Abstract
A narrow-spectrum clavulanic acid-inhibited class A β-lactamase, BOR-1, was identified in a Bordetella bronchiseptica clinical isolate. It shared 45% amino acid identity with L-2 from Stenotrophomonas maltophilia. An identical β-lactamase gene was found in B. bronchiseptica and Bordetella parapertussis reference strains that may contribute only in part to their resistance phenotype.
Bordetella bronchiseptica and Bordetella parapertussis are closely related gram-negative rods that may colonize the respiratory tract of mammals (4). B. bronchiseptica causes chronic respiratory infections in a wide range of animals but is rarely implicated as a pathogen in humans, mostly in immunocompromised patients that are often exposed to animals. B. parapertussis may cause a mild whooping cough (8).
B. bronchiseptica DOR was recovered from the respiratory tract secretions of a 26-year-old immunocompromised woman who was admitted to a hospital in the Paris area (Montreuil, France) for persistent bronchitis in April 2004. The organism was identified as B. bronchiseptica by using the API32GN and API20NE systems (bioMérieux, Marcy-L'Etoile, France), confirmed by partial sequencing of the 16S rRNA gene, which showed a 99% identity with the sequence of B. bronchiseptica RB50 (GenBank accession no. NC002927). B. bronchiseptica DOR was susceptible to amoxicillin, ticarcillin, piperacillin, cephalothin, imipenem, amikacin, gentamicin, tobramycin, trimethoprim-sulfamethoxazole, and ciprofloxacin and resistant to cefoxitin, cefuroxime, cefotaxime, aztreonam, and erythromycin by the disk diffusion method (2). The patient was treated with amoxicillin-clavulanic acid given orally. She denied close exposure to animals.
The complete genome sequences of closely related species B. bronchiseptica RB50 (5.3 Mb) and B. parapertussis 12822 (4.7 Mb) have been published recently (4) (GenBank accession no. NC002927 and NC002928, respectively). Thus, in silico analysis of these sequences identified the same open reading frame (ORF) in both genomes that shared 45% amino acid identity with that of an Ambler class A β-lactamase gene of Stenotrophomonas maltophilia (7). This ORF was not found in the Bordetella pertussis genome (4) (GenBank accession no. NC002929). We have cloned the corresponding gene of B. bronchiseptica DOR, biochemically characterized this protein, and determined its spectrum of activity. Escherichia coli reference strain DH10B and plasmid pCR-Blunt II-TOPO (Invitrogen, Life Technologies, Cergy-Pontoise, France) were used for cloning and expression experiments. Total DNA of B. bronchiseptica DOR was extracted as described previously (6). A PCR experiment was performed using primers PREBOR-1A (5′-ATACGCTGACGCAGACATTG-3′) and PREBOR-1B (5′-AGAAGGCCGTGGTCACCTTC-3′), designed according to the database sequence of B. bronchiseptica RB50 to amplify the entire β-lactamase gene. The corresponding 1,084-bp PCR product was cloned in the plasmid pCR-Blunt II-TOPO (Invitrogen, Life Technologies, Cergy-Pontoise, France), giving rise to recombinant plasmid pFL-1 and transformed into E. coli DH10B, according to the manufacturer's instructions. Selection of recombinant clones was performed on Mueller-Hinton (MH) plates containing 50 μg of amoxicillin and 30 μg of kanamycin per ml. Once cloned, the PCR product was sequenced on both strands as described previously (6), and the β-lactamase gene sequence revealed identity with that found in the databases for B. bronchiseptica RB50. Disk diffusion susceptibility testing performed for E. coli DH10B (pFL-1) suggested that the cloned gene encoded a functional β-lactamase named BOR-1. The blaBOR-1-like genes of two reference strains of B. bronchiseptica (CIP 102314 and CIP 52.125) and of B. parapertussis CIP 63.33 were sequenced after the PCR experiment using whole-cell DNAs as templates. A blaBOR-1 gene identical to that of B. bronchiseptica RB50 was found in these three strains except for four silent mutations in the B. parapertussis strain, in which the sequence was identical to that of the B. parapertussis 12822. The G+C content of the blaBOR-1 gene was 70%, which fits with the average G+C value of genes of Bordetella spp. (68%) (4). Within the deduced protein of this ORF, characteristic elements of Ambler class A β-lactamases were identified (Fig. 1). BOR-1 shared 42 to 45% amino acid identity with the most closely related enzymes, the naturally occurring β-lactamases L-2 from S. maltophilia, PenA from Burkholderia cepacia, FAR-1 from Nocardia farcinica, AST-1 from Nocardia asteroides, YENT from Yersinia enterocolitica, and SHV-1 from Klebsiella pneumoniae, and the plasmid-mediated β-lactamase TEM-1 (3, 6, 7). The surrounding sequences of the blaBOR-1 gene in B. bronchiseptica and B. parapertussis were identical. No LysR-type regulator gene was identified in the upstream DNA sequences of the blaBOR-1 gene.
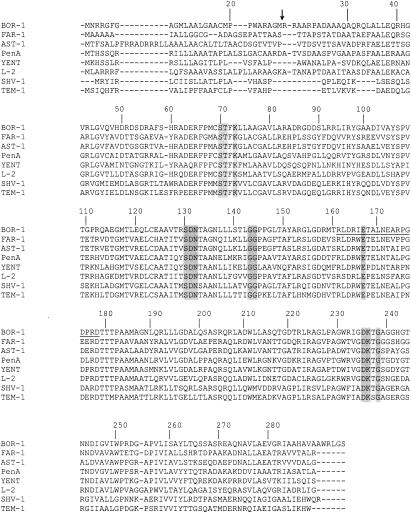
FIG. 1.
Alignment of the BOR-1 amino acid sequence with those of closely related enzymes: FAR-1 from Nocardia farcinica, AST-1 from Nocardia asteroides, PenA from Burkholderia cepacia, YENT from Yersinia enterocolitica, L-2 from Stenotrophomonas maltophilia, SHV-1 from Klebsiella pneumoniae, and TEM-1 from Escherichia coli (3, 6, 7). Numbering is according to the work of Ambler et al. (1). Structural elements characteristic of class A β-lactamases are shaded. The amino acid residues of the omega loop of Ambler class A β-lactamases are underlined. The arrow indicates the cleavage site for the leader peptide of β-lactamase BOR-1.
Susceptibility testing of B. parapertussis CIP 63.33 and of B. bronchiseptica DOR strains was performed on blood-containing MH plates at 35°C with the E-test technique (AB Biodisk, Solna, Sweden), since the former species is unable to grow on MH plates. B. parapertussis CIP 63.33 was susceptible to all β-lactams except cefoxitin, whereas B. bronchiseptica was susceptible to amino-, carboxy-, ureidopenicillins, cephalothin, and carbapenems but resistant to broad-spectrum cephalosporins (Table 1). Specific activity of a culture extract of B. bronchiseptica DOR obtained as described previously (6) using nitrocefin as a substrate revealed that this strain had a detectable β-lactamase activity. Using subinhibitory concentrations of imipenem as inducers (2), the induction of BOR-1 biosynthesis from B. bronchiseptica DOR was not evidenced (data not shown).
TABLE 1.
MICs of β-lactams for B. bronchiseptica DOR, B. parapertussis CIP 63.33, E. coli DH10B harboring recombinant plasmid pFL-1 expressing BOR-1 from B. bronchiseptica DOR, and the E. coli DH10B reference strain
| β-Lactam(s)b | MIC (μg/ml) for:
|
|||
|---|---|---|---|---|
| B. bronchiseptica DOR | B. parapertussis CIP 63.33a | E. coli DH10B (pFL-1)a | E. coli DH10B | |
| Amoxicillin | 8 | 2 | 512 | 2 |
| Amoxicillin + CLA | 2 | 0.12 | 8 | 2 |
| Ticarcillin | 8 | 2 | 512 | 1 |
| Ticarcillin + CLA | 2 | <0.06 | 64 | 1 |
| Piperacillin | 0.25 | <0.06 | 8 | 1 |
| Piperacillin + TZB | 0.06 | <0.06 | 4 | 1 |
| Cephalothin | 8 | 8 | 4 | 2 |
| Imipenem | 0.25 | 0.25 | 0.25 | 0.25 |
| Cefoxitin | 512 | 64 | 2 | 2 |
| Cefuroxime | 512 | 2 | 4 | 2 |
| Cefotaxime | 512 | 0.5 | <0.06 | <0.06 |
| Ceftazidime | 16 | <0.06 | <0.06 | <0.06 |
| Cefepime | 16 | <0.06 | <0.06 | <0.06 |
| Cefpirome | 512 | <0.06 | <0.06 | <0.06 |
| Aztreonam | 512 | 0.06 | 0.06 | 0.06 |
| Moxalactam | 1 | 0.12 | 0.12 | 0.12 |
B. parapertussis CIP 63.33 and E. coli DH10B (pFL-1) produced β-lactamase BOR-1.
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
E. coli DH10B (pFL-1) gave a clavulanic acid-inhibited narrow-spectrum penicillinase phenotype, conferring resistance to narrow-spectrum penicillins, sparing cephalosporins (Table 1).
Isoelectric focusing analyses were performed with extracts of B. bronchiseptica DOR and B. parapertussis CIP 63.33 strains, grown with or without subinhibitory concentrations of imipenem (0.06 to 0.24 μg/ml) and E. coli DH10B (pFL-1). They revealed that these strains produced only a single β-lactamase with an alkaline pI value (>9).
Purification of β-lactamase BOR-1 was performed with culture of E. coli DH10B harboring recombinant plasmid pFL-1, as described previously (6). Briefly, culture extracts were subjected to two purification steps using ion-exchange chromatography. First, a Q-Sepharose column equilibrated with 20 mM bis-Tris buffer (pH 7.0) was used, and the enzyme was eluted with a linear NaCl gradient (0 to 0.5 M). Then, the fractions containing the β-lactamase activity were dialyzed against 50 mM bicine buffer (pH 8.5) and loaded on an S-Sepharose column preequilibrated with the same buffer. The β-lactamase activity was eluted with a linear NaCl gradient (0 to 0.5 M). After purification from culture extracts of E. coli DH10B(pFL-1), the specific activity of β-lactamase BOR-1, measured with 100 μmol of cephalothin as the substrate at 30°C in 100 mM sodium phosphate (pH 7.0), was 1 U/mg of protein, and its purification factor was 30-fold (one unit of enzyme activity being defined as the activity that hydrolyzed 1 μmol of cephalothin per min). Protein purity was estimated to be >95% by sodium-dodecyl-sulfate-polyacrylamide gel electrophoresis analysis(data not shown). The relative molecular mass of BOR-1 determined with the purified enzyme was ca. 32 kDa (data not shown). The signal peptide cleavage site was identified as described previously (2). N-terminal amino acid sequencing of the mature protein revealed that the cleavage site for the leader peptide is between amino acid positions 27 and 28 (M-RRAAR). Purified β-lactamase extracts were used for kinetic measurements performed at 30°C in 100 mM sodium phosphate (pH 7.0). The kcat and Km values were determined by analyzing β-lactam hydrolysis under initial-rate conditions with a UV spectrophotometer and by using the Eadie-Hofstee linearization of the Michaelis-Menten equation, as previously described (5). The β-lactamase BOR-1 significantly hydrolyzed benzylpenicillin, ampicillin, ticarcillin, and piperacillin, as do the naturally occurring β-lactamases L-2, AST-1, FAR-1, and YENT (3, 6, 7). BOR-1 hydrolyzed cephalothin at a lower level. Contrary to the case with β-lactamases L2 and FAR-1, hydrolysis of extended-spectrum cephalosporins was not detected (Table 2) (3, 7). Activity inhibition measured by determination of 50% inhibitory concentrations showed that BOR-1 was inhibited by clavulanic acid (0.2 μM), tazobactam (2 μM), and sulbactam (100 μM) (6).
TABLE 2.
Kinetic parameters of purified β-lactamase BOR-1 and comparison with catalytic efficiences of FAR-1 enzyme from N. farcinicaa
| Substrate | kcat (s−1) | Km (μM) |
kcat/Km (s−1 · mM−1)
|
|
|---|---|---|---|---|
| BOR-1 | FAR-1 | |||
| Benzylpenicillin | 6 | 20 | 300 | 5,500 |
| Amoxicillin | 10 | 500 | 20 | 3,800 |
| Ampicillin | 40 | 400 | 100 | —b |
| Ticarcillin | 1 | 10 | 100 | 1,600 |
| Piperacillin | 70 | 350 | 200 | 9,200 |
| Cephalotin | 0.1 | 30 | 5 | 130 |
| Cefuroxime | <0.1 | NDc | ND | <5 |
| Cefoxitin | <0.1 | ND | ND | — |
| Cefotaxime | <0.1 | ND | ND | <5 |
| Ceftazidime | <0.1 | ND | ND | ND |
| Cefpirome | <0.1 | ND | ND | — |
| Aztreonam | <0.1 | ND | ND | <20 |
| Moxalactam | <0.1 | ND | ND | — |
| Imipenem | <0.1 | ND | ND | — |
Data are the means from three independent experiments. Standard deviations were within 10% of the means. Data for FAR-1 enzyme are from the work of Laurent et al. (3).
—, data not available.
ND, no detectable hydrolysis.
Conclusion.
The entire genome sequences of B. pertussis, B. parapertussis, and B. bronchiseptica have been published recently (4). B. pertussis and B. parapertussis each are reported to derive from a B. bronchiseptica-like ancestor. In addition, B. bronchiseptica and B. parapertussis share a significant degree of genome identity that may explain why the β-lactamase BOR-1 was found in these species and absent in B. pertussis. The MICs of amino- and carboxy-penicillins might be explained by the expression of the β-lactamase BOR-1 in both species. Other nonenzymatic mechanisms of resistance to β-lactams, such as impermeability, efflux, or penicillin binding protein affinities, might be associated in B. bronchiseptica.
This study indicated that B. bronchiseptica and B. parapertussis harbor an identical and naturally occurring penicillinase that may contribute in part to their β-lactam resistance phenotype.
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, and by the European Community 6th PCRD, LSHM-CT-2003-503335.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frère, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girlich, D., T. Naas, and P. Nordmann. 2004. Biochemical characterization of the naturally occurring oxacillinase OXA-50 of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48:2043-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent, F., L. Poirel, T. Naas, E. B. Chaibi, R. Labia, P. Boiron, and P. Nordmann. 1999. Biochemical-genetic analysis and distribution of FAR-1, a class A β-lactamase from Nocardia farcinica. Antimicrob. Agents Chemother. 43:1644-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 5.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poirel, L., F. Laurent, T. Naas, R. Labia, P. Boiron, and P. Nordmann. 2001. Molecular and biochemical analysis of AST-1, a class A β-lactamase from Nocardia asteroides sensu stricto. Antimicrob. Agents Chemother. 45:878-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh, T. R., A. P. MacGowan, and P. M. Bennett. 1997. Sequence analysis and enzyme kinetics of the L2 serine β-lactamase from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 41:1460-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolfrey, B. F., and J. A. Moody. 1991. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 4:243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]