Abstract
Hemorrhagic fever of arenaviral origin is a frequently fatal infectious disease of considerable priority to the biodefense mission. Historically, the treatment of arenaviral infections with alpha interferons has not yielded favorable results. Here we present evidence that interferon alfacon-1, a nonnaturally occurring bioengineered alpha interferon approved for the treatment of chronic hepatitis C, is active against Pichinde and Tacaribe arenaviruses in cell culture. In the hamster model of Pichinde virus (PCV) infection, interferon alfacon-1 treatment significantly protected animals from death, prolonged the survival of those that eventually died, reduced virus titers, and limited liver damage characteristic of PCV-induced disease. Moreover, interferon alfacon-1 also demonstrated therapeutic activity, to a lesser degree, when the initiation of treatment was delayed up to 2 days post-virus challenge. Despite the observed advantages of interferon alfacon-1 therapy, efforts to stimulate the immune system with the known interferon inducer poly(I:C12U) (Ampligen) offered only limited protection against lethal PCV challenge. Taken together, these data suggest that the increased potency of the bio-optimized interferon alfacon-1 molecule may be critical to the observed antiviral effects. These data are the first report demonstrating efficacious treatment of acute arenaviral disease with alpha interferon therapy, and further study is warranted.
Several viruses of Arenaviridae family pose a considerable threat to humans. Infection by Lassa, Junin (Argentina), Machupo (Bolivia), Guanarito (Venezuela), and Sabia (Brazil) arenaviruses can lead to hemorrhagic fever which is often fatal (8). With the exception of ribavirin treatment of Lassa fever (27) and a candidate experimental vaccine for Argentine hemorrhagic fever (25), no treatments or vaccines exist for these deadly viruses. In light of the description above and the potential for intentional release and adverse public health impact, these viruses have been classified as select agents by the Department of Health and Human Services and have been prioritized as category A pathogens by the National Institute for Allergy and Infectious Diseases and the Centers for Disease Control and Prevention. In many cases, because of the rapid onset of disease, postexposure prophylaxis from vaccines may not be beneficial. Thus, measures to treat these infections must be sought.
Interferon alfacon-1 (Infergen) is an expanded-spectrum cytokine that was engineered to contain the most frequently occurring amino acids among the nonallelic alpha interferon (IFN-α) subtypes. In cell culture models, interferon alfacon-1, also known as consensus interferon, demonstrates increased potency compared to naturally occurring alpha interferons (4) and is a more potent inhibitor of hepatitis C virus replication in comparative clinical trials with naturally occurring alpha interferons (44). Further, there is considerable evidence that consensus IFN-α is efficacious against herpesvirus infections in hamsters (14, 15). More recently, the beneficial activity of interferon alfacon-1 in hamsters challenged with West Nile virus was shown, with a measurable increase in survival and reduction of disease symptoms (30, 31). Hepatitis C and West Nile viruses are members of the Flaviviridae family, suggesting that interferon alfacon-1 may be an effective therapy for treatment of flavivirus infections. Interferon alfacon-1 is currently approved by the Food and Drug Administration for treating chronic hepatitis C worldwide.
A number of independent studies have shown that acute arenaviral infections are relatively insensitive to interferon treatment in vitro and in animal models (7, 11, 24, 35, 42). However, a recent report demonstrated that IFN-α can inhibit the replication of several strains of Lassa virus in vitro (3). Due to the requirement of maximum-level containment facilities and the dangers associated with working with arenaviruses that cause hemorrhagic fever in humans, the activity of interferon alfacon-1 was evaluated against related, less biohazardous arenaviruses in cell culture and in vivo in the hamster model of Pichinde virus (PCV) infection previously described (41). The PCV hamster model of acute infection serves as a surrogate model system for identifying potential prophylactic and therapeutic agents that may be efficacious against the more biohazardous arenaviruses. Others have demonstrated that PCV infection of hamsters and guinea pigs provides suitable models for human arenaviral hemorrhagic fever diseases (2, 6, 9, 10, 21, 33). This study was undertaken to provide insight into the potential use of consensus IFN-α for the treatment of acute arenaviral disease.
MATERIALS AND METHODS
Viruses and cells.
PCV, strain An 4763, was kindly provided by David Gangemi (Clemson University, Clemson, South Carolina). The virus used for in vivo studies was passaged once through hamsters. Virus stocks were prepared from pooled liver homogenates from infected hamsters. For cell culture experiments, PCV was routinely passaged in the African green monkey kidney cell line BS-C-1 (American Type Culture Collection [ATCC], Manassas, VA). Amapari (AMV), Latino (LAV), Tacaribe (TAV), and Tamiami (TMV) arenaviruses were obtained from ATCC, and were also passaged in BS-C-1 cells for preparation of stocks for in vitro use. BS-C-1 cells were maintained in minimal essential medium (MEM; Gibco, Invitrogen, Carlsbad, CA) supplemented with 0.1% NaHCO3, 50 μg/ml gentamicin (Sigma, St. Louis, MO), and 10% fetal bovine serum (HyClone, Logan, UT). Cells were plated the day prior to the start of experiments so that they would be ∼95% confluent at the time of treatment.
Animals.
Weanling female 45- to 55-g golden Syrian hamsters were obtained from Charles River Laboratories (Wilmington, MA) and quarantined for 48 h prior to use. In one experiment designed to evaluate poly(I:C12U) (Ampligen) in older animals, 91- to 100-g hamsters were purchased and allowed to acclimate 6 days prior to the start of the study. Hamsters were fed standard hamster chow and tap water ad libitum. All animal procedures used in this study complied with guidelines set by the U.S. Department of Agriculture and Utah State University's Institutional Animal Care and Use Committee.
Materials.
Interferon alfacon-1 was kindly provided by InterMune, Inc. (Brisbane, CA), and had a reported activity of 109 units/mg. Poly(I:C12U) was generously provided by Hemispherx Biopharma, Inc. (Philadelphia, PA). Loxoribine was purchased from Invivogen (San Diego, CA) and prepared in 2% NaHCO3 solution, pH 8.7. Ribavirin, previously shown to be effective against PCV infection in hamsters (41), was used as the positive control and supplied by ICN Pharmaceuticals, Inc. (Costa Mesa, CA). With the exception of loxoribine, all drugs were prepared in sterile saline for use in vivo.
Cytopathic effect (CPE) reduction assays and toxicity determination.
Antiviral activity studies were all performed with 96-well microplates. Half-log dilutions of interferon alfacon-1 diluted in MEM supplemented with 2% fetal bovine serum were added to test wells (100 μl/well) 24 h prior to infection with viruses. Equal volumes of virus were diluted in MEM supplemented with 2% fetal bovine serum to an optimal multiplicity of infection that would give rise to maximal CPE in BS-C-1 cells by visual examination in preliminary virus titration experiments. Ribavirin treatment was done in the same manner except that it was added at the time of infection. For toxicity determinations, drugs were added in the absence of viral challenge. Plates were incubated at 37°C with 5% CO2 until virus-infected control wells were observed to have complete viral CPE, at which time the plates were scored for toxicity and CPE in the drug-treated test wells. Following visual inspection, 0.034% neutral red (NR) solution was added to each test well and incubated for 2 h at 37°C with 5% CO2. After incubation, the NR solution was removed, the wells were rinsed and dried, and the dye was extracted for 30 min at room temperature in the dark with absolute ethanol buffered with Sorenson's citrate buffer. Plates were read at 540 nm on a Bio-Tek EL 309 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT), and the absorbance values were expressed as percentages of uninfected, untreated control cells. The 50% virus-inhibitory or effective concentration (EC50) and the 50% cytotoxic concentration (IC50) were determined by regression analysis and selectivity index (SI) values were calculated as IC50/EC50. In certain cases, EC50 values appear much higher than their respective IC50 values. This occurs as a consequence of the normalization procedure incorporated into the evaluation of NR data in which cell death associated with drug toxicity is subtracted from the parallel antiviral test and the EC50 is determined based on the remaining population of viable cells.
In vivo studies.
Hamsters were randomly assigned to groups that were treated by the intraperitoneal (i.p.) route (see Tables 2 and 3) with interferon alfacon-1, ribavirin, or saline (placebo) prior to or after i.p. inoculation with 650 PFU of PCV. Interferon alfacon-1 was administered once a day, and ribavirin twice a day, for 7 days. Where indicated, the initiation of interferon alfacon-1 therapy was delayed until 24 or 48 h post-virus challenge (see Table 3). Five animals from each group were sacrificed on day 6, since they became moribund and began to die by day 7. Liver and serum were collected from each hamster for virus titer determinations and serum liver enzyme analysis, the latter as an indicator of liver damage. The remaining 10 animals (20 for the saline placebo group) were observed for 21 days for death. To assess potential toxicity associated with treatment, three uninfected hamsters from each group were observed for death and their weights measured the day of initial treatment and 24 h after the cessation of treatment. Studies with poly(I:C12U) were conducted similarly but primarily with a single-dose treatment 48, 24, and 4 h pre-virus inoculation and 24 h post-virus inoculation with the indicated doses. For the experiment using older hamsters, poly(I:C12U) was given once every 4 days starting 24 h before virus challenge and ending on day 7.
TABLE 2.
Effect of intraperitoneal interferon alfacon-1 treatmenta on hamsters challenged with Pichinde virus
| Drug | Dosage (mg/kg/day) | No. of survivors/total | Mean day of deathb ± SD | Mean virus titer ± SD
|
Mean serum ALTef (IU/liter) ± SD | |
|---|---|---|---|---|---|---|
| Liverc | Serumd | |||||
| Interferon alfacon-1 | 0.01 | 8/10*** | 12.5 ± 2.1* | 4.6 ± 0.1** | 4.6 ± 0.1** | 24 ± 18** |
| Interferon alfacon-1 | 0.001 | 4/10** | 9.5 ± 1.0* | 7.8 ± 0.5* | 6.7 ± 0.9* | 65 ± 33** |
| Ribavirin | 40 | 10/10*** | 4.5 ± 0.0** | 4.8 ± 0.5** | 24 ± 15** | |
| Saline | 0/20 | 8.2 ± 0.8 | 9.0 ± 0.4 | 8.6 ± 0.8 | 294 ± 127 | |
Once daily for 7 days beginning 4 h pre-virus inoculation (ribavirin administered twice daily). *indicates a P of <0.05, **indicates a P of <0.01, and *** indicates a P of <0.001 in comparison to saline-treated controls.
Mean day of death of hamsters dying prior to day 21.
log10 cell culture 50% infectious dose/g.
log10 cell culture 50% infectious dose/ml.
ALT, alanine aminotransferase; measured in international units per liter.
ALT and virus titers were determined for five hamsters/group sacrificed on day 6.
TABLE 3.
Effect of delayed intraperitoneal interferon alfacon-1 therapya on Pichinde virus-infected hamsters
| Drug | Dosage (mg/kg/day)/start of treatment (h) | No. of survivors/total | Mean day of deathb ± SD | Mean virus titer ± SD
|
Mean serum ALTef (IU/liter) ± SD | |
|---|---|---|---|---|---|---|
| Liverc | Serumd | |||||
| Interferon alfacon-1 | 0.02/−4 | 8/10*** | 17.5 ± 0.7* | 4.5 ± 0.0** | ≤2.8** | 29 ± 18* |
| 0.01/−4 | 8/10*** | 12.5 ± 2.1* | 4.4 ± 1.0* | ≤2.8** | 17 ± 4** | |
| 0.02/24 | 7/10*** | 9.7 ± 0.6 | 6.5 ± 0.9 | 5.9 ± 0.7 | 62 ± 43 | |
| 0.01/24 | 4/10** | 10.8 ± 2.8* | 7.1 ± 1.5 | 5.4 ± 1.7 | 281 ± 200 | |
| 0.02/48 | 2/10 | 9.1 ± 1.6 | 7.4 ± 0.5 | 6.5 ± 0.8 | 46 ± 13* | |
| 0.01/48 | 2/10 | 9.8 ± 3.9 | 8.1 ± 1.1 | 7.4 ± 1.1 | 99 ± 72 | |
| Ribavirin | 40/−4 | 10/10*** | 4.7 ± 0.2 | 3.4 ± 0.9** | 30 ± 8** | |
| Saline | /−4 | 0/20 | 8.8 ± 2.8 | 7.6 ± 1.7 | 7.0 ± 1.0 | 455 ± 584 |
| Sham-infected | 3/3 | 42 ± 11* | ||||
Once daily for 7 days beginning at indicated times pre-virus inoculation (ribavirin administered twice daily). *indicates a P of <0.05, **indicates a P of <0.01, and ***indicates a P of <0.001 in comparison to saline-treated controls.
Mean day of death of hamsters dying prior to day 21.
log10 cell culture 50% infectious dose/g.
log10 cell culture 50% infectious dose (assay detection limit was 2.8 log10 CCID50/ml).
ALT, alanine aminotransferase, is measured in international units per liter.
ALT and virus titers were determined for five hamsters/group sacrificed on day 6.
Virus titer and serum alanine aminotransferase (ALT) determinations.
Virus titers were assayed using an infectious cell culture assay as previously described (41). Briefly, a specific volume of liver homogenate or serum was serially diluted and added to triplicate wells of BS-C-1 cell monolayers in 96-well microplates. The viral CPE was determined 7 days post-virus inoculation, and the 50% endpoints were calculated as described previously (36). Serum ALT levels were measured using the ALT (serum glutamic pyruvic transaminase) reagent set purchased from Pointe Scientific, Inc. (Lincoln Park, MI), following the manufacturer's recommendations. The assay was modified for analysis on 96-well microplates.
Assay for alpha interferon.
Induction of alpha interferon by poly(I:C12U) was determined using a bioassay adapted for the hamster system from previously described methods (37). Briefly, Chinese hamster ovary (CHO-K1) cells (CCL-61 [ATCC]) seeded in 96-well microtiter plates were preincubated with diluted serum samples obtained from 100-g hamsters treated with poly(I:C12U) or loxoribine. After a 6-h preincubation period, the Indiana strain of vesicular stomatitis virus (VSV) was added (2 × 103 cell culture 50% infectious dose [CCID50]/well) and the ability of the samples to inhibit CPE was evaluated visually and by NR assay. Interferon alfacon-1 was included as a positive control.
Statistical analysis.
The Fisher's exact test (two-tailed) was used for evaluating increases in total survivors. The Mann-Whitney test (two-tailed) was performed to analyze the differences in the mean days of death (MDD), virus titers, and serum ALT levels.
RESULTS
In vitro antiviral activity of interferon alfacon-1.
Despite the fact that historically the treatment of acute arenaviral infections with alpha interferon or interferon inducers has met with little success, interferon alfacon-1 was found to be active in vitro against PCV and TAV by the NR uptake assay (Table 1). Visual analysis of CPE reduction was consistent with these findings, and activity against both interferon alfacon-1-sensitive viruses was further verified by virus yield reduction (data not shown). For comparison, the activity of interferon alfacon-1 against VSV was also evaluated. As expected, EC50 values were markedly lower against VSV (<0.032 ng/ml), known to be highly sensitive to the effects of alpha interferon. Although the effect of interferon treatment was considerably more refractory against TAV than PCV, ribavirin was equally effective, as indicated by the similar SI values (Table 1). Not surprisingly, extended incubation resulted in increased toxicity from ribavirin treatment. With Vero 76 cells, similar results were obtained for PCV and TAV (data not shown), while the other arenaviruses tested did not produce discernible CPEs. BS-C-1-cell CPEs caused by AMV, LAV, and TMV appeared similar and were characterized predominantly by cell elongation, granularity, and extended incubation (∼12 to 13 days) before monolayer damage and limited clearing was evident. In contrast, PCV and TAV caused cell rounding and more dramatic destruction of the cell monolayers. However, there were clear differences in the degrees of rounding and the times required for maximal CPEs to develop, which were 7 days for PCV and 10 days for TAV.
TABLE 1.
Neutral red determination of inhibitory effects of interferon alfacon-1 against New World arenaviruses in vitroa
| Virus | Strain | Incubation period (days) | Drug characteristicb
|
|||||
|---|---|---|---|---|---|---|---|---|
| Interferon alfacon-1
|
Ribavirin
|
|||||||
| IC50 ± SD (ng/ml) | EC50 ± SD (ng/ml) | SI | IC50 ± SD (μg/ml) | EC50 ± SD (μg/ml) | SI | |||
| Amapari | Be An 70563 | 13 | >83 ± 29 | >100 | 1 | 67 ± 30 | 664 ± 291 | 0 |
| Latino | 10924 | 12 | >80 ± 35 | >100 | 1 | 110 ± 63 | 260 ± 143 | 0 |
| Pichinde | An 4763 | 7 | >98 ± 4 | 2.0 ± 0.3 | >49 | 376 ± 209 | 37 ± 10 | 10 |
| Tacaribe | TRVL 11573 | 10 | >100 | 0.4 ± 0.3 | >250 | 110 ± 51 | 9 ± 5 | 12 |
| Tamiami | W-10777 | 13 | >100 | >100 | 1 | 90 ± 47 | 405 ± 138 | 0 |
Data are the means and standard deviations from three or four independent NR experiments.
SI, calculated as IC50/EC50, is the selectivity (therapeutic) index.
In vivo efficacy of interferon alfacon-1 against PCV.
On the basis of several independent studies that showed marked antiviral activity against PCV in vitro, the efficacy of interferon alfacon-1 was evaluated in vivo. In the initial investigation, two doses were chosen based on previous reports (1, 14, 30). Hamsters treated with interferon alfacon-1, ribavirin, or a placebo were challenged with a lethal dose of PCV as described in Materials and Methods. At a dosage of 0.01 mg/kg of body weight/day, interferon alfacon-1 protected 80% of the animals, and those that died survived appreciably longer than the saline-treated hamsters (Table 2). At the lower dose (0.001 mg/kg/day), interferon alfacon-1 was still able to afford protection to 40% of infected animals and significantly extend their MDD (Table 2). No obvious toxicity in uninfected, toxicity control animals treated with interferon alfacon-1 was seen. They were apparently healthy and steadily gained weight (data not shown). In addition to the group used for the survival analysis, another group of animals undergoing interferon alfacon-1, ribavirin, or placebo therapy were sacrificed at day 6 for the analysis of additional disease parameters. As shown in Table 2, interferon alfacon-1 was comparable to ribavirin in its ability to reduce liver and serum virus titers. As an indirect method for evaluating liver damage resulting from viral disease, ALT activity in serum samples from sacrificed animals was assayed. It has previously been demonstrated that elevated levels of ALT are associated with PCV infection in hamsters and that treatment with ribavirin effectively abrogates this increase in ALT activity (41). Interferon alfacon-1 was equally effective as ribavirin in its capacity to prevent elevation of serum ALT levels as seen with the saline-treated animals (Table 2). The 0.001-mg/kg/day treatment was also effective in reducing virus titers and serum ALT but, consistent with the findings mentioned above, to a lesser degree (Table 2). Thus, although not as effective as the 0.01-mg/kg/day treatment regimen, the lower dose was beneficial in that it was able to ameliorate the infection outcome and significantly lessen the liver pathology and viral load.
Since interferon alfacon-1 was efficacious in our initial investigation, the study was expanded to evaluate additional treatment regimens. Since we were unable to achieve 100% protection in the first experiment, a higher dose (0.020 mg/kg/day) of interferon alfacon-1 was tested. Moreover, we evaluated whether treatment initiated 24 or 48 h post-PCV challenge would still have a beneficial impact on disease outcome. The results of the second experiment are summarized in Table 3. When treatment was started 4 h prior to PCV challenge, the 0.01- and 0.02-mg/kg/day doses protected the same percentage of animals (80%); however, the higher dose extended the MDD by 5 days. Starting treatment 24 h after virus inoculation also resulted in significant increases in the numbers of survivors at both doses of interferon alfacon-1, with the most dramatic effect observed with the higher dose, protecting almost as well as the treatments initiated 4 h prior to challenge. When the start of interferon alfacon-1 therapy was delayed until 48 h post-PCV challenge, there was a slight increase in the percentage of survivors and the MDD for these animals, but these results were not statistically significant in comparison to the saline-treated placebo group. Ribavirin treatment, initiated 4 h prior to virus challenge, protected 100% of infected animals. Additional therapy schedules with ribavirin were not evaluated. As observed in the initial study, interferon alfacon-1 had a remarkable effect on various disease parameters used to measure disease severity associated with PCV infection (Table 3). Although slight reductions were evident therapeutically, only treatment initiated prior to PCV challenge was significantly helpful in limiting liver and serum virus titers, as observed with ribavirin. With the exception of the 0.01-mg/kg/day dose initiated 24 h postchallenge, interferon alfacon-1 dramatically lowered the levels of serum ALT compared to those present in the infected placebo-treated animals (Table 3). It remains unclear as to why this exceptional group would present with higher levels than those seen with the 48-h-treatment groups. As with the first experiment, both doses of interferon alfacon-1 were well tolerated by the hamsters, with no apparent weight loss in toxicity controls during and after the course of treatment (data not shown).
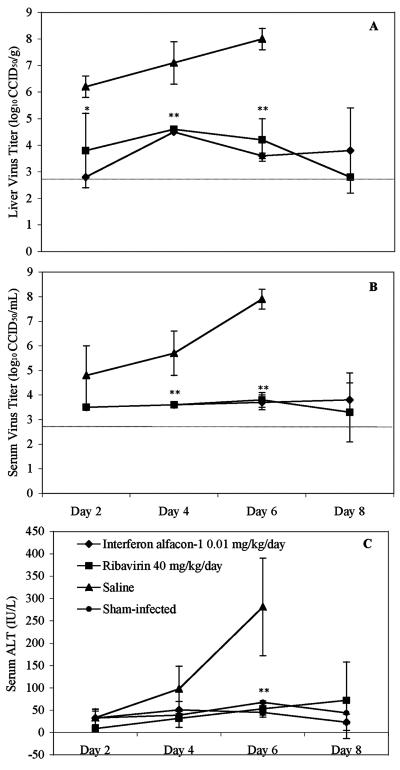
Despite the ability of interferon alfacon-1 to reduce viral loads and liver disease equal to that of the positive-control drug, ribavirin, complete protection (100% survival rate) could not be achieved with the treatment regimens tested. To further investigate potential differences between the two treatments that may account for the slight disparity in survival, a time-course study evaluating virus titers and serum ALT, every other day through the course of treatment initiated 4 h prechallenge, was conducted. As shown in Fig. 1A and B, liver virus titers slightly decrease over time, whereas serum virus titers remain fairly constant following interferon alfacon-1 and ribavirin treatment. Although there appear to be slightly lower virus titers with the ribavirin group, the differences were not statistically significant in this analysis limited to five hamsters per group. There were no appreciable differences between the two treatment groups when comparing serum ALT levels (Fig. 1C). Collectively, these data do not show any remarkable differences in these disease parameters in a comparison of interferon alfacon-1 and ribavirin.
FIG. 1.
Analysis of PCV viral load (A and B) and serum ALT levels (C) following treatment with interferon alfacon-1 or ribavirin initiated 4 h prechallenge. The limit of detection for the virus titer assays was 2.8 log10 CCID50 per g of liver tissue (A) or ml of serum (B) and is indicated by the hatched gray lines. Values represent the means and standard deviations of groups of five animals (three for the sham-infected controls; ALT) sacrificed on the indicated days. Values were not obtained for the infected saline-treated placebo group for the day 8 time point, since there were no survivors. * indicates a P of <0.05 and ** indicates a P of <0.01 in comparison to saline-treated controls.
Effectiveness of poly(I:C12U) against PCV challenge.
In light of the beneficial effects seen with consensus IFN-α, the ability of poly(I:C12U), a known interferon inducer, to confer similar protection against PCV challenge was investigated. For mice, poly(I:C12U) has been shown to dramatically increase alpha interferon levels within a few hours of administration, and a single dose is sufficient to provide complete protection against Punta Toro virus, a member of the Bunyaviridae family of viruses (40). In the initial experiment, only the 1-mg/kg/day dose, given 4 h prior to virus inoculation, provided significant protection as indicated by the number of survivors, higher MDD, and reduced virus titers (Table 4). When treatment with the same dose of poly(I:C12U) was delayed until 24 h after challenge, no protection was observed (Table 4). In the second experiment, lower doses of poly(I:C12U) were chosen based on our initial findings and the drug was given 1 and 2 days before virus challenge. As shown in Table 4, only the lowest dosage of poly(I:C12U) (0.25 mg/kg/day), administered 24 h before PCV challenge, protected a significant number of animals from death. For treatment of humans, the standard for poly(I:C12U) administration is twice per week (W. M. Mitchell, personal communication). Therefore, a third experiment using older hamsters and a multidose treatment regimen was also conducted in efforts to obtain a more robust immunostimulatory effect. Although a slight protective effect was evident for both multidose poly(I:C12U) treatment groups (Table 4), the results were not statistically significant, and overt toxicity was observed with the 1-mg/kg/day group as all three animals in the parallel toxicity study died on days 14 (two animals) and 15 (one animal) after the initiation of treatment (data not shown). No toxicity was evident with the lower multidose or any of the single dose poly(I:C12U) treatments, as the animals gained weight and appeared healthy throughout the course of the experiments (data not shown). Although a slight protective effect was evident with some of the treatment doses and schedules tested, collectively, the results were disappointing, as our attempt to beneficially modulate the antiviral immune response in hamsters with poly(I:C12U) treatment met with very limited success.
TABLE 4.
Effect of intraperitoneal poly(I:C12U) treatment on hamsters challenged with Pichinde virus
| Drug | Dosage (mg/kg/day)/ start of treatment (h) | No. of survivors/total | Mean day of deathc ± SD | Mean virus titer ± SD
|
Mean serum ALTfg (IU/liter) ± SD | |
|---|---|---|---|---|---|---|
| Liverd | Serume | |||||
| Experiment 1 | ||||||
| poly(I:C12U)a | 4/−4 | 0/10 | 11.0 ± 4.0 | 7.9 ± 0.9 | 7.3 ± 1.5 | 233 ± 164 |
| 4/24 | 0/10 | 9.6 ± 1.6 | 7.4 ± 1.3 | 6.9 ± 1.0* | 240 ± 67 | |
| 1/−4 | 3/10* | 10.3 ± 2.2* | 6.9 ± 0.8* | 6.6 ± 1.2* | 89 ± 40 | |
| 1/24 | 0/10 | 9.7 ± 3.4 | 8.5 ± 0.3 | 8.2 ± 0.6 | 434 ± 217 | |
| Ribavirin | 40/−4 | 10/10*** | 4.7 ± 1.2** | 4.7 ± 1.2** | 34 ± 25 | |
| Salinea | /−4 | 0/20 | 9.0 ± 2.3 | 8.3 ± 0.5 | 8.2 ± 0.4 | 139 ± 25 |
| Sham-infected | 3/3*** | 60 ± 43 | ||||
| Experiment 2 | ||||||
| poly(I:C12U)a | 1/−24 | 1/10 | 11.0 ± 0.8 | 7.4 ± 0.7 | 6.1 ± 1.5 | 85 ± 24* |
| 0.25/−24 | 3/10* | 10.1 ± 0.4 | 7.2 ± 1.6 | 6.9 ± 1.7 | 159 ± 55 | |
| 1/−48 | 0/10 | 11.5 ± 1.2* | 7.8 ± 1.0 | 6.3 ± 1.2 | 182 ± 40 | |
| 0.25/−48 | 1/10 | 11.7 ± 1.4* | 8.2 ± 1.3 | 6.6 ± 2.0 | 551 ± 238 | |
| Ribavirin | 40/−4 | 10/10*** | 4.5 ± 0.0** | 3.7 ± 0.1** | 80 ± 22* | |
| Salinea | /−24 | 0/20 | 10.5 ± 0.9 | 8.2 ± 0.7 | 6.6 ± 0.8 | 258 ± 68 |
| Sham-infected | 3/3*** | 35 ± 7* | ||||
| Experiment 3 | ||||||
| poly(I:C12U)b | 1/−24 | 2/10 | 10.6 ± 1.8 | 6.1 ± 1.7 | 5.2 ± 1.5 | 854 ± 1,031 |
| 0.1/−24 | 2/10 | 10.5 ± 1.4* | 5.5 ± 1.0 | 4.3 ± 1.2 | 707 ± 792 | |
| Ribavirin | 40/−4 | 10/10*** | 4.7 ± 0.3 | 3.5 ± 0.0 | 43 ± 25** | |
| Salineb | /−24 | 0/20 | 9.5 ± 1.1 | 5.2 ± 1.4 | 4.4 ± 1.3 | 1,528 ± 302 |
| Sham-infected | 4/4*** | 38 ± 11* | ||||
Single dose; 50-g hamsters.
Treatment every 4 days (−1, 3, and 7); 100-g hamsters.
Mean day to death of hamsters dying prior to day 21.
log10 cell culture 50% infectious dose/g.
log10 cell culture 50% infectious dose/ml.
ALT, alanine aminotransferase, is measured in international units per liter.
ALT and virus titers were determined for five hamsters/group sacrificed on day 6 for experiments 1 and 2 and on day 7 for experiment 3.
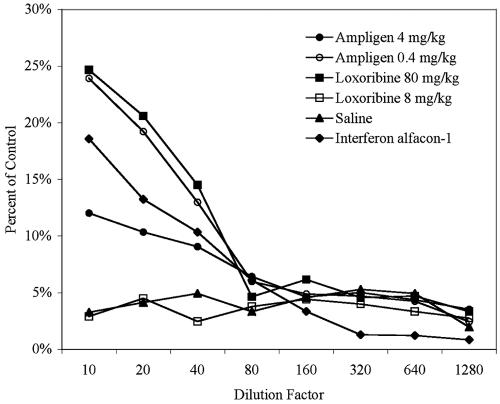
It is possible that poly(I:C12U) does not elicit a strong interferon response in hamsters, as has been reported with mice (40). To test the ability of poly(I:C12U) to elicit alpha/beta interferon, a bioassay was employed to assay for interferon in serum collected from animals treated with several doses of poly(I:C12U). Loxoribine, a guanosine nucleoside analog known to induce interferon (23), was included for comparison. As shown in Fig. 2, both doses of poly(I:C12U) were able to induce the production of interferon in serum, as indicated by the protection of CHO-K1 cells from VSV-induced cytotoxicity. However, the lower dose (0.4 mg/kg) elicited considerably more interferon activity, which was greater than the protection observed with treatment of CHO-K1 cells with interferon alfacon-1 treatment (Fig. 2). These data are consistent with the findings of the PCV challenge experiments in which the lowest doses offered the most significant protection to hamsters (Table 4, experiments 1 and 2). As expected, the 80-mg/kg dose of loxoribine elicited significant levels of interferon that were comparable to that observed with poly(I:C12U). On the other hand, the 8-mg/kg dose yielded no detectable activity (Fig. 2). Similar profiles were obtained from samples collected 1 or 4 h posttreatment and visual evaluation of CPE reduction concurred with the NR assay results (data not shown). Taken together, these data suggest that poly(I:C12U) is capable of eliciting alpha/beta interferon production in hamsters.
FIG. 2.
Poly(I:C12U) induction of alpha/beta interferon in hamsters. poly(I:C12U) (4 or 0.4 mg/kg) or loxoribine (80 or 8 mg/kg) was injected i.p. into 7-week-old hamsters and serum was collected after 2 h. Values are represented as percentages of NR uptake of control CHO-K1 cells and are a measure of the ability of diluted serum samples (triplicate) to protect cells from VSV-induced cytotoxicity. Interferon alfacon-1 (200 ng/ml) was included as a positive control and diluted as indicated.
DISCUSSION
There are more than a dozen closely related IFN-α subtypes. The antiviral activity of this multigene family has been well documented, and several IFN-α-based drugs have been approved by the Food and Drug Administration for clinical use against hepatitis B and C viruses (38). Of these drugs, interferon alfacon-1 (Infergen) is currently indicated as a treatment for chronic hepatitis C virus infection. In efforts to identify new therapeutics for arenaviruses of biodefense interest, this drug was evaluated in vitro against a cohort of New World arenaviruses related to those that cause hemorrhagic fever and are often lethal. Interferon alfacon-1 was found to be effective against two of the five arenaviruses tested (Table 1). There were clear differences, upon visual inspection of CPEs, between arenaviruses that were sensitive to interferon alfacon-1 (PCV and TAV) and those that were refractory to its effects (AMV, LAV, and TMV). PCV caused visible cell damage, rounding, and monolayer deterioration in BS-C-1 cells by day 7 in culture. Cells infected with TAV became rounded and refractory in appearance; however, this CPE took about 10 days to develop. The other arenaviruses all needed 12 or more days to grossly impact monolayers, and the CPE pattern was distinct, in that the cells became noticeably elongated and granular instead of rounded, as seen with PCV and TAV. Since we do not have an acceptable positive-control drug for AMV, LAV, and TMV, the observed negative results indicate failure of interferon alfacon-1 only under the test conditions examined. It is conceivable that since much lower dilutions, and likely higher multiplicities of infection of virus, were required to produce maximal CPE with AMV, LAT, and TMV, the beneficial antiviral action of interferon alfacon-1 or ribavirin was overwhelmed by the amount of challenge virus. Moreover, the requirement of extended incubation (≥12 days) to visualize CPE could also account for the observed lack of activity. This notion is supported by the fact that with PCV, if cultures are allowed to go out to 10 days, much of the protection seen on day seven is lost (data not shown). Thus, until a more robust assay system is developed for AMV, LAV, and TMV, we cannot conclude that interferon alfacon-1 is inactive against these viruses in vitro. Perhaps an as-yet-unidentified, more permissive cell line that would require far less virus and less time to produce CPE would yield more favorable results. We are actively working on real-time reverse transcription-PCR-based strategies for the detection of these viruses in BS-C-1 and other cell lines that would eliminate the need for CPE development to assess antiviral efficacy. Currently, animal models have not been developed for these viruses.
Since promising results against PCV and TAV in our cell-based assay system were obtained, the efficacy of interferon alfacon-1 was examined in vivo. It is clear that interferon is crucial to the induction of the host antiviral state (22). Generally, there are considerable problems associated with evaluating potential human interferon-based therapeutics in animal models due to weak activity, if any, across species (5, 46). Interferon alfacon-1 seems to have bypassed the cross-reactivity problem that is evident across certain rodent and human systems. Although interferon alfacon-1 is inactive in mice, others have previously reported activity in hamsters (1, 14, 15, 30). Although TAV was more sensitive to the effects of interferon alfacon-1 in vitro, the virus does not produce any signs of disease in hamsters (B. B. Gowen, unpublished data), making the PCV hamster model of acute arenaviral infection the model system of choice to conduct in vivo studies. For the most part, at the tested dosages, route, and schedule, interferon alfacon-1 was remarkably active against PCV (Tables 2 and 3). Significant reduction in mortality, increased survival time, abrogation of virus-induced liver damage, and decreased virus titers were observed. PCV targets the liver in our hamster disease model in a way similar to that of other arenaviruses that cause hemorrhagic fever (12, 28). Interferon alfacon-1 reduced liver virus titers and abrogated PCV-induced liver damage as indicated by reduced serum ALT levels. Remarkably, the 0.001-mg/kg/day dose, although suboptimal, still protected animals and significantly reduced all disease parameters evaluated (Table 2). The results from experiments in which the initiation of treatment was delayed indicate that there may be a limited window of opportunity to treat the infection in the hamster disease model (Table 3). However, since interferon alfacon-1 was designed for use with humans, our results with hamsters are likely an underestimate of the beneficial activity of the drug for treatment of human disease. In the case of Lassa fever in humans, it is reported that the incubation period can be as long as 18 to 24 days (18). Due to this apparent slower disease progression, therapeutic use of interferon alfacon-1 may be beneficial if an early diagnosis can be made. However, due to the nature of the development of arenaviral hemorrhagic disease, by the time that most physicians can make the appropriate diagnosis, it may be too late for effective treatment. With that said, a clinical study indicated that the treatment of Lassa fever patients with ribavirin initiated by day 6 dramatically reduced death rates, and even administration on day 7 or later significantly reduced mortality (27). In such cases, the combination of interferon alfacon-1 and ribavirin therapy could potentially further decrease the case fatality rates associated with Lassa and other hemorrhagic fevers of arenaviral origin. It is likely that the greatest protection against arenaviral diseases, and potentially other infectious diseases of viral etiology, would be seen in a situation in which intentional release has occurred, rapid identification of the infectious agent has been made, and interferon alfacon-1 can be given 1 to 2 days postexposure.
Interestingly, although interferon alfacon-1 performed well in vivo against PCV, we were not able to achieve 100% protection, as evidenced with ribavirin. It is possible that interferon alfacon-1 may not be as effective as ribavirin in earlier stages of disease progression, contributing to the slight disparity in survival, even though by the time of sampling (day 6), the two treatments have become equally effective, resulting in similar disease profiles. Our findings in investigating the development of several disease parameters over time do not support this theory (Fig. 1). Another possibility is that the combination of cytokines and factors elicited by PCV infection with interferon alfacon-1 were deleterious for a small percentage of the animals. Alternatively, it is possible that following the cessation of interferon alfacon-1 treatment, most of the hamsters were well on their way to clearing the virus; however, in a small group, the infection was able to reestablish itself, resulting in extended survival times and 20% mortality. If this were the case, extending the treatment from 7 days to 10 or 14 days may have allowed for complete resolution of the infection. It is also possible that host cells become refractory to daily treatment with interferon, as is seen with cell culture systems. However, it is difficult to compare in vitro cell culture data with in vivo data given the dosing of interferon that can be achieved in animals. It is unlikely that, in vivo, local concentrations of interferon alfacon-1 ever achieve anything close to those concentrations used in vitro, due to rapid renal elimination. In the mouse, an animal similar to the hamster, the terminal half-life of interferon alfacon-1 is approximately 30 min. Thus, giving the drug once a day is the minimal amount one would administer to achieve good concentrations of the drug in vivo. These pharmacokinetic issues are the reason that some interferon manufacturers have added polyethylene glycol (PEG) to their products, resulting in improved pharmacokinetic characteristics with constant exposure of PEG-interferon for up to 7 days following a single administration. For the treatment of hepatitis C virus infection, PEG-interferon has led to improved efficacy despite constant interferon levels present in the blood. While it remains unclear as to why we were not able to achieve complete protection, it should be noted that previous experiments have demonstrated substantially reduced activity of interferon alfacon-1 on hamster cells in vitro (20). Nevertheless, the collective results of our investigation with interferon alfacon-1 clearly demonstrate its beneficial effects for the treatment of PCV infection in the hamster model.
Since interferon alfacon-1 was highly effective against PCV, we also examined the capacity of double-stranded RNA (dsRNA), a potent interferon inducer (13, 17), for protecting animals challenged with PCV. We have previously tested poly(I:C12U), a form of dsRNA, against PCV in the hamster model and found it to be ineffective under the multidose treatment regimen used (41). In this study, the investigation with poly(I:C12U) was modified so that the tested treatment schedules should have removed or minimized the possibility of inducing a hyporesponsive state from repeated administration. Moreover, in the experiment in which a multidose schedule was used, older hamsters were also evaluated, since they are likely to be more immunologically developed than weanlings. In contrast to our previous analysis (41), a slight but statistically significant protective effect was seen with several of the treatments (Table 4). Our findings could not be accounted for by inactivity of the lot of poly(I:C12U) used in these experiments, since its potency was verified by testing against Punta Toro virus infection in mice (data not shown), which we have previously reported to be sensitive to poly(I:C12U) therapy (39, 40). It is also possible that poly(I:C12U) does not elicit a measurable alpha interferon response in hamsters, thus resulting in the observed limited activity against PCV. Lack of efficacy did not seem to be a result of failure to induce alpha/beta interferon activity, since elevated levels of these cytokines in animals treated with poly(I:C12U) could be detected and these levels were comparable to those stimulated by loxoribine (Fig. 2), a immunomodulator and known interferon inducer (23). It was somewhat of a surprise that treatment with lower doses of poly(I:C12U) yielded the best disease outcomes and elicited greater levels of interferon activity. It would seem that toxicity at the higher doses likely contributes to the observed results.
Due to the distinct nature of the two drugs evaluated in this study, one being an interferon inducer and activator of dsRNA-dependent pathways and the other a bio-optimized IFN-α, the administration schedules were also different and chosen to achieve optimal performance based on previous experience. The main objective of this work was not to provide a direct comparison of these drugs but simply to expand our findings with interferon alfacon-1 to see whether induction of native interferon would also protect hamsters from PCV infection. Our findings suggest that the endogenous interferon induced by poly(I:C12U) may not have the same potency as the bioengineered consensus IFN-α molecule. However, a simpler explanation could be that fluctuations or differences in the levels of interferon induced by poly(I:C12U), as opposed to the steady infusion of interferon alfacon-1 (given on a daily basis), allowed the virus to overcome host defenses. Moreover, we cannot rule out the possibility that PCV may be blocking the production of interferon, since a number of viruses have evolved this capacity (16). Further investigation into this matter is warranted.
There is evidence that IFN-α and IFN-β play an important role in antiviral resistance to lymphocytic choriomeningitis virus (26, 34, 45). Moreover, there appear to be various degrees of resistance, across various strains of this prototypical arenavirus, that correlate with their ability to establish persistent infection in mice (32). Lymphocytic choriomeningitis virus disease is largely due to immune-mediated pathology, whereas pathology from arenaviruses that produce acute disease (hemorrhagic fever) results from direct cytopathic events (35). Unfortunately, unlike with mice, genetically modified hamsters lacking IFN-α/β receptors are not currently available. The aforementioned genetic tools would be of great value to investigations aimed at delineating the role of alpha/beta interferons in combating viral diseases in hamster models of infection, which are emerging as models that more closely resemble human viral diseases (6, 19, 29, 31, 43, 47, 48). Our results with interferon alfacon-1 are significant, considering that alpha interferon treatment has not been previously reported to be effective against acute arenaviral diseases (7, 11, 24, 35, 42). These findings should spur increased efforts in designing alpha interferon-based therapeutics for treatment of arenaviral infections. Considering the threat of malicious intentional release of hemorrhagic fever-causing arenaviruses, combinatorial studies employing alpha interferon-based drugs with ribavirin are necessary and may lead to more effective treatment regimens for fighting these frequently fatal infections.
Acknowledgments
We thank William Mitchell (School of Medical Pathology, Vanderbilt University) for critical review of the manuscript. We also thank John Morrey for helpful discussions about interferon alfacon-1 and Chris de la Houssaye and Matt Heiner for cell culture and animal technical support, respectively.
This work was supported by contract NO1-AI-15435 and NO1-AI-30048 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Altrock, B. W., K. D. Fagin, H. R. Hockman, E. N. Fish, L. Goldstein, D. Chang, K. Duker, and N. Stebbing. 1986. Antiviral and antitumor effects of a human interferon analog, IFN-alpha Con 1, assessed in hamsters. J. Interferon Res. 6:405-415. [DOI] [PubMed] [Google Scholar]
- 2.Aronson, J. F., N. K. Herzog, and T. R. Jerrells. 1994. Pathological and virological features of arenavirus disease in guinea pigs. Comparison of two Pichinde virus strains. Am. J. Pathol. 145:228-235. [PMC free article] [PubMed] [Google Scholar]
- 3.Asper, M., T. Sternsdorf, M. Hass, C. Drosten, A. Rhode, H. Schmitz, and S. Gunther. 2004. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J. Virol. 78:3162-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatt, L. M., J. M. Davis, S. B. Klein, and M. W. Taylor. 1996. The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J. Interferon Cytokine Res. 16:489-499. [DOI] [PubMed] [Google Scholar]
- 5.Bluyssen, H. A., N. Nakamura, R. J. Vlietstra, E. M. Smit, A. Hagemeijer, and J. Trapman. 1995. Isolation, properties and chromosomal localization of four closely linked hamster interferon-alpha-encoding genes. Gene 158:295-300. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier, M. J., and W. E. Rawls. 1977. Variation between strains of hamsters in the lethality of Pichinde virus infections. Infect. Immun. 16:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canonico, P. G., M. Kende, B. J. Luscri, and J. W. Huggins. 1984. In-vivo activity of antivirals against exotic RNA viral infections. J. Antimicrob. Chemother. 14(Suppl. A):27-41. [DOI] [PubMed] [Google Scholar]
- 8.Charrel, R. N., and X. de Lamballerie. 2003. Arenaviruses other than Lassa virus. Antivir. Res. 57:89-100. [DOI] [PubMed] [Google Scholar]
- 9.Connolly, B. M., A. B. Jenson, C. J. Peters, S. J. Geyer, J. F. Barth, and R. A. McPherson. 1993. Pathogenesis of Pichinde virus infection in strain 13 guinea pigs: an immunocytochemical, virologic, and clinical chemistry study. Am. J. Trop. Med. Hyg. 49:10-24. [DOI] [PubMed] [Google Scholar]
- 10.Cosgriff, T. M., P. B. Jahrling, J. P. Chen, L. A. Hodgson, R. M. Lewis, D. E. Green, and J. I. Smith. 1987. Studies of the coagulation system in arenaviral hemorrhagic fever: experimental infection of strain 13 guinea pigs with Pichinde virus. Am. J. Trop. Med. Hyg. 36:416-423. [DOI] [PubMed] [Google Scholar]
- 11.de Guerrero, L. B., M. C. Boxaca, E. Malumbres, C. Dejean, and E. Caruso. 1985. Early protection to Junin virus of guinea pig with an attenuated Junin virus strain. Acta Virol. 29:334-337. [PubMed] [Google Scholar]
- 12.Elsner, B., E. Schwarz, O. G. Mando, J. Maiztegui, and A. Vilches. 1973. Pathology of 12 fatal cases of Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 22:229-236. [PubMed] [Google Scholar]
- 13.Field, A. K., A. A. Tytell, G. P. Lampson, and M. R. Hilleman. 1967. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl. Acad. Sci. USA 58:1004-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fish, E. N., K. Banerjee, H. L. Levine, and N. Stebbing. 1986. Antiherpetic effects of a human alpha interferon analog, IFN-alpha Con1, in hamsters. Antimicrob. Agents Chemother. 30:52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish, E. N., K. Banerjee, and N. Stebbing. 1985. Efficacy of consensus interferon alpha against HSV-2 infections. Antivir. Res. 5(Suppl. 1):191-197. [DOI] [PubMed] [Google Scholar]
- 16.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 17.Greene, J. J., J. L. Alderfer, I. Tazawa, S. Tazawa, P. O. Ts'o, J. A. O'Malley, and W. A. Carter. 1978. Interferon induction and its dependence on the primary and secondary structure of poly(inosinic acid).poly(cytidylic acid). Biochemistry (Moscow) 17:4214-4220. [DOI] [PubMed] [Google Scholar]
- 18.Gunther, S., and O. Lenz. 2004. Lassa virus. Crit. Rev. Clin. Lab. Sci. 41:339-390. [DOI] [PubMed] [Google Scholar]
- 19.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6-14. [DOI] [PubMed] [Google Scholar]
- 20.Hu, C. J., O. N. Ozes, S. B. Klein, L. M. Blatt, and M. W. Taylor. 1995. Comparison of the in vitro host range of recombinant met-interferon-con1, interferon-alpha 2b, and interferon-beta. J. Interferon Cytokine Res. 15:231-234. [DOI] [PubMed] [Google Scholar]
- 21.Jahrling, P. B., R. A. Hesse, J. B. Rhoderick, M. A. Elwell, and J. B. Moe. 1981. Pathogenesis of a Pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect. Immun. 32:872-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 23.Lee, J., T. H. Chuang, V. Redecke, L. She, P. M. Pitha, D. A. Carson, E. Raz, and H. B. Cottam. 2003. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 100:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucia, H. L., D. H. Coppenhaver, and S. Baron. 1989. Arenavirus infection in the guinea pig model: antiviral therapy with recombinant interferon-alpha, the immunomodulator CL246,738 and ribavirin. Antivir. Res. 12:279-292. [DOI] [PubMed] [Google Scholar]
- 25.Maiztegui, J. I., K. T. McKee, Jr., J. G. Barrera Oro, L. H. Harrison, P. H. Gibbs, M. R. Feuillade, D. A. Enria, A. M. Briggiler, S. C. Levis, A. M. Ambrosio, N. A. Halsey, C. J. Peters, et al. 1998. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J. Infect. Dis. 177:277-283. [DOI] [PubMed] [Google Scholar]
- 26.McClary, H., R. Koch, F. V. Chisari, and L. G. Guidotti. 2000. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74:2255-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick, J. B., I. J. King, P. A. Webb, C. L. Scribner, R. B. Craven, K. M. Johnson, L. H. Elliott, and R. Belmont-Williams. 1986. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 28.McCormick, J. B., D. H. Walker, I. J. King, P. A. Webb, L. H. Elliott, S. G. Whitfield, and K. M. Johnson. 1986. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am. J. Trop. Med. Hyg. 35:401-407. [DOI] [PubMed] [Google Scholar]
- 29.Milazzo, M. L., E. J. Eyzaguirre, C. P. Molina, and C. F. Fulhorst. 2002. Maporal viral infection in the Syrian golden hamster: a model of hantavirus pulmonary syndrome. J. Infect. Dis. 186:1390-1395. [DOI] [PubMed] [Google Scholar]
- 30.Morrey, J. D., C. W. Day, J. G. Julander, L. M. Blatt, D. F. Smee, and R. W. Sidwell. 2004. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir. Chem. Chemother. 15:101-109. [DOI] [PubMed] [Google Scholar]
- 31.Morrey, J. D., C. W. Day, J. G. Julander, A. L. Olsen, R. W. Sidwell, C. D. Cheney, and L. M. Blatt. 2004. Modeling hamsters for evaluating West Nile virus therapies. Antivir. Res. 63:41-50. [DOI] [PubMed] [Google Scholar]
- 32.Moskophidis, D., M. Battegay, M. A. Bruendler, E. Laine, I. Gresser, and R. M. Zinkernagel. 1994. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J. Virol. 68:1951-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, F. A., M. J. Buchmeier, and W. E. Rawls. 1977. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab. Investig. 37:502-515. [PubMed] [Google Scholar]
- 34.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters, C. J., C. T. Liu, G. W. Anderson, Jr., J. C. Morrill, and P. B. Jahrling. 1989. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev. Infect. Dis. 11(Suppl. 4):S743-S749. [DOI] [PubMed] [Google Scholar]
- 36.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 37.Rubinstein, S., P. C. Familletti, and S. Pestka. 1981. Convenient assay for interferons. J. Virol. 37:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidwell, R. W., J. H. Huffman, D. L. Barnard, D. F. Smee, R. P. Warren, M. A. Chirigos, M. Kende, and J. Huggins. 1994. Antiviral and immunomodulating inhibitors of experimentally-induced Punta Toro virus infections. Antivir. Res. 25:105-122. [DOI] [PubMed] [Google Scholar]
- 40.Sidwell, R. W., J. H. Huffman, D. F. Smee, J. Gilbert, A. Gessaman, A. Pease, R. P. Warren, J. Huggins, and M. Kende. 1992. Potential role of immunomodulators for treatment of phlebovirus infections of animals. Ann. N. Y. Acad. Sci. 653:344-355. [DOI] [PubMed] [Google Scholar]
- 41.Smee, D. F., J. Gilbert, J. A. Leonhardt, B. B. Barnett, J. H. Huggins, and R. W. Sidwell. 1993. Treatment of lethal Pichinde virus infections in weanling LVG/Lak hamsters with ribavirin, ribamidine, selenazofurin, and ampligen. Antivir. Res. 20:57-70. [DOI] [PubMed] [Google Scholar]
- 42.Stephen, E. L., S. K. Scott, G. A. Eddy, and H. B. Levy. 1977. Effect of interferon on togavirus and arenavirus infections of animals. Tex. Rep. Biol. Med. 35:449-454. [PubMed] [Google Scholar]
- 43.Tesh, R. B., H. Guzman, A. P. da Rosa, P. F. Vasconcelos, L. B. Dias, J. E. Bunnell, H. Zhang, and S. Y. Xiao. 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 183:1431-1436. [DOI] [PubMed] [Google Scholar]
- 44.Tong, M. J., K. R. Reddy, W. M. Lee, P. J. Pockros, J. C. Hoefs, E. B. Keeffe, F. B. Hollinger, E. J. Hathcote, H. White, R. T. Foust, D. M. Jensen, E. L. Krawitt, H. Fromm, M. Black, L. M. Blatt, M. Klein, J. Lubina, et al. 1997. Treatment of chronic hepatitis C with consensus interferon: a multicenter, randomized, controlled trial. Hepatology 26:747-754. [DOI] [PubMed] [Google Scholar]
- 45.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weck, P. K., S. Apperson, N. Stebbing, P. W. Gray, D. Leung, H. M. Shepard, and D. V. Goeddel. 1981. Antiviral activities of hybrids of two major human leukocyte interferons. Nucleic Acids Res. 9:6153-6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong, K. T., I. Grosjean, C. Brisson, B. Blanquier, M. Fevre-Montange, A. Bernard, P. Loth, M. C. Georges-Courbot, M. Chevallier, H. Akaoka, P. Marianneau, S. K. Lam, T. F. Wild, and V. Deubel. 2003. A golden hamster model for human acute Nipah virus infection. Am. J. Pathol. 163:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao, S. Y., H. Zhang, Y. Yang, and R. B. Tesh. 2001. Pirital virus (Arenaviridae) infection in the syrian golden hamster, Mesocricetus auratus: a new animal model for arenaviral hemorrhagic fever. Am. J. Trop. Med. Hyg. 64:111-118. [DOI] [PubMed] [Google Scholar]