Abstract
Partial sequence analysis of a tet(O) plasmid from a multiple-drug-resistant clinical isolate of Campylobacter jejuni revealed 10 genes or pseudogenes encoding different aminoglycoside inactivating enzymes, transposase-like genes, and multiple unknown genes from a variety of pathogenic and commensal bacteria. The plasmid could be mobilized by a P incompatibility group plasmid into Escherichia coli, where it apparently integrated into the chromosome and expressed high-level resistance to multiple aminoglycoside antibiotics. This work provides new information about both the nature of drug resistance in C. jejuni and the ability of C. jejuni to exchange genes with other bacterial species.
Campylobacter jejuni is among the most frequent causes of diarrheal disease worldwide (2, 23), and the incidence is particularly high in Thailand, where both antimicrobial resistance and multidrug resistance are increasing problems (6). Although the levels of resistance to the macrolides erythromycin and azithromycin have remained low, the incidence of resistance to the quinolones has risen to 96% in a 1998 study of U.S. military personnel (22). Plasmid-mediated resistance to tetracycline, chloramphenicol, and kanamycin has been well documented in C. jejuni (25, 31, 29), but no other plasmid-encoded resistance genes have been reported, other than a very recent report of streptomycin and streptothricin resistance (12). Recently, comparison of the sequences of transferable tet(O) plasmids from a C. jejuni strain and a C. coli strain isolated on different continents more than 20 years apart revealed high conservation (4, 5). Surveys of clinical isolates of C. jejuni in Thailand in our laboratories have revealed a high incidence of both multiple drug resistance and plasmids (unpublished data). Given these observations, we hypothesized that at least some of these antibiotic resistances in recent Thai isolates were plasmid encoded. Here we describe the partial genetic structure of a multiple-drug-resistant plasmid from a Thai clinical isolate of C. jejuni. The plasmid is related to other tet(O) plasmids but is novel in that it also contains multiple aminoglycoside-inactivating enzymes and transposon-like sequences from a variety of bacteria that have not previously been reported in C. jejuni.
(This work was done in partial fulfillment of the requirements for a Ph.D. degree from Mahidol University by W.N.)
MATERIALS AND METHODS
Bacterial strains and plasmids.
C. jejuni strain CG8245 was isolated from a diarrhea case of a U.S. soldier deployed to Thailand during a military exercise in 1999. Escherichia coli DH5α was used as the host for cloning experiments, and pBluescript (Stratagene, La Jolla, CA) was used as the cloning vector. For conjugation experiments with another C. jejuni strain, a mutant of C. jejuni NCTC 11168 that had been insertionally inactivated with a chloramphenicol resistance (cat) cassette (32) in the Cj1316c gene was used as the recipient (P. Guerry, unpublished data). For conjugation experiments with E. coli, E. coli C600(RK212.2) (Tetr Ampr) (9) and E. coli DH5α (Nalr) were used.
Bacterial growth conditions.
Bacterial strains were maintained at −80°C in brucella broth (Difco) supplemented with 40% glycerol. C. jejuni was grown routinely on Mueller-Hinton (MH) agar under microaerobic conditions at 37°C, while E. coli was grown on LB agar or MacConkey agar under aerobic conditions at 37°C. Antibiotics were added when appropriate to the following concentrations: 50 μg of kanamycin per ml, 50 μg of spectinomycin per ml, 100 μg of hygromycin per ml, 20 μg of tetracycline per ml, 50 μg of gentamicin per ml, 25 μg of streptomycin per ml, 50 μg of nalidixic acid per ml, 20 μg of chloramphenicol per ml, 62.5 μg of ampicillin per ml.
DNA manipulations.
Plasmid DNA from C. jejuni strain CG8245 was purified as previously described (3). Plasmid DNA from CG8245 was digested separately with BglII, BclI, and SacII (New England Biolabs, Beverly, Mass.) and cloned into pBluescript that had been digested with either BamHI or SacII. Transformants were selected by blue/white screening on LB supplemented with ampicillin and 1.2 mg/plate of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), kanamycin, or spectinomycin.
DNA sequence analysis.
DNA sequencing was facilitated by transposition mutagenesis of the cloned fragments with a previously described (13) EZ::TN MOD transposon containing the cat resistance cassette from pRY109 (32). Additional sequencing was done by primer walking, and some contigs were closed using PCR with Pfu Ultra High-Fidelity DNA polymerase (Stratagene), followed by cloning into pPCR-Script (Stratagene).
DNA sequencing was done using Big Dye sequencing kits (Applied Biosystems, Foster City, Calif.) and analyzed on an Applied Biosystems 3100 sequencer. Open reading frames (ORFs) predicted to encode peptides of greater than 30 residues were evaluated based on the presence of a suitable initiation codon with appropriate spacing to the ribosome-binding site, as well as physical location with respect to other ORFs. Similarity searches to the predicted proteins were performed using a BLAST algorithm against all available public databases via the National Center for Biotechnology Information, Bethesda, MD.
Mutagenesis of drug resistance genes.
Site-specific mutagenesis of each drug resistance gene was performed using clones generated from in vitro transposition as described above. The clones selected had the cat gene inserted in the same orientation as the target gene to minimize possible polarity. Selected plasmids were used to electroporate C. jejuni CG8245 (14) with selection on MH agar supplemented with chloramphenicol. The successful mutation of each drug resistance gene was confirmed to have occurred by a double-crossover event by PCR using primers flanking the insertion point.
MIC and antibiotic susceptibility testing.
MICs were determined using the agar dilution technique as recommended by the National Committee for Clinical Laboratory Standards (20a). Semiquantitative in vitro susceptibility testing by the agar disk diffusion method was performed according to the manufacturer's instructions (BBL, Becton, Dickinson and Company, Sparks, MD).
Conjugation experiments.
Conjugation experiments between strains of C. jejuni were done using a modification (5) of the method of Taylor (24). The transconjugants were selected by incubation overnight at 37°C under microaerobic conditions on medium that contained chloramphenicol and tetracycline or chloramphenicol and kanamycin.
For conjugation experiments between C. jejuni and E. coli strains, C. jejuni CG8245 was grown overnight on MH plates, while two strains of E. coli, C600 and DH5α, were grown overnight on LB plates. The donor and two recipient strains were resuspended in MH broth to an optical density at 600 nm of 1.0 and mixed in a ratio of 1:1:1 before spotting onto MH agar. The MH agar was incubated for 6 h at 37°C under microaerobic conditions. Bacterial growth was scraped from the plates and resuspended in 1 ml of MH broth. Aliquots of 100 μl were plated onto MacConkey agar containing kanamycin, spectinomycin, hygromycin, gentamicin, or streptomycin. The cultures were incubated aerobically at 37°C overnight.
Nucleotide sequence accession number.
The sequences of the genes described in this paper have been submitted to GenBank under accession number AY701528.
RESULTS
Antibiogram of CG8245.
C. jejuni strain CG8245, which was serotyped as Lior 19, was resistant to three fluoroquinolones: nalidixic acid (30 μg), ciprofloxacin (5 μg), and ofloxacin (16 μg). It was also resistant to ampicillin (10 μg) and bactrim (25 μg). Table 1 shows that CG8245 was also resistant to tetracycline and six aminoglycosides. The strain was sensitive to chloramphenicol (30 μg), colistin (10 μg), and azithromycin (15 μg).
TABLE 1.
MICs for CG8245, DH5α and mutants
| Substrate | Gene/plasmida | MIC (μg/ml)b for:
|
||||
|---|---|---|---|---|---|---|
| CG8245 | CJ mutant | DH5α | EC clone | EC mutant | ||
| Tetracycline | tet(O)/ORF5/pJW101 | 150 | 0.064 | 1 | 24 | 0.75 |
| Streptomycin | aadE/ORF2/pJW101 | 96 | 0.094 | 0.38 | 12 | 1 |
| aadE/ORF23/pJW102 | 96 | 96 | 0.38 | 0.38 | 0.38 | |
| aadA/ORF11/pJW102 | 96 | 96 | 0.38 | 0.38 | 0.38 | |
| Spectinomycin | aad9/ORF8/pJW101 | ≥1,024 | 2 | 2 | ≥1,024 | 2 |
| aadA/ORF11/pJW102 | ≥1,024 | ≥1,024 | 2 | 2 | 2 | |
| Kanamycin | aac/ORF12/pJW106 | 3,500 | 2,500 | 0.5 | 1,500 | 0.75 |
| aphA-3/ORF25/pJW107 | 3,500 | 700 | 0.5 | 1,500 | 0.5 | |
| aacA/aphD/ORF13/pJW108 | 3,500 | 2,500 | 0.5 | 1,500 | 6 | |
| Gentamicin | aph2/ORF10/pJW102 | 400 | 400 | 0.047 | 0.125 | 0.125 |
| aacA/aphD/ORF13/pJW102 | 400 | 0.38 | 0.047 | 0.125 | 0.125 | |
| Streptothricin | sat4/ORF24/pJW102 | 25 | ≤1.56 | ≤1.56 | 150 | ≤1.56 |
| Hygromycin | hph/ORF21/pJW102 | ≥2,800 | 1,400 | 50 | ≥2,800 | 50 |
The plasmid used for mutational analysis for each gene is indicated and shown schematically in Fig. 2, and the transposon insertion point for each mutant allele is shown in Table 3.
MICs were determined for wild-type C. jejuni CG8245 or a Cmr insertional mutant of each gene (CJ mutant); MICs were determined for DH5α, DH5α carrying the indicated plasmid (EC clone), and the Cmr insertional mutant of each plasmid gene (EC mutant) that was used to generate the C. jejuni mutant.
Cloning and sequence analysis of drug resistance genes from pCG8245.
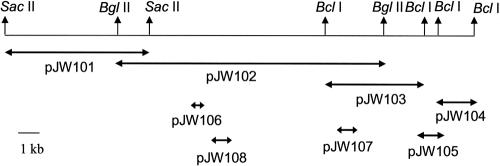
C. jejuni strain CG8245 contained a single plasmid that hybridized to both tet(O) and aphA-3 probes in Western blot assays (data not shown). Based on these blots, a BglII fragment encoding resistance to kanamycin was cloned into pBluescript and called pJW102. The inserts in this clone and overlapping clones, shown in Fig. 1 and comprising 25.7 kb of contiguous DNA, were fully sequenced. The overall G+C content of this region of the plasmid was 33.4%, which was higher than the two sequenced strains of C. jejuni (30.6% and 30.3%, respectively) (11, 21). The G+C content of this region of pCG8245 was also higher than that of the pTet plasmid of strain 81-176 (29.1%) (4, 5) but similar to that of several cryptic Campylobacter plasmids (19).
FIG. 1.
Schematic of the 25.7-kb region of pCG8245 that was sequenced. DNA sequencing was done on the overlapping regions of DNA either cloned into pBluescript (pJW101, pJW102, pJW103, and pJW104) or PCR amplified and cloned into pPCR-Script (pJW105, pJW106, pJW107, and pJW108). Plasmids pJW106, pJW107, and pJW108 are PCR clones of three different kanamycin resistance genes that were generated for mutational analyses as described in the text.
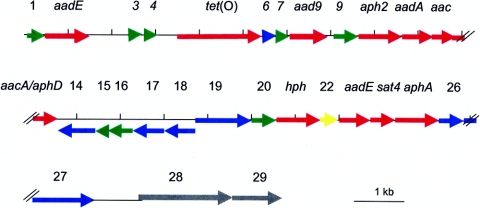
This region of pCG8245 encoded 29 ORFs, as summarized in Table 2 and Fig. 2. Among the ORFs surrounding the tet(O) gene were 10 genes predicted to encode aminoglycoside modifying enzymes. These 10 genes are discussed in more detail below.
TABLE 2.
Annotation of genes found on the 25 kb sequenced region of pCG8245
| ORF | Gene | Codon region (bp) | %G + C content | Length (aa)a | Length (aa)b | % Identity/% similarity (no. of aa) | Annotation/accession no. |
|---|---|---|---|---|---|---|---|
| 1 | Unknown | 2-316 | 37.8 | 181 | 92 | 85/95 (92) | Unknown/Arcanobacterium pyogenes/AAQ94626 |
| 2 | aadE | 492-1358 | 35.5 | 302 | 281 | 60/75 (281) | Streptomycin aminoglycoside 6-adenyltransferase/E. faecium/NP_863159 |
| 3 | cpp50 | 2599-2811 | 26.3 | 473 | 70 | 100/100 (70) | Unknown of pTet plasmid/C. jejuni strain 81-176/YP_063493 |
| 4 | cpp51 | 2830-3009 | 23.9 | 59 | 59 | 83/83 (59) | Unknown of pTet plasmid/C. jejuni strain 81-176/YP_063494 |
| 5 | tet(O) | 3368-5287 | 40.3 | 639 | 639 | 99/99 (639) | Tet(O)/C. jejuni/YP_063446 |
| 6 | Unknown Tn916 | 5339-5512 | 36.7 | 62 | 57 | 66/82 (57) | ORF6 of Tn916/E. faecalis/AAB60023 |
| 7 | Unknown | 5841-5990 | 37.3 | Unknown (no significant match in database) | |||
| 8 | aad9 | 6015-6791 | 35.8 | 242 | 235 | 55/71 (235) | Spectinomycin adenyltransferase/E. faecalis/AAL05551 |
| 9 | Unknown | 6991-7536 | 37.7 | 181 | 180 | 80/88 (180) | Unknown/Arcanobacterium pyogenes/AAQ94626 |
| 10 | aph2 | 7652-8449 | 23.7 | 301 | 227 | 22/41 (227) | Aminoglycoside 3′-phosphotransferase, type III/E. casseliflavus/AAC14693 |
| 11 | aadA | 8450-9244 | 25.6 | 261 | 249 | 33/57 (250) | Streptomycin 3"-adenylyltransferase/E. faecalis V583/NP_814808 |
| 12 | aac | 9268-9696 | 25.1 | 130 | 127 | 50/75 (127) | Acetyltransferase GNAT family/E. faecalis V583/NP-816983 |
| 13 | aacA/aphD | 9696-10589 | 23.8 | 479 | 281 | 79/88 (281) | Bifunctional aminoglycoside modifying enzyme/S. warneri/CAD60199 |
| 14 | Transposase | 10829-11332c | 25.2 | 431 | 125 | 40/56 (125) | Transposase IS232/B. thuringiensis/Q99335 |
| 15 | Transcriptional regulator | 11399-11710c | 44.9 | 117 | 91 | 48/72 (91) | Transcriptional regulator/C. acetobutylicum ATCC 824/NP_349177 |
| 16 | Unknown | 11710-12111c | 44.7 | 150 | 116 | 33/54 (118) | Conserved hypothetical/C. thermocellum/ZP00311549 |
| 17 | Transposon related | 12233-12991c | 30.2 | 250 | 248 | 58/76 (248) | IS232 putative ATP-binding protein/B. thuringiensis/Q99338 |
| 18 | Transposase | 12954-13622c | 28.8 | 431 | 179 | 40/53 (184) | Transposase IS232/B. thuringiensis/Q99335 |
| 19 | Unknown | 13896-14933 | 39.8 | 204 | 143 | 42/63 (147) | Unknown/C. acetobutylicum/NP-348122 |
| 20 | Unknown | 14960-15505 | 39.6 | 181 | 181 | 76/81 (181) | Unknown/Arcanobacterium pyogenes/AAQ94626 |
| 21 | hph | 15565-16482 | 47.6 | 341 | 296 | 37/57 (303) | Hygromycin B phosphotransferase (aph7")/E. coli/P00557 |
| 22 | pcp | 16487-16906 | 49.3 | 213 | 136 | 53/78 (136) | Pyrrolidone carboxylate peptidase/E. faecalis V583/NP_814190 |
| 23 | aadE | 17168-17683 | 39.5 | 302 | 163 | 100/100 (163) | Streptomycin aminoglycoside 6-adenyltransferase/E. faecium/NP_863159 |
| 24 | sat4 | 17692-18222 | 37.7 | 180 | 176 | 94/94 (176) | Streptothricin acetyltransferase/E. faecium/AAM77897 |
| 25 | aphA-3 | 18315-19109 | 44.9 | 264 | 264 | 100/100 (264) | Aminoglycoside 3′-phosphotransferase, type III/E. faecium/CAD36021 |
| 26 | tnp | 19400-19873 | 29.3 | 132 | 127 | 65/79 (129) | Transposase/C. tetani/NP_781296 |
| 27 | tnpB | 19860-21098 | 27.3 | 442 | 442 | 52/67 (442) | IS606 transposase/H. pylori/NP_223544 |
| 28 | pTet13a or cpp14 | 22645-24315 | 31.0 | 1,932 | 550 | 100/100 (550) | pTet13/C. jejuni strain 81-176; homolog of H. pylori JHP0928/YP_063459 |
| 29 | pTet13b or cpp14 | 24458-25711 | 28.0 | 1,932 | 417 | 99/99 (417) | pTet13/C. jejuni strain 81-176; homolog of H. pylori JHP0928/YP_063459 |
Size of the ortholog protein. aa, amino acids.
Length of the pCG8245 peptide.
FIG. 2.
Schematic of the coding regions within the sequenced 25.7-kb region of pCG8245. The ORFs are color coded based on the putative function of the predicted encoded proteins. Red, antibiotic resistance genes; blue, transposases and transposon associated; green, unknown proteins; gray, similar to genes from the plasticity zones of H. pylori; yellow, known but not associated with antibiotic resistance. Vertical hash marks, below the linear plasmid, are located every 1 kb.
Mutational analyses of antibiotic markers.
Transposon insertions into each of the putative drug resistance genes were generated during sequence analysis of the plasmids shown in Fig. 1, and the insertions used to generate mutants of C. jejuni by electroporation are summarized in Table 3. The MICs for the C. jejuni CG8245 wild-type strain and the site-specific mutation in each drug gene against its predicted substrate(s) are shown in Table 1. Table 1 also shows the MICs for DH5α, DH5α carrying different clones shown in Fig. 2, and the corresponding mutated allele of each gene in the respective clones.
TABLE 3.
Insertion points of cat transposon cassette within antibiotic resistance genes
| ORF | Gene | Coding region (bp) | Insertion point (bp) |
|---|---|---|---|
| 2 | aadE | 492-1358 | 861 |
| 5 | tet(O) | 3368-5287 | 4101 |
| 8 | aad9 | 6015-6791 | 6703 |
| 10 | aph2 | 7652-8449 | 8258 |
| 11 | aadA | 8450-9244 | 8785 |
| 12 | aac | 9268-9696 | 9293 |
| 13 | aacA/aphD | 9696-10589 | 9977 |
| 21 | hph | 15565-16482 | 15760 |
| 23 | aadE | 17168-17683 | 17564 |
| 24 | sat4 | 17692-18222 | 17743 |
| 25 | aphA-3 | 18315-19109 | 18822 |
There were three genes predicted to encode alleles of streptomycin-inactivating enzymes from Enterococcus species. Two genes, ORF2 and ORF23, encoded predicted proteins with similarity to AadE, a streptomycin 6-adenyltransferase, although the homology of the protein encoded by ORF23 appeared limited to the carboxy-terminal 163 amino acids (Table 2). This change apparently resulted in loss of function since ORF23 did not express resistance in either E. coli or C. jejuni. However, ORF2 functioned in both hosts. ORF11 encoded a predicted streptomycin/spectinomycin 3"-adenylyltransferase, whose closest homolog was AadA [or Ant(3")-Ia] from Enterococcus faecalis, but the gene appeared not to function in either E. coli or C. jejuni (Table 1). ORF8, however, encoding an ortholog of Aad-9 or Ant(9)-I from E. faecalis, an enzyme that catalyzes ATP-dependent adenylation of the position 9 hydroxyl group of spectinomycin, functioned in both E. coli and C. jejuni.
There were three genes predicted to encode resistance to kanamycin, all of which functioned in both E. coli and C. jejuni. ORF12 encoded a protein related to an acetyltransferase enzyme GNAT (GCN5-related N-acetyltransferase) family member from E. faecalis. ORF25 encoded a type III aminoglycoside 3′-phosphotransferase or AphA-3, which has been reported on campylobacter plasmids previously (18). ORF13, encoded a homolog of a staphylococcal bifunctional enzyme with acetyltransferase activity at the amino-terminal domain and phosphotransferase activity at the carboxy-terminal domain. However, the homology of the ORF13 product was restricted to the carboxy-terminal region of this bifunctional enzyme, suggesting only phosphotransferase activity. ORF13 also conferred gentamicin resistance on C. jejuni, but not on E. coli (Table 1). Another gene, ORF10 or aph2, encoded an aminoglycoside 3′-phosphotransferase predicted to be active against gentamicin, but the gene did not function in either E. coli or C. jejuni.
ORF24 encoded a protein with 94% identity to a streptothricin acetyltransferase from Enterococcus. ORF21 encoded a protein with 37% identity and 57% similarity to a hygromycin B phosphotransferase (Aph-7") from E. coli. This gene was recently found on another tet(O) plasmid during genomic sequencing of a C. coli strain (11). ORF24 and ORF21 conferred resistance to streptothricin and hygromycin, respectively, in both E. coli and C. jejuni.
Genes encoding transposases and insertion sequences.
Several transposon-related sequences from a variety of bacteria were found on pCG8245. ORF26 encoded a predicted protein with 65% identity to a transposase from Clostridium tetani strain E88 (7). The protein also showed 41% identity to OrfA from the Helicobacter pylori transposon ISHp608 (16). ORF27 encoded a predicted protein whose best match was OrfB from IS606 from H. pylori strain J99 (52% identity) (15). ORF6 encoded a predicted protein with 66% identity to a protein of unknown function from the conjugative transposon Tn916 of E. faecalis (10). There were three ORFs related to an insertion sequence, IS232, from Bacillus thuringiensis (20). ORF14 and ORF18 have homology to the amino and carboxy regions of the IS232 transposase, respectively. ORF14 encoded a predicted protein with 40% identity to this transposase, but the homology is restricted to the amino end (amino acids 34 to 158 out of 431). ORF18 encoded a protein with 40% identity over 42% of the total length of the IS232 transposase (amino acids 220 to 398 out of 431). Thus, the N and C termini of the IS232 transposase are split and appear reversed, as if there had been a duplication of the IS232 gene, followed by a complex rearrangement. ORF17, which maps between ORF14 and ORF18, encoded a protein that showed 58% identity and 76% similarity to a putative ATP-binding protein from IS232 over 99% of the total length of the protein (amino acids 3 to 250 out of 250). Also mapping between the apparently truncated transposase were two genes encoding proteins with homologs from other species of Clostridium (see below).
Other sequenced genes in this region of pCG8245.
There were three copies of a gene encoding an unknown protein of 181 amino acids from Arcanobacterium pyogenes strain OX-7 (ORF1, -9, and -20). The three alleles encoded proteins that were 75 to 86% identical to each other. PCR was done using primers within these genes and adjacent genes to confirm the presence of these three copies at the indicated positions (data not shown). A homolog of the predicted proteins encoded by ORF1, -9, and -20 has recently been described in C. coli RM2228 (11).
ORF3 and ORF4 encoded predicted proteins that showed high levels of similarity to Cpp50 and Cpp51, respectively, from the pTet plasmid of C. jejuni 81-176 (5). ORF7 encoded a predicted protein with no significant match in the databases.
ORF15 encoded a predicted protein with a helix-turn-helix motif annotated as a transcriptional regulator in C. acetobutylicum. ORF16 encoded a conserved hypothetical protein found in numerous bacteria, with the best match being to a protein from another species of Clostridium, C. thermocellum.
At the end of the sequenced region are two genes encoding predicted proteins with high homology to the protein encoded by cpp14 of the pTet plasmid of C. jejuni 81-176 (5). The protein encoded by cpp14 is a large protein (1,932 amino acids) with 99% identity to a protein encoded by JHP0928, a putative methylase encoded in the plasticity zone of Helicobacter pylori strain J99 (1). ORF28 of pCG8245 encodes a protein with 100% identity to amino acids 1 to 550 of Cpp14; ORF29 of pCG8245 encodes a protein with 99% identity to amino acids 606 to 1022 of Cpp14. Thus, these two genes appear to represent a split of the larger cpp14 ORF into two smaller ORFs, the latter of which encodes the putative methylase domain.
Conjugal transfer of pCG8245 to another strain of C. jejuni.
Similar to previous reports on conjugation of campylobacter tet(O) plasmids, pCG8245 could be transferred conjugatively to C. jejuni NCTC 11168 Cmr at a frequency of 4.2 × 10−5/donor cell. The frequency was slightly lower (6.0 × 10−6/donor cell) when the mating was done in the presence of DNase to eliminate natural transformation. All transconjugants tested, regardless of whether DNase was added to the mating plates or the selective medium used (chloramphenicol and kanamycin or chloramphenicol and tetracycline) also expressed resistance to hygromycin, spectinomycin, kanamycin, tetracycline, and chloramphenicol. The restriction pattern of plasmid DNA from selected transconjugants was identical to that of pCG8245 (data not shown).
Transfer of drug resistance markers from pCG8245 to E. coli.
Attempts to directly transfer pCG8245 to E. coli DH5α with selection on kanamycin were uniformly unsuccessful. However, a triple cross among C. jejuni CG8245, E. coli DH5α (Lac−), and E. coli C600 (Lac+) containing P incompatibility plasmid RK212.2, encoding resistance to ampicillin and tetracycline (9), was done with selection on MacConkey agar supplemented with either streptomycin, kanamycin, spectinomycin, or hygromycin incubated aerobically. Both Lac+ and Lac− E. coli transconjugants were obtained at frequencies of approximately 10−6 to 10−8/donor cell. Regardless of the selection used, all transconjugants examined were phenotypically resistant to kanamycin, spectinomycin, hygromycin, spectinomycin, and streptomycin. Attempts to isolate plasmid DNA from both Lac− (DH5α) and Lac+ (C600/RK212.2) transconjugants yielded plasmids that appeared identical to RK212.2 by restriction analysis (data not shown). However, PCR analysis with primers specific for the pCG8245-encoded genes aadE, tet(O), aad9, aph2, aadA, aac, aacA/aphD, hph, sat4, and aphA-3 indicated that all of these genes were present in both classes of transconjugants.
DISCUSSION
The plasmid from C. jejuni strain CG8245 appears related to pTet from C. jejuni strain 81-176 in that, in addition to tet(O), it contains several homologs of other pTet genes in the region sequenced. However, it differs from any previously described tet(O) plasmid in that it contains 10 ORFs that encode copies of aminoglycoside-inactivating enzymes from both gram-negative and gram-positive sources. Three of these genes are not functional in either C. jejuni or E. coli (aph2/ORF10, aadA/ORF11, and aadE/ORF23).
There have been two other recent reports on tet(O) plasmids with additional antibiotic resistance markers, although neither was as extensive a repertoire as that found on pCG8245. Gibreel et al. (12) reported on C. jejuni plasmids containing the aadE-sat4-aphA-3 gene cluster that was originally described in gram positives, a cluster similar to that present on pCG8245 as ORF23, -24, and -25. However, in the case of pCG8245, the aadE gene copy located adjacent to sat4 is nonfunctional. A second, full-length, functional copy of aadE, ORF2, is located >15 kb upstream. The aadE-sat4-aphA-3 gene cluster was originally described as part of a transposon structure, Tn5405 from staphylococci (8). The version of this gene cluster with an intact aadE gene reported for C. jejuni plasmids from Canada, Egypt, and Sweden was associated with IS607 from H. pylori. In the case of pCG8245, the aadE-sat4-aphA-3 gene cluster mapped next to an apparent hybrid of two H. pylori transposons, ISHp608 and IS606 (16, 15). Genomic sequencing of a chicken isolate of C. coli has recently revealed a 110-kb plasmid encoding resistance to tetracycline, kanamycin, and hygromycin (11).
Conjugative plasmids of C. jejuni are generally considered unable to transfer to other genera (26-30). However, recently Gibreel et al. (12) were able to conjugatively transfer a plasmid encoding tet(O) and aphA-3 from C. jejuni to E. coli, where the plasmid integrated into the chromosome. The plasmid from CG8245 could be transferred conjugatively to another strain of C. jejuni, but we were unable to detect direct transfer from C. jejuni to E. coli. We could, however, detect mobilization in a triple cross that included P incompatibility plasmid RK212.2 (9). It has been recognized for some time that P incompatibility plasmids can conjugatively transfer from E. coli into C. jejuni but cannot replicate vegetatively in C. jejuni, an observation that has been used in genetic studies to mobilize campylobacter shuttle vectors from E. coli into C. jejuni (17). Although the molecular details remain to be elucidated, it appears that RK212.2 was able to mobilize pCG8245 from C. jejuni into an E. coli recipient. It appears that most, if not all, of pCG8245 integrated into the E. coli chromosome, similar to the situation described by Gibreel et al. (12), rendering the E. coli recipient resistant to multiple aminoglycosides.
The partial DNA sequence of pCG8245 reported here is an eclectic collection of genes from a wide variety of bacterial genera. There are drug resistance genes that appear to have originated from Enterococcus, Staphylococcus, Streptococcus, and E. coli. There are homologs of transposons or insertion sequences from two species of Clostridium, Bacillus thuringiensis, and H. pylori and additional genes from Enterococcus, Clostridium, Arcanobacterium pyogenes, and the plasticity zone of H. pylori. This is particularly surprising given the fact that C. jejuni has not been reported to exchange genes outside of its own genus. Since aminoglycosides are not used clinically to treat C. jejuni infections, the high level of resistance in C. jejuni strain CG8245 and other Thai isolates is likely due to environmental exposure, perhaps the result of veterinary use. The data suggest that C. jejuni can serve as a reservoir of drug resistance that can be disseminated to E. coli and perhaps other bacteria.
Acknowledgments
This research was supported by In-House Laboratory Independent Research and by Military Infectious Diseases Research Program work unit 6000.RAD1.DA3.A0308.
We thank Prapon Wilairat for valued suggestions and the staffs of both AFRIMS and NMRC for technical assistance.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon, D. J., R. A. Alm, D. H. Burr, L. Hu, D. J. Kopecko, C. P. Ewing, T. J. Trust, and P. Guerry. 2000. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 68:4384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon, D. J., R. A. Alm, L. Hu, T. E. Hickey, C. P. Ewing, R. A. Batchelor, T. J. Trust, and P. Guerry. 2002. DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 81-176. Infect. Immun. 70:6242-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batchelor, R. A., B. M. Pearson, L. M. Friis, P. Guerry, and J. M. Wells. 2004. Nucleotide sequences and comparison of two large conjugative plasmids from different Campylobacter species. Microbiology 150:3507-3517. [DOI] [PubMed] [Google Scholar]
- 6.Bodhidatta, L., N. Vithayasai, B. Eimpokalarp, C. Pitarangsi, O. Serichantalergs, and D. W. Isenbarger. 2002. Bacterial enteric pathogens in children with acute dysentery in Thailand: increasing importance of quinolone-resistant Campylobacter. Southeast Asian J. Trop. Med. Public Health 33:752-757. [PubMed] [Google Scholar]
- 7.Bruggemann, H., S. Baumer, W. F. Fricke, A. Wiezer, H. Liesegang, I. Decker, C. Herzberg, R. Martinez-Arias, R. Merkl, A. Henne, and G. Gottschalk. 2003. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc. Natl. Acad. Sci. USA 100:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derbise, A., S. Aubert, and N. El Solh. 1997. Mapping the regions carrying the three contiguous antibiotic resistance genes aadE, sat4, and aphA-3 in the genomes of staphylococci. Antimicrob. Agents Chemother. 41:1024-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flannagan, S. E., L. A. Zitzow, Y. A. Su, and D. B. Clewell. 1994. Nucleotide sequence of the 18-kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid 32:350-354. [DOI] [PubMed] [Google Scholar]
- 11.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Dougherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. Public Library Sci. Biol. 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibreel, A., O. Skold, and D. E. Taylor. 2004. Characterization of plasmid-mediated aphA-3 kanamycin resistance in Campylobacter jejuni. Microb. Drug Resist. 10:98-105. [DOI] [PubMed] [Google Scholar]
- 13.Guerry, P., C. P. Ewing, T. E. Hickey, M. M. Prendergast, and A. P. Moran. 2000. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect. Immun. 68:6656-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerry, P., R. Yao, R. A. Alm, D. H. Burr, and T. J. Trust. 1994. Systems of experimental genetics for Campylobacter species. Methods Enzymol. 235:474-481. [DOI] [PubMed] [Google Scholar]
- 15.Kersulyte, D., N. S. Akopyants, S. W. Clifton, B. A. Roe, and D. E. Berg. 1998. Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. Gene 223:175-186. [DOI] [PubMed] [Google Scholar]
- 16.Kersulyte, D., B. Velapatino, G. Dailide, A. K. Mukhopadhyay, Y. Ito, L. Cahuayme, A. J. Parkinson, R. H. Gilman, and D. E. Berg. 2002. Transposable element ISHp608 of Helicobacter pylori: nonrandom geographic distribution, functional organization, and insertion specificity. J. Bacteriol. 184:992-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert, T., G. Gerbaud, P. Trieu-Cuot, and P. Courvalin. 1985. Structural relationship between the genes encoding 3′-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann. Inst. Pasteur Microbiol. 136B:135-150. [DOI] [PubMed] [Google Scholar]
- 19.Luo, N., and Q. Zhang. 2001. Molecular characterization of a cryptic plasmid from Campylobacter jejuni. Plasmid 45:127-133. [DOI] [PubMed] [Google Scholar]
- 20.Menou, G., J. Mahillon, M. M. Lecadet, and D. Lereclus. 1990. Structural and genetic organization of IS232, a new insertion sequence of Bacillus thuringiensis. J. Bacteriol. 172:6689-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing: twelfth informational supplement, M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 22.Sanders, J. W., D. W. Isenbarger, S. E. Walz, L. W. Pang, D. A. Scott, C. Tamminga, B. A. Oyofo, W. C. Hewitson, J. L. Sanchez, C. Pitarangsi, P. Echeverria, and D. R. Tribble. 2002. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am. J. Trop. Med. Hyg. 67:533-538. [DOI] [PubMed] [Google Scholar]
- 23.Tauxe, R. V. 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. ASM Press, Washington, D.C.
- 24.Taylor, D. E. 1992. Genetics of Campylobacter and Helicobacter. Annu. Rev. Microbiol. 46:35-64. [DOI] [PubMed] [Google Scholar]
- 25.Taylor, D. E. 1986. Plasmid-mediated tetracycline resistance in Campylobacter jejuni: expression in Escherichia coli and identification of homology with Streptococcal class M determinant. J. Bacteriol. 165:1037-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor, D. E., and J. H. Bryner. 1984. Plasmid content and pathogenicity of Campylobacter jejuni and Campylobacter coli strains in the pregnant guinea pig model. Am. J. Vet. Res. 45:2201-2202. [PubMed] [Google Scholar]
- 27.Taylor, D. E., S. A. De Grandis, M. A. Karmali, and P. C. Fleming. 1981. Transmissible plasmids from Campylobacter jejuni. Antimicrob. Agents Chemother. 19:831-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor, D. E., R. S. Garner, and B. J. Allan. 1983. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 24:930-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenover, F. C., and P. M. Elvrum. 1988. Detection of two different kanamycin resistance genes in naturally occurring isolates of Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 32:1170-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., S. Williams, K. P. Gordon, C. Nolan, and J. J. Plorde. 1985. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 27:37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]
- 32.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]