checkerboard titration method by using 96-well polypropylene microtiter plates. The fractionary inhibitory concentration (FIC) index for combinations of two antimicrobials was calculated according to the following equation: FIC index = FICA + FICB = A/MICA + B/MICB, where A and B are the MICs of drug A and drug B in the combination, MICA and MICB are the MICs of drug A and drug B alone, and FICA and FICB are the FICs of drug A and drug B. The FIC indexes were interpreted as follows: <0.5, synergy; 0. to 4.0, indifferent; and >4.0, antagonism (8).
Results.
All isolates were inhibited by citropin 1.1 at concentrations of 1 to 32 mg/liter. In details, for the control strain S. aureus ATCC 29213, S. aureus ATCC 43300, E. faecalis ATCC 29212, E. faecalis ATCC 51299, and S. pyogenes ATCC 19615 the peptide exhibited MIC of 8 mg/liter, 8 mg/liter, 16 mg/liter, 16 mg/liter, and 4 mg/liter and MBC of 16 mg/liter, 16 mg/liter, 32 mg/liter, 32 mg/liter, and 8 mg/liter, respectively. Overall, high rates of resistance to the clinically used antibiotics were demonstrated for multiresistant strains with the exception of linezolid. The results are summarized in Table 1.
TABLE 1.
MICs and MBCs of citropin 1.1 and other clinically used antibiotics for 90 clinical isolates
| Strains (n) and agent | MIC (mg/liter)
|
MBC (mg/liter)
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| MR S. aureus (20) | ||||||
| Citropin 1.1 | 1-16 | 4 | 8 | 2-32 | 8 | 16 |
| AMX/CLVa | 2-128 | 16 | 128 | 8-128 | 64 | 256 |
| Doxycycline | 0.50-16 | 4 | 8 | 2-64 | 8 | 32 |
| Clarithromycin | 0.50-16 | 4 | 16 | 2-128 | 32 | 128 |
| Linezolid | 0.25-4 | 0.5 | 2 | 0.50-2 | 0.5 | 2 |
| Vancomycin | 0.12-4 | 1 | 2 | 0.50-4 | 1 | 2 |
| MS S. aureus 20 | ||||||
| Citropin 1.1 | 1-16 | 4 | 8 | 2-16 | 8 | 16 |
| AMX/CLVa | 0.25-8 | 2 | 4 | 0.5-32 | 8 | 16 |
| Doxycycline | 0.50-8 | 4 | 8 | 2-32 | 8 | 32 |
| Clarithromycin | 0.50-16 | 2 | 8 | 2-64 | 16 | 64 |
| Linezolid | 0.12-2 | 0.5 | 2 | 0.50-2 | 0.5 | 2 |
| VS Enterococcus faecalis 30 | ||||||
| Citropin 1.1 | 2-32 | 8 | 16 | 2-32 | 16 | 32 |
| AMX/CLVa | 1-64 | 8 | 16 | 4-256 | 16 | 64 |
| Doxycycline | 1-32 | 8 | 16 | 4-128 | 16 | 64 |
| Clarithromycin | 4-128 | 8 | 32 | 8-256 | 16 | 128 |
| Linezolid | 0.50-4 | 1 | 2 | 0.5-4 | 2 | 4 |
| Vancomycin | 0.25-4 | 1 | 2 | 1-4 | 2 | 4 |
| VR Enterococcus faecalis 12 | ||||||
| Citropin 1.1 | 2-32 | 8 | 16 | 2-64 | 16 | 32 |
| AMX/CLVa | 4-128 | 16 | 32 | 8-256 | 64 | 128 |
| Doxycycline | 2-64 | 16 | 32 | 8-64 | 16 | 64 |
| Clarithromycin | 8-128 | 16 | 64 | 16-256 | 32 | 128 |
| Linezolid | 0.50-4 | 1 | 2 | 1-4 | 4 | 4 |
| Vancomycin | 32-128 | 32 | 64 | 64-256 | 64 | 256 |
| Streptococcus pyogenes 20 | ||||||
| Citropin 1.1 | 1-8 | 2 | 4 | 2-16 | 4 | 8 |
| AMX/CLVa | 0.25-2 | 0.50 | 1 | 0.5-4 | 1 | 4 |
| Doxycycline | 0.50-8 | 4 | 8 | 1-32 | 8 | 16 |
| Clarithromycin | 0.50-8 | 2 | 8 | 1-32 | 8 | 32 |
| Linezolid | 0.12-2 | 0.5 | 1 | 0.50-2 | 1 | 2 |
| Vancomycin | 0.25-2 | 0.5 | 1 | 0.50-2 | 1 | 2 |
AMX/CLV, amoxicillin-clavulanate, ratio 7:1.
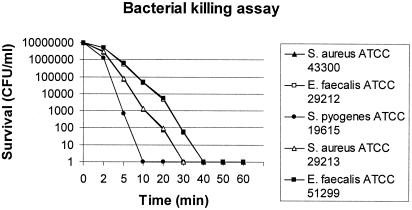
Killing by citropin 1.1 was shown to be very rapid: its activity was complete after 10 to 40 min at a concentration of 2× MIC (Fig. 1).
FIG. 1.
Time-kill kinetics of citropin 1.1 against the quality control strains methicillin-susceptible (MR) S. aureus ATCC 29213, methicillin-resistant (MR) S. aureus ATCC 43300, vancomycin-susceptible (VS) E. faecalis ATCC 29212, vancomycin-resistant (VR) E. faecalis ATCC 51299, and S. pyogenes ATCC 19615.
Synergy was never observed, with the exception of the combinations between citropin 1.1 and doxycycline or clarithromycin. FIC indexes of 0.385, 0.385, and 0.312 were observed by testing citropin 1.1 combined to doxycycline for S. aureus, E. faecalis, and S. pyogenes, respectively. Moreover, FIC indexes ranging from 0.312 to 0.458 were observed when citropin 1.1 was combined with clarithromycin (Table 2).
TABLE 2.
Results of interaction studies between citropin 1.1 and other drugsa
| Agent | FIC indexes for citropin 1.1 (range)
|
||
|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis | Streptococcus pyogenes | |
| AMX/CLV | 0.917 (0.750-1.250) | 0.927 (0.750-1.250) | 1.292 (0.750-2.00) |
| Doxycycline | 0.385 (0.312-0.50) | 0.312 (0.187-0.50) | 0.385 (0.312-0.50) |
| Clarithromycin | 0.312 (0.187-0.50) | 0.312 (0.187-0.50) | 0.458 (0.312-0.50) |
| Linezolid | 1.50 (1.00-2.00) | 1.458 (1.250-2.00) | 1.50 (1.00-2.00) |
| Vancomycin | 1.167 (1.00-1.50) | 1.833 (1.50-2.00) | 1.292 (0.750-2.00) |
The ranges of concentrations tested were: 0.125 to 64 mg/liters for citropin 1.1 and 0.25 to 256 mg/liters for the other antimicrobial agents. AMX/CLV, amoxicillin-clavulanate.
Discussion.
In the present study, in vitro experiments with citropin 1.1 were performed to determine its bactericidal activity and if synergism, antagonism, or indifference would be the predominant response when the combinations with other clinically used antibiotics were tested against gram-positive cocci.
Interestingly, citropin 1.1 demonstrated to be equally active against both susceptible and multiresistant clinical isolates. Time-killing studies have shown a rapid bactericidal effect, even if the inactivation of E. faecalis appears to be slower than that observed for the other gram-positive cocci.
Combination studies showed that citropin 1.1 acted synergistically with hydrophobic antibiotics. The interaction between peptides and macrolides or tetracycline has not yet been extensively studied. The mechanism of this positive interaction remains largely unknown, even though it is probable that the damage of membrane induced by the peptide can allow a maximal entry of the hydrophobic substrates (18). Citropin 1.1 might increase the access of the hydrophobic antibiotics to the cytoplasmic membrane following breakdown of peptidoglycan while, on the other hand, it is possible that hydrophobic antibiotics create new sites in the biological membranes for peptide entry. In addition, some peptides are thought to inhibit synthesis of DNA, RNA, and cellular proteins and to be able to insert themselves into the cytoplasmic membrane, triggering the activity of bacterial murein hydrolases and leading to degradation of the peptidoglycan with lysis of the cell.
Citropin 1.1 may be a promising antibacterial drug with a new mechanism of action and an adjuvant for antimicrobial therapy.
Acknowledgments
This work was supported by the Italian Ministry of Education, University, and Research (PRIN 2003).
REFERENCES
- 1.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, B. I. Eisenstein, and the daptomycin 98-01 and 99-01 Investigator. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. [DOI] [PubMed] [Google Scholar]
- 2.Bevins, C. L., and M. Zasloff. 1990. Peptides from frog skin. Annu. Rev. Biochem. 59:395-414. [DOI] [PubMed] [Google Scholar]
- 3.Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334:240-245. [DOI] [PubMed] [Google Scholar]
- 4.Cannon, M. 1987. A family of wound healers. Nature 328:478. [DOI] [PubMed] [Google Scholar]
- 5.Cormican, M. G., and R. N. Jones. 1996. Emerging resistance to antimicrobial agents in Gram-positive bacteria. Enterococci, staphylococci and nonpneumococcal streptococci. Drugs 51:S6-S12. [DOI] [PubMed] [Google Scholar]
- 6.DiNubile, M. J., and B. A. Lipsky. 2004. Complicated infections of skin and structures: when the infection is more than skin deep. J. Antimicrob. Chemother. 53(Suppl. 2):ii37-ii50. [DOI] [PubMed] [Google Scholar]
- 7.Doyle, J., C. S. Brinkworth, K. L. Wegener, J. A. Carver, L. E. Llewellin, I. N. Olver, J. H. Bowie, P. A. Wabnitz, and M. J. Tyler. 2003. nNOS inhibition, antyimicrobial and anticancer activity of the amphibian skin peptide, citropin 1.1 and synthetic modifications. The solution structure of a modified citropin 1.1. Eur. J. Biochem. 270:1141-1153. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-393. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 9.Elston, D. M. 2004. Epidemiology and prevention of skin and soft tissue infections. Cutis 73:S3-S7. [PubMed] [Google Scholar]
- 10.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Peptide Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 11.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn, R. M., and E. J. C. Goldstein. 1993. Common bacterial skin infections: diagnostic clues and therapeutic options. Postgrad. Med. 93:175-182. [DOI] [PubMed] [Google Scholar]
- 14.Linden, P. K. 2002. Treatment options for vancomycin-resistant enterococcal infections. Drugs 62:425-441. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 16.Raghavan, M., and P. K. Linden. 2004. Newer treatment options for skin and soft tissue infections. Drugs 64:1621-1642. [DOI] [PubMed] [Google Scholar]
- 17.Tenover, F. C., J. W. Biddle, and M. V. Lancaster. 2001. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus Emerg. Infect. Dis. 7:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaara, M., and M. Porro. 1996. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob. Agents Chemother. 40:1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wabnitz, P. A., J. H. Bowie, J. C. Wallace, and M. J. Tyler. 1999. The antibiotic citropin peptides from the Australian tre frog Litoria citropa. Structure determination using electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 13:1724-1732. [DOI] [PubMed] [Google Scholar]