Abstract
Macrolide antibiotics have an outstanding ability to concentrate within host cells, particularly phagocytes. In the study described in this paper five different macrolide antibiotics were compared regarding the uptake and release kinetics in human peripheral blood polymorphonuclear neutrophils (PMNs) and three different cell lines, two phagocytic cell lines (RAW 264.7 and THP-1) and an epithelial cell line (MDCK). Based on the results obtained, the substances tested could be clustered into different groups. Azithromycin constituted the first group, characterized by rapid and nonsaturable uptake into phagocytic cells and a high degree of retention in the preloaded cells. The second group included erythromycin and clarithromycin. These two substances do not exhibit cell specificity; consequently, they are taken up to a similar extent and are released by all cell types studied. Ketolides constituted the last group. Their uptake was saturable in cells of monocytic lineage as well as in nondifferentiated cells of myeloid lineage, and they were rapidly released from all the cell lines studied. However, in PMNs, ketolide uptake was not saturable; and unlike telithromycin, cethromycin rapidly egressed from the loaded cells.
Macrolide antibiotics are a well-established class of antimicrobial agents characterized by the presence of a highly substituted macrocyclic lactone ring. Extensive chemical modifications of a natural compound, erythromycin A, have led to the development of numerous semisynthetic derivatives with increased bioavailabilities and antimicrobial spectra (azalides) (3). A new subclass, ketolides, has recently emerged to overcome the problems of inducible macrolide-lincosamide-streptogramin B resistance (6, 19).
Macrolide antibiotics possess several interesting features, among which are exceptionally high levels of accumulation and retention in cells and tissues, in particular, phagocytes. A beneficial consequence of macrolide accumulation within cells is increased activity against intracellular pathogens (16). In addition, phagocytes serve as a vehicle that transports macrolides to the site of infection (tissue-directed pharmacokinetics) (8, 9). Therefore, various in vitro accumulation and release studies of macrolides have been performed. The cells preferentially analyzed in those experiments were phagocytes, in particular, human polymorphonuclear neutrophils (PMNs) (11, 13, 20, 36). Based on the results obtained, Labro (17) has proposed classification of these antibiotics into two groups. The first group includes dibasic molecules, such as azithromycin, which are massively accumulated within PMNs, with no saturation in uptake during a 3-h incubation period, and which are slowly released from the preloaded cells. The second group consists of monobasic molecules, such as erythromycin, whose uptake is rapid but saturable and which rapidly egress from the loaded cells. Interestingly, ketolides display intermediate characteristics between these two groups (34, 36). Despite the many uptake studies that have been conducted so far, the mechanism of entry and accumulation of macrolides has not been elucidated yet. However, more and more data support the idea of the existence of an active transport system for macrolides. Factors include dependence of the uptake on cell viability, temperature, pH, and extracellular Ca2+; competition among macrolides for entry into cells; and interindividual variability in the kinetics of uptake (22, 23, 34, 36). At the same time, considerably fewer data on the release mechanisms of macrolides from the host cells are available (11, 34, 36). To overcome the differences in the kinetics of uptake and efflux that have been observed as a result of interindividual variability among PMNs from different donors, studies of various phagocytic and nonphagocytic cell lines have begun (1, 2, 4, 22, 28, 29, 35).
In the present study, five different macrolide antibiotics, azithromycin, erythromycin, clarithromycin, telithromycin (HMR 3647), and cethromycin (ABT-773), were compared regarding their uptake and release kinetics in human PMNs and three different cell lines. The cell lines used were RAW 264.7, derived from murine macrophages; THP-1, a promonocytic cell line; and MDCK, a canine kidney epithelium cell line. This is the first study in which these macrolides have been compared simultaneously in a variety of different cell lines.
MATERIALS AND METHODS
Antibiotics.
Erythromycin was purchased from Sigma. Clarithromycin, azithromycin, and cethromycin (ABT-773) (internally synthesized by Dražen Pavlović, as described by Ma et al. [19]) were provided by PLIVA Research Institute Ltd. Telithromycin (HMR 3647) was from GlaxoSmithKline, Italy (internally synthesized by Federica Damiani, as described by Denis et al. [6]). All the substances tested were dissolved in dimethyl sulfoxide (DMSO; Kemika, Croatia) at a concentration of 25 mg/ml and were further diluted in RPMI 1640 medium (Institute of Immunology, Croatia) to the desired concentrations.
Human PMNs.
PMNs were obtained from the venous blood of healthy volunteers by sedimentation on 2% dextran T-500 (Amersham Biosciences, Sweden), followed by Ficoll-Paque (Amersham Biosciences) density gradient centrifugation and hypotonic lysis of residual erythrocytes.
Cell lines.
The RAW 264.7 and THP-1 cell lines were obtained from the European Collection of Cell Cultures, United Kingdom, while MDCK cells were from the American Type Culture Collection (ATCC). RAW 264.7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin, 50 μg/ml streptomycin, and 2.5 μg/ml amphotericin B (Fungizone). The THP-1 cell line was maintained in RPMI 1640 medium containing 10% heat-inactivated FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2.5 μg/ml amphotericin B. MDCK cells were grown in minimum essential medium (MEM) supplemented with 10% heat-inactivated FBS, 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2.5 μg/ml amphotericin B. All media were purchased from the Institute of Immunology, Croatia, while all medium supplements were from Gibco, Australia.
Antibiotic uptake.
PMNs were resuspended in RPMI 1640 medium at a concentration of 5 × 106 cells per 3 ml. The cells were incubated at 37°C for up to 3 h in a shaking water bath with 10 μg/ml of the tested macrolide. Free and cell-bound macrolide was separated by centrifugation (18,400 × g, 5 min, 10°C) through poly(dimethylsiloxane-co-diphenylsiloxane), dihydroxy terminated (Aldrich Chemical Company). The cell pellet was disrupted by sonication (output power 3, 1 min; Microson Ultrasonic Cell Disruptor XL; Misonix) in the presence of 0.5% Triton X-100 (Sigma).
RAW 264.7 cells were seeded in a six-well plate in DMEM with 10% FBS at a concentration of 5 × 106 cells per well. The cells were allowed to adhere to the well for 2 h. Afterwards, the medium was removed from the wells and the cells were washed with phosphate-buffered saline (PBS). The cells were incubated at 37°C in an atmosphere containing 5% CO2 for up to 3 h with 10 μg/ml of the tested macrolide in DMEM with a total volume of incubation of 3 ml. After incubation, the cells were washed four times with ice-cold PBS, scraped off and placed in 0.5% Triton X-100, and disrupted by sonication.
A total of 1 × 107 THP-1 cells were incubated in 3 ml of a 10-μg/ml macrolide solution in RPMI 1640 medium in a closed glass tube. The samples were incubated in a water bath at 37°C with shaking for up to 3 h. After incubation, the cells were removed from the incubation medium by centrifugation (359 × g, 10 min, 10°C) and were washed three times with ice-cold PBS. The cell pellet was lysed in 0.5% Triton X-100 by sonication.
MDCK cells were seeded in a six-well plate in MEM with 10% FBS, 0.1 mM MEM nonessential amino acids, and 1 mM sodium pyruvate at a density of about 3 × 106 cells per well. The cells were allowed to adhere to the well overnight. Afterwards, the medium was removed from the wells and the cells were washed with PBS. The samples were incubated for up to 3 h with 10 μg/ml of the tested substance in MEM with a total volume of incubation of 3 ml. After incubation, the cells were washed four times with ice-cold PBS, scraped off and placed in 0.5% Triton X-100, and disrupted by sonication.
Drug-naïve cells were resuspended in different concentrations of macrolide solutions. The spiked cells were then immediately sonicated and analyzed in the same manner as all the other samples. The results obtained were used for determination of the recovery of the substance after interaction with the cell lysate.
Drug-naïve cells were resuspended in 0.5% Triton X-100 and disrupted by sonication. The inhibitory zones of samples prepared in this way represent the influence on bacterial growth of the cell lysates themselves and were therefore subtracted from all the results obtained.
Antibiotics were also added to drug-naïve cells immediately before they were washed to determine the amount of background that may arise from substance that had remained on the surfaces of the cells or the walls of the dishes. With all macrolides, the background for samples prepared in this way was the same as that for the cell lysates of drug-naïve cells, indicating that the samples were thoroughly washed (data not shown).
Antibiotic release.
PMNs and THP-1 cells were preloaded with 10 μg/ml of the tested substance for 1 and 3 h, respectively, as described above for the procedure for uptake. Afterwards, they were removed from the drug-containing medium by centrifugation (359 × g, 10 min, 10°C). The cells were washed in ice-cold PBS; resuspended in 5 ml of ice-cold RPMI 1640 medium; and incubated for up to 3 and 2.5 h, respectively, at 37°C in a water bath with shaking. The cells were then washed three times in ice-cold PBS and disrupted by sonication.
RAW 264.7 and MDCK cells were incubated for 3 h with 10 μg/ml of the substance, as described above for the uptake experiments. Afterwards, they were washed three times with ice-cold PBS and incubated for up to 3 h in 4 ml of drug-free medium. After the incubation, the cells were washed twice with ice-cold PBS, scraped off and placed in 0.5% Triton X-100, and disrupted by sonication.
Macrolide quantitation.
The quantity of the cell-associated macrolide was determined by the microbiological method of diffusion in agar (7, 12) by using Micrococcus luteus (ATCC 9341). Standard curves were prepared from stock solutions in DMSO, which were diluted to the desired concentration in 0.5% Triton X-100.
Cell recovery and viability.
In each experiment at least three samples per substance were incubated with the macrolide tested to check cell recovery and viability after incubation. These samples were handled in the same way as all the other drug-loaded samples. The only exception was that at the end the cells were resuspended in medium. The cell number was determined with a hematological analyzer (SF-3000; Sysmex, Japan). In all experiments with RAW 264.7 cells, cell recovery was always greater than 50%, with an average of about 75%. The rate of recovery of MDCK cells after incubation was greater than 80%. Such a high recovery rate is probably due to the fact that the cells were seeded 1 day before the beginning of the experiment, giving some of them enough time to divide once more. In experiments with PMNs and THP-1 cells, the rate of cell recovery was about 50%.
Cell viability after incubation, as assessed by trypan blue dye exclusion, was about 80% for RAW 264.7 cells and about 90% for PMNs and THP-1 cells. The viability of MDCK cells was not determined, since the cells were mechanically removed from the wells after incubation, and most of them were damaged in this process. Scraping was preferred over trypsinization, because incubation of the cells with trypsin at 37°C for at least 3 min might promote the release of accumulated substances from the cells. Instead, after incubation the cells were examined under a microscope to determine the existence of detached and, therefore, probably dead cells.
None of the substances tested had any significant effect on cell recovery or viability.
Data analysis.
Each experiment was repeated at least three times, with all the samples taken in triplicate. The concentration of cell-associated antibiotic was determined based on the knowledge of the mean number of cells in the experiment and the cell volume. According to the literature, 106 cells are considered to have volumes of 0.24 μl for PMNs (24), 1.068 μl for RAW 264.7 cells (2), 2.7 μl for MDCK cells (31), and 1.2 μl for THP-1 (22). Uptake of macrolides was expressed as the ratio of the intracellular concentration to the extracellular concentration of antibiotic (I/E), where the extracellular concentration was taken as constant (10 μg/ml) due to the large relative volume of incubation medium. The efflux of macrolides was expressed as the percentage of drug remaining associated with the cells after incubation in drug-free medium.
RESULTS
Incorporation of macrolides into cells.
Cells were incubated in macrolide-containing medium. At the end of the incubation period, the amount of accumulated substance was determined by the microbiological method of diffusion in agar. Based on the knowledge of the mean number of cells in the experiment and the cell volume, the I/E ratio was calculated.
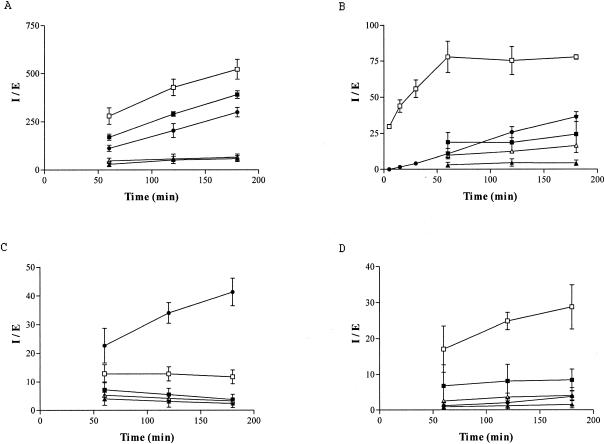
Azithromycin, telithromycin, and cethromycin were extensively taken up by PMNs without saturation during a 3-h incubation period. Cethromycin showed the strongest accumulation, with an I/E ratio exceeding 500 after 3 h. On the other hand, uptake of erythromycin and clarithromycin was moderate and reached a plateau within 60 min (Fig. 1A).
FIG. 1.
Kinetics of intracellular accumulation of azithromycin (solid circles), erythromycin (solid triangles), clarithromycin (open triangles), telithromycin (solid squares), and cethromycin (open squares) in PMNs (A) and in the RAW 264.7 (B), THP-1 (C), and MDCK (D) cell lines. Data are expressed as mean I/E ratios ± standard deviations. Four experiments were carried out with azithromycin and three experiments were carried out with all the other substances, with all samples taken in triplicate.
In RAW 264.7 cells incubated with azithromycin, the intracellular concentration of the macrolide was about 35 times higher than the extracellular concentration after 3 h of incubation. The uptake was nonsaturable even after 24 h (data not shown). Contrary to azithromycin, erythromycin, clarithromycin, and telithromycin were only moderately taken up by the cells. Their uptake was saturable and reached a plateau within 60 min. Further incubation of the cells for up to 3 h did not enhance the uptake of these drugs. Cethromycin rapidly penetrated the cells within the first hour of incubation, and afterwards the I/E ratio remained almost the same (Fig. 1B).
Azithromycin concentrated within THP-1 cells, with no saturation during the whole incubation period. On the other hand, erythromycin and clarithromycin were very poorly accumulated within the cells, with I/E values not exceeding 5 after a 3-h incubation. Uptake of telithromycin and cethromycin was moderate and reached a plateau within 60 min. Interestingly, as already observed (22), the plateau of telithromycin decreased with time (Fig. 1C).
Cethromycin was substantially concentrated in the MDCK cells, with no saturation during the whole incubation period. On the other hand, azithromycin, clarithromycin, and erythromycin were poorly taken up by the cells, with I/E ratios lower than 5 in the first 3 h of incubation. Uptake of telithromycin was moderate and reached a plateau in the first 60 min of incubation (Fig. 1D).
Release of macrolides.
Macrolide-loaded cells were placed in a drug-free medium, and the amount of substance that remained within the cells was determined by bioassay. The results are expressed as the percentage of the drug that remained cell associated at the end of incubation.
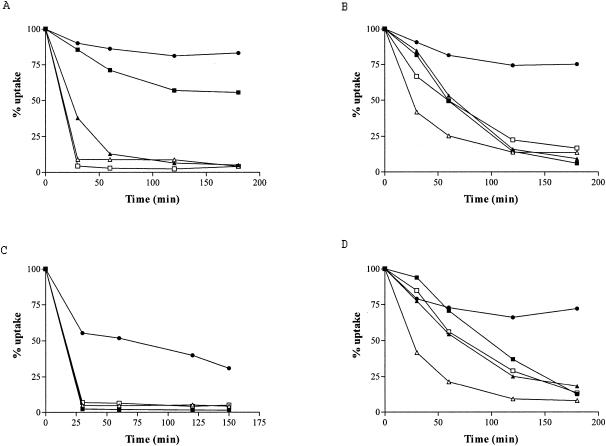
Azithromycin and telithromycin were slowly released from PMNs after the removal of extracellular drug. After 3 h, approximately 80% and 55% of the accumulated drugs, respectively, remained trapped within the cells. In contrast, the efflux of erythromycin, clarithromycin, and cethromycin was rapid; about 90% of the substance was exported into the medium during the first hour of incubation (Fig. 2A).
FIG. 2.
Kinetics of release of the macrolides azithromycin (solid circles), erythromycin (solid triangles), clarithromycin (open triangles), telithromycin (solid squares), and cethromycin (open squares) from PMNs (A) and RAW 264.7 (B), THP-1 (C), and MDCK (D) cells. Data are expressed as the percentage of the drug that has remained cell associated (% uptake). Three experiments were carried out for each substance, with all samples taken in triplicate.
Azithromycin was slowly released from the preloaded RAW 264.7 cells. About 20% of the accumulated substance egressed during the first hour of incubation in a drug-free medium. Further incubation of the cells for up to 3 h did not increase the drug efflux, and 75% of the substance remained cell associated. The other substances tested, erythromycin, clarithromycin, telithromycin, and cethromycin, showed massive and rapid release from the cells. The cell-associated antimicrobial levels were less than 20% after 2 h of incubation (Fig. 2B).
The substances tested were rapidly released from the preloaded THP-1 cells. However, azithromycin showed a gradual but continuous egress during the whole incubation period. The other substances massively egressed from the cells, with about 90% of the drug released in the first 30 min of incubation (Fig. 2C).
Elution of azithromycin from MDCK cells was slow; after a 3-h incubation of loaded cells in drug-free medium, about 70% of the substance remained trapped within the cells. On the contrary, erythromycin, clarithromycin, telithromycin, and cethromycin were substantially released from the cells, with about 20% of the substance remaining cell associated at the end of incubation (Fig. 2D).
DISCUSSION
In this study five different macrolide antibiotics, azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin, were compared regarding their uptake and release kinetics in PMNs and three different cell lines, two phagocytic cell lines and an epithelial cell line. Based on the results obtained, the substances tested could be divided into three groups.
In the first group, azithromycin was concentrated within all cells tested, with no saturation during a 3-h incubation period. It accumulated much more in phagocytic cell lines than in nonphagocytic cell lines. This correlates well with the results published by Pascual et al. (29), who compared the uptake kinetics of azithromycin in human PMNs, peritoneal macrophages, and two nonphagocytic cell lines, McCoy cells (murine fibroblasts) and HEp-2 cells (tissue-cultured epithelial cells).
When the results for the phagocytic cells tested were compared, it was found that mature phagocytes (PMNs and RAW 264.7) accumulated more azithromycin than nondifferentiated phagocytic precursors (THP-1). Similar findings were reported by Munic et al. (26) for azithromycin uptake into PMNs, their nondifferentiated precursors (HL-60 cell line), and HL-60 cells differentiated toward granulocytes with retinoic acid, and by Abdelghaffar et al. (1) for azithromycin uptake into PMNs, their nondifferentiated precursors (PLB-985 cell line), and PLB-985 cells differentiated toward granulocytes. This suggests that during the process of differentiation, cells acquire some additional characteristics that are involved in the entry of macrolide antibiotics into cells and the accumulation of macrolide antibiotics in cells. Our data are in contrast to those of Hall et al. (10). In their studies, the uptake of azithromycin into THP-1 cells was very high even after only 30 min (I/E ratio, nearly 200 with an extracellular concentration of 4 μg/ml). These findings are not in agreement with either our data or other published data on azithromycin uptake into cultured phagocytic cell lines, which show that uptake occurs gradually over several hours (2, 4).
In our study, azithromycin was slowly released from the loaded PMNs, murine macrophages, and MDCK cells. Efflux from promonocytic cells (THP-1) was substantial. This actually agrees with the findings of Hall et al. (10), who showed that the efflux of azithromycin from THP-1 cells was complete after 4 h of incubation in drug-free medium. The high level of retention of azithromycin in cells is probably due to its weakly basic nature, which results in protonation and trapping of the substance within acidic compartments. This is supported by the observation that substances that neutralize the lysosomal pH and that therefore prevent protonation and the subsequent trapping of azithromycin markedly increase its efflux from the PMNs (11).
The second group of compounds included erythromycin and clarithromycin. For these macrolides, uptake was poor and reached a plateau within the first hour of incubation. However, if we compare the two substances, clarithromycin accumulated slightly more than erythromycin in all cell types. Phagocytes accumulated somewhat more substance than their progenitors and epithelial cells. Contrary to azithromycin, erythromycin and clarithromycin did not seem to be tightly cell associated and they did not exhibit cell specificity, as they were taken up to a similar extent and released by all cell lines studied. These results are in agreement with the data reported by Martin et al. (21), who studied the kinetics of accumulation and release of erythromycin in various tissue culture cells.
Telithromycin and cethromycin constitute the last group. Their uptake was saturable in the promonocytic cell line (THP-1), which is in line with the earlier findings of Vazifeh and Labro (35). A saturation uptake profile was also observed in murine macrophages (RAW 264.7 cell line). Miossec-Bartoli et al. (22) reported similar findings in their experiments on the accumulation of telithromycin in human peripheral blood mononuclear cells and the THP-1, NB4, and K562 cell lines. Therefore, uptake of ketolides is saturable in cells of the monocytic lineage as well as in nondifferentiated cells of myeloid lineage. Although all-trans-retinoic acid-differentiated promyelocytes (HL-60 cell line) accumulate more telithromycin than their undifferentiated counterparts, they still display saturable uptake kinetics (26). Moreover, Miossec-Bartoli et al. (22) showed in their experiment with telithromycin and the NB4 cell line (a promyelocytic cell line) that upon differentiation of the cells, there are changes not only in the extent of accumulation but also in the intracellular location of the substance.
Ketolides were rapidly released from the preloaded cells. This is in agreement with data published by Miossec-Bartoli et al. (22), who showed that telithromycin is specifically trapped in the azurophilic granules of mature PMNs and is quickly released from other cell types. On the other hand, cethromycin egressed rapidly even from the PMNs. Although in comparison to telithromycin, cethromycin accumulated more in all the cells tested, it did not remain tightly bound or trapped within cellular compartments. This observation can be explained by the different cellular locations of the two ketolides tested. Unlike telithromycin, which accumulates in the granular compartment of PMNs (22, 36), cethromycin is mainly located in the cytosol (18).
In addition, our results demonstrate the selective in vitro behaviors of compounds that in vivo are all widely distributed throughout the body (5, 7, 25, 30). Extensive accumulation in vitro of established macrolide antibacterials within cells shows in general an inverse correlation with serum concentrations in vivo. This is illustrated by the fact that the peak serum concentration of azithromycin after a single 500-mg oral dose is approximately 0.4 mg/ml (7). This is four- to fivefold lower than the serum concentration achieved with a comparable dose of erythromycin (1.5 mg/liter) (15) or clarithromycin (2.3 mg/liter) (14). The slow release from the cells that was observed leads to prolonged retention of the substance in the body and probably influences the terminal half-life of the drug and, subsequently, the dosing regimen. The terminal half-lives after a single oral dose are about 40 h for azithromycin (7) and about 7 h for telithromycin (27), which thus allow once-daily dosing. On the other hand, clarithromycin and erythromycin, which have terminal half-lives of 4 h and 3 h, respectively (30), require multiple doses daily. These half-lives show a general inverse correlation with the in vitro release rates described in our study. In addition, infections caused by obligate or facultative intracellular pathogens can be eradicated as a result of the intracellular penetration and accumulation of substances (32, 33). Consequently, the differences in the uptake and the release kinetics of macrolide antibacterials observed in vitro could, among other factors, account for the differences in the terminal half-lives and the in vivo potencies of the substances. This in vitro-in vivo inverse correlation may well be applicable to newer agents, including ketolides. However, further comparative in vivo studies are needed to confirm the validity of such an in vitro-to-in vivo correlation.
In conclusion, as described in numerous papers published over the last few years, macrolide antibiotics have an outstanding ability to concentrate within host cells. Labro (17) has proposed classification of these antibiotics into two groups, according to their pharmacokinetic profiles in human PMNs. However, ketolides display intermediate characteristics between these two groups (34, 36). The results presented in this paper confirm this behavior and extend the observations of the kinetics of the accumulation and the release of macrolides to three different cell lines, in which, again, it was shown that ketolides do not entirely fit into either of the other two groups.
Acknowledgments
We are grateful to Federica Damiani (GlaxoSmithKline) and Dražen Pavlović (PLIVA Research Institute Ltd.) for providing telithromycin and cethromycin, respectively. We thank Dubravka Županić, Ksenija Štajcer, and Vesna Matek for exceptional technical assistance.
All authors are employees of PLIVA Inc., the manufacturer of azithromycin and the sponsor of this study.
REFERENCES
- 1.Abdelghaffar, H., A. Soukri, C. Babin-Chevaye, and M.-T. Labro. 2003. Interaction of macrolides and ketolides with the phagocytic cell line PLB-985. J. Chemother. 15:350-356. [DOI] [PubMed] [Google Scholar]
- 2.Blais, J., D. Beauchamp, and S. Chamberland. 1994. Azithromycin uptake and intracellular accumulation by Toxoplasma gondii-infected macrophages. J. Antimicrob. Chemother. 34:371-382. [DOI] [PubMed] [Google Scholar]
- 3.Bright, G. M., A. A. Nagel, J. Bordner, K. A. Desai, J. N. Dibrino, J. Nowakowska, L. Vincent, R. M. Watrous, F. C. Sciavolino, A. R. English, J. A. Retsema, M. R. Anderson, L. A. Brennan, R. J. Borovoy, C. R. Cimochowski, J. A. Faiella, A. E. Girard, D. Girard, C. Herbert, M. Manousos, and R. Mason. 1988. Synthesis, in vitro and in vivo activity of novel 9-deoxo-9a-aza-9a-homoerythromycin A derivatives; a new class of macrolide antibiotics, the azalides. J. Antibiot. 41:1029-1047. [DOI] [PubMed] [Google Scholar]
- 4.Carlier, M. B., I. Garcia-Luque, J. P. Montenez, P. M. Tulkens, and J. Piret. 1994. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue React. 16:211-220. [PubMed] [Google Scholar]
- 5.Conte, J. E., Jr., J. A. Golden, J. Kipps, and E. Zurlinden. 2004. Steady-state plasma and intrapulmonary pharmacokinetics and pharmacodynamics of cethromycin. Antimicrob. Agents Chemother. 48:3508-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denis, A., C. Agouridas, J. M. Auger, Y. Benedetti, A. Bonnefoy, F. Bretin, J. F. Chantot, A. Dussarat, C. Fromentin, S. G. D'Ambrieres, S. Lachaud, P. Laurin, O. Le Martret, V. Loyau, N. Tessot, J. M. Pejac, and S. Perron. 1999. Synthesis and antibacterial activity of HMR 3647, a new ketolide highly potent against erythromycin-resistant and susceptible pathogens. Bioorg. Med. Chem. Lett. 9:3075-3080. [DOI] [PubMed] [Google Scholar]
- 7.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 8.Frank, M. O., G. W. Sullivan, H. T. Carper, and G. L. Mandell. 1992. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 36:2584-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladue, R. P., G. M. Bright, R. E. Isaacson, and M. F. Newborg. 1989. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 33:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall, I. H., U. E. Schwab, E. S. Ward, J. D. Butts, E. T. Wolford, and T. J. Ives. 2002. Disposition and intracellular activity of azithromycin in human THP-1 acute monocytes. Int. J. Antimicrob. Agents 20:348-360. [DOI] [PubMed] [Google Scholar]
- 11.Hand, W. L., and D. L. Hand. 2001. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents 18:419-425. [DOI] [PubMed] [Google Scholar]
- 12.Idid, S. Z., N. R. Damanhury, Z. Ismail, and M. Arif. 2000. The absorption and distribution pattern of azithromycin in mice determined by the agar diffusion bioassay method. Asia Pacific J. Pharmacol. 14:79-82. [Google Scholar]
- 13.Ishiguro, M., H. Koga, S. Kohno, T. Hayashi, K. Yamaguchi, and M. Hirota. 1989. Penetration of macrolides into human polymorphonuclear leucocytes. J. Antimicrob. Chemother. 24:719-729. [DOI] [PubMed] [Google Scholar]
- 14.Kees, F., M. Wellenhofer, and H. Grobecker. 1995. Serum and cellular pharmacokinetics of clarithromycin 500 mg q.d. and 250 mg b.i.d. in volunteers. Infection 23:168-172. [DOI] [PubMed] [Google Scholar]
- 15.Kroboth, P. D., A. Brown, J. A. Lyon, F. J. Kroboth, and R. P. Juhl. 1982. Pharmacokinetics of single-dose erythromycin in normal and alcoholic liver disease subjects. Antimicrob. Agents Chemother. 21:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labro, M. T. 1996. Intracellular bioactivity of macrolides. Clin. Microbiol. Infect. 1(Suppl. 1):S24-S30. [DOI] [PubMed] [Google Scholar]
- 17.Labro, M. T. 1997. Effects of macrolides on leukocytes and inflammation, p. 101-116. In S. H. Zinner, L. S. Young, J. F. Acar, and H. C. Neu (ed.), Expanding indications for the new macrolides, azalides, and streptogramins. Marcel Dekker, Inc., New York, N.Y.
- 18.Labro, M. T., H. Abdelghaffar, and C. Babin-Chevaye. 2004. Interaction of the new ketolide ABT-773 (cethromycin) with human polymorphonuclear neutrophils and the phagocytic cell line PLB-985 in vitro. Antimicrob. Agents Chemother. 48:1096-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma, Z., R. F. Clark, A. Brazzale, S. Wang, M. J. Rupp, L. Li, G. Griesgraber, S. Zhang, H. Yong, L. T. Phan, P. A. Nemoto, D. T. Chu, J. J. Plattner, X. Zhang, P. Zhong, Z. Cao, A. M. Nilius, V. D. Shortridge, R. Flamm, M. Mitten, J. Meulbroek, P. Ewing, J. Alder, and Y. S. Or. 2001. Novel erythromycin derivatives with aryl groups tethered to the C-6 position are potent protein synthesis inhibitors and active against multidrug-resistant respiratory pathogens. J. Med. Chem. 44:4137-4156. [DOI] [PubMed] [Google Scholar]
- 20.Mandell, G. L., and E. Coleman. 2001. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 45:1794-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, J. R., P. Johnson, and M. F. Miller. 1985. Uptake, accumulation, and egress of erythromycin by tissue culture cells of human origin. Antimicrob. Agents Chemother. 27:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miossec-Bartoli, C., L. Pilatre, P. Peyron, E. N. N′Diaye, V. Collart-Dutilleul, I. Maridonneau-Parini, and A. Diu-Hercend. 1999. The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonucelar cells. Antimicrob. Agents Chemother. 43:2457-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mtairag, E. M., H. Abdelghaffar, C. Douhet, and M. T. Labro. 1995. Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob. Agents Chemother. 39:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mtairag, E. M., H. Abdelghaffar, and M. T. Labro. 1994. Investigation of dirithromycin and erythromycylamine uptake by human neutrophils in vitro. J. Antimicrob. Chemother. 33:523-536. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Serieys, C., J. Andrews, F. Vacheron, and C. Cantalloube. 2004. Tissue kinetics of telithromycin, the first ketolide antibacterial. J. Antimicrob. Chemother. 53:149-157. [DOI] [PubMed] [Google Scholar]
- 26.Munic, V., M. Bosnar, Z. Kelneric, D. Zupanic, V. Erakovic, M. J. Parnham, and F. Damiani. 2002. Macrolide uptake and release by HL-60 and human polymorphonuclear (PMN) cells, abstr. 4.06, p. 120. In Program and abstracts of 6th Int. Conf. Macrolides, Azalides, Streptogramins, Ketolides and Oxazolidinones.
- 27.Namour, F., D. H. Wessels, M. H. Pascual, D. Reynolds, E. Sultan, and B. Lenfant. 2001. Pharmacokinetics of the new ketolide telithromycin (HMR 3647) administered in ascending single and multiple doses. Antimicrob. Agents Chemother. 45:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pascual, A., S. Ballesta, I. García, and E. J. Perea. 2001. Uptake and intracellular activity of ketolide HMR 3647 in human phagocytic and non-phagocytic cells. Clin. Microbiol. Infect. 7:65-69. [DOI] [PubMed] [Google Scholar]
- 29.Pascual, A., J. Rodriguez-Baño, S. Ballesta, I. García, and E. J. Perea. 1997. Azithromycin uptake by tissue cultured epithelial cells. J. Antimicrob. Chemother. 39:293-295. [DOI] [PubMed] [Google Scholar]
- 30.Rodvold, K. A. 1999. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 37:385-398. [DOI] [PubMed] [Google Scholar]
- 31.Rothen-Rutishauser, B., S. D. Krämer, A. Braun, M. Günthert, and H. Wunderli-Allenspach. 1998. MDCK cell cultures as an epithelial in vitro model: cytoskeleton and tight junctions as indicators for the definition of age-related stages by confocal microscopy. Pharm. Res. 15:964-971. [DOI] [PubMed] [Google Scholar]
- 32.Segreti, J., P. Meyer, and K. Kapell. 1996. In vitro activity of macrolides against intracellular Legionella pneumophila. Diagn. Microbiol. Infect. Dis. 25:123-126. [DOI] [PubMed] [Google Scholar]
- 33.Strigl, S., P. M. Roblin, T. Reznik, and M. R. Hammerschlag. 2000. In vitro activity of ABT 773, a new ketolide antibiotic, against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 44:1112-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazifeh, D., H. Abdelghaffar, and M. T. Labro. 1997. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob. Agents Chemother. 41:2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazifeh, D., and M. T. Labro. 1999. Investigation of the uptake of HMR 3647, HMR 3004 and roxithromycin by myelomonocytic cell lines, abstr. 1929, p. 48. In Program abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 36.Vazifeh, D., A. Preira, A. Bryskier, and M. T. Labro. 1998. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 42:1944-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]