Abstract
This study extends earlier reports regarding the in vitro and in vivo efficacies of the nitroimidazopyran PA-824 against Mycobacterium tuberculosis. PA-824 was tested in vitro against a broad panel of multidrug-resistant clinical isolates and was found to be highly active against all isolates (MIC < 1 μg/ml). The activity of PA-824 against M. tuberculosis was also assessed grown under conditions of oxygen depletion. PA-824 showed significant activity at 2, 10, and 50 μg/ml, similar to that of metronidazole, in a dose-dependent manner. In a short-course mouse infection model, the efficacy of PA-824 at 50, 100, and 300 mg/kg of body weight formulated in methylcellulose or cyclodextrin/lecithin after nine oral treatments was compared with those of isoniazid, rifampin, and moxifloxacin. PA-824 at 100 mg/kg in cyclodextrin/lecithin was as active as moxifloxacin at 100 mg/kg and isoniazid at 25 mg/kg and was slightly more active than rifampin at 20 mg/kg. Long-term treatment with PA-824 at 100 mg/kg in cyclodextrin/lecithin reduced the bacterial load below 500 CFU in the lungs and spleen. No significant differences in activity between PA-824 and the other single drug treatments tested (isoniazid at 25 mg/kg, rifampin at 10 mg/kg, gatifloxacin at 100 mg/kg, and moxifloxacin at 100 mg/kg) could be observed. In summary, its good activity in in vivo models, as well as its activity against multidrug-resistant M. tuberculosis and against M. tuberculosis isolates in a potentially latent state, makes PA-824 an attractive drug candidate for the therapy of tuberculosis. These data indicate that there is significant potential for effective oral delivery of PA-824 for the treatment of tuberculosis.
Therapy for tuberculosis (TB) is arduous due to its long duration and the need to use multidrug regimens. The current standard regimen of isoniazid (INH), rifampin (RIF), and pyrazinamide (PZA) requires 6 to 8 months of daily treatment. In part due to noncompliance with treatment, therapy is now further complicated by the emergence of drug-resistant strains, with the global prevalence of drug resistance being from 1 to 3% (27). A further, equally important issue with tuberculosis therapy is the treatment of patients in which the infection may be in a latent state. Supposedly, 1:3 people throughout the world harbor latent bacilli, which have the potential to reactivate and cause active disease (21, 23). Current anti-TB drugs are mainly effective against replicating and metabolically active bacteria, and therefore, there is an urgent need for novel drugs that are also effective against persisting or latent bacterial infections, as well as those that can overcome the increasing problem of drug resistance.
A series of bicyclic nitroimidazofurans, originally investigated as radiosensitizers for use in cancer chemotherapy (1), were found to possess activity against cultured replicating Mycobacterium tuberculosis and had significant in vivo activity in a murine infection model (3, 17, 25). A subsequent series of 3-substituted nitroimidazopyrans (NAPs) were synthesized, and more than 100 compounds were found to possess substantial antitubercular activity. This activity was found to be highly specific for the M. tuberculosis complex and showed only modest or no activity against mycobacteria outside the M. tuberculosis complex (M. avium, M. smegmatis, M. chelonae, and M. fortuitum). One NAP compound, PA-824, exhibited a submicromolar MIC against M. tuberculosis (22). Both mono- and multidrug-resistant (MDR) strains of M. tuberculosis exhibited comparable susceptibilities to PA-824, indicating that there is no cross-resistance with current anti-TB drugs. The activity of NAPs against nonreplicating M. tuberculosis isolates in an anaerobic culture model was also compared with that of metronidazole, a structurally related antibiotic used to treat anaerobic infections, which possesses activity against static M. tuberculosis isolates that survive under anaerobic conditions (26). PA-824 exhibited bactericidal activity comparable to that of the related compound metronidazole.
The compound PA-824 was not the most potent NAP in the series against cultured M. tuberculosis clinical isolates, but it was found to be the most active in infected mice after oral administration. Mouse studies with PA-824 at 25, 50, and 100 mg/kg of body weight daily for 10 days or 60 days resulted in reductions of the mycobacterial burden in both spleen and lung tissues that were comparable to those produced by INH at 25 mg/kg (22). In an M. tuberculosis aerosol challenge guinea pig model, oral administration of PA-824 at 40 mg/kg once daily for 30 days resulted in statistically significant reductions in the numbers of M. tuberculosis bacilli in the lungs and spleens, comparable to the effect of INH compared to its effect in controls. The concentration of PA-824 that resulted in therapeutic activity in murine and guinea pig infection models was also well under the acute and chronic toxic threshold concentrations of PA-824 in mice. Toxic thresholds were determined to be greater than 1,000 mg/kg when it was administered as a single dose (acute toxicity test) and greater than 500 mg/kg when it was administered daily for 28 days (chronic toxicity test).
In 2002 the Global Alliance for TB Drug Development licensed PA-824 and related nitroimidazole compounds for further development. PA-824 has three key characteristics: it has a unique mechanism of action, a narrow spectrum of activity, and activity in an acute TB mouse infection model (22). Currently, PA-824 is nearing completion of the required preclinical safety testing and is expected to enter the clinic in the second quarter of 2005. In the study described in this report, we tested PA-824 against a broader panel of MDR M. tuberculosis clinical isolates retrieved internationally and demonstrated that the drug had significant activity against all strains tested. In addition, PA-824 was tested against M. tuberculosis isolates grown under conditions of transient oxygen depletion (26), again, with promising results. Finally, the efficacy of PA-824 was tested in different formulations in studies of short-term and long-term mouse models of infection, and the results were compared to those obtained with several clinically available anti-TB drugs.
MATERIALS AND METHODS
M. tuberculosis.
The H37Rv strain (Trudeau Institute, Saranac Lake, NY) was grown in Proskauer-Beck liquid medium containing 0.05% Tween 80 (Sigma-Aldrich, St. Louis, MO) to mid-log phase, aliquoted, and frozen at −80°C until use in further in vitro assays (2). Aliquots of frozen bacteria were used to start aerobic cultures in Dubos medium (Becton Dickinson, Sparks, MD), prepared according to the manufacturer's directions and supplemented with 0.05% Tween 80. The cultures were grown in 10-ml volumes in screw-cap tubes (150 by 25 mm) at 37°C with rapid stirring for 7 to 10 days until they reached mid-log phase (optical density at 600 nm [OD600] = 0.4 to 0.6).
The virulent M. tuberculosis strain Erdman (TMCC 107) was grown to mid-log phase in Proskauer-Beck liquid medium containing 0.05% Tween 80 and frozen in aliquots at −70°C until needed. This isolate has been used as the standard strain in tests of the activities of drugs in animal models in our laboratory at the Department of Microbiology, Immunology and Pathology, Colorado State University (CSU) (11).
MDR M. tuberculosis clinical isolates.
All MDR M. tuberculosis strains tested were provided to CSU between 1990 and 1992 and are now part of the National Institutes of Health Materials Contract strain repository. Strains CSU 19 and CSU 21 originated from pulmonary TB patients from South Korea and India, respectively, and were provided to Colorado State University by the Yonsei University College of Medicine, Seoul, Korea. Strains CSU 25, CSU 31, CSU 34, CSU 36, CSU 40, and CSU 45 originated from tuberculosis patients throughout the United States and were provided to Colorado State University by the National Jewish Center in Denver, Colorado. Table 1 presents the reported resistance of the strains from the hospitals at the time of sputum collection and their current resistance profile from recent susceptibility tests performed at the Mycobacteriology Laboratory of the National Jewish Center (June 2004) by L. Heifets et al.
TABLE 1.
In vitro activities against drug-resistant M. tuberculosis clinical isolates
| Strain name(s) | Origin | % Resistance
|
MIC (μg/ml)
|
||
|---|---|---|---|---|---|
| Reporteda | Testedb | INH | PA-824 | ||
| CSU 19, C1 | South Korea | 65% R to PZA at 25 μg/ml | |||
| 100% R to EMB at 10 μg/ml | 0% R to any of the tested drugs | 0.078 | 0.031 | ||
| CSU 21, I5 | India | 100% R to INH at 5 μg/ml | 100% R to INH at 1 μg/ml | 5.0 | 0.531 |
| 100% R to RIF at 10 μg/ml | 100% R to RIF at 1 μg/ml | ||||
| 65% R to EMB at 7.5 μg/ml | 25% R to EMB at 7.5 μg/ml | ||||
| 100% R to STR at 10 μg/ml | 100% R to STR at 4 μg/ml | ||||
| 100% R to RBT at 4 μg/ml | |||||
| CSU 25 | NJCc collection | 10% R to RBT at 2 μg/ml | 0% R to any of the tested drugs | 0.078 | 0.375 |
| 30% R to PZA at 25 μg/ml | |||||
| CSU 31 | NJC collection | 33% R to INH at 5 μg/ml | Pending | 0.039 | 0.25 |
| 100% R to RIF at 5 μg/ml | |||||
| CSU 34 | NJC collection | 3% R to INH at 5 μg/ml | 100% R to INH at 1 μg/ml | 2.5 | 0.063 |
| 100% R to STR at 10 μg/ml | 50% R to RIF at 1 μg/ml | ||||
| 100% R to PZA at 25 μg/ml | 100% R to STR at 4 μg/ml | ||||
| CSU 36 | NJC collection | 40% R to RIF at 10 μg/ml | 100% R to RIF at 1 μg/ml | 0.039 | 0.063 |
| CSU 40 | NJC collection | 10% R to INH at 1 μg/ml | 100% R to INH at 1 μg/ml | 2.5 | 0.25 |
| CSU 45 | NJC collection | 8% R to INH at 5 μg/ml | 100% R to INH at 1 μg/ml | 2.5 | 0.063 |
| 100% R to RIF at 10 mg/ml | 100% R to RIF at 1 μg/ml | ||||
| H37Rv | Trudeau Institute | None | None | 0.039 | 0.039 |
Resistance (R) as reported by the hospital where the isolate was collected (most isolates were collected from 1990 to 1992). RBT, rifabutin; EMB, ethambutol.
Recent testing of the susceptibility profile by the TB laboratory at the National Jewish Center (May and June 2004). Isolate susceptibilities to the following compounds were tested: isoniazid at 0.2 μg and 1.0 μg, rifampin at 1.0 μg, ethambutol at 7.5 μg, ethionamide at 10.0 μg, streptomycin at 2.0 μg and 4.0 μg, capreomycin at 10.0 μg, kanamycin at 6.0 μg, amikacin at 6.0 μg, cycloserine at 60.0 μg, and para-aminosalicylic acid at 8.0 μg; the pyrazinamide MIC was determined at pH 6.0 (all MICs were less than 300 μg/ml).
NJC, National Jewish Center.
The bacteria were grown in glycine alanine salt liquid medium and frozen at −80°C with 20% glycerol for long-term storage. Strain H37Rv was originally obtained from the Trudeau Institute, Saranac Lake, NY, and was grown in Proskauer-Beck liquid medium containing 0.05% Tween 80 to mid-log phase, aliquoted, and frozen at −80°C until use. All bacterial cultures were serially diluted in saline and plated onto 7H11/oleic acid-albumin-dextrose-catalase (OADC) agar to determine the numbers of CFU/ml. To prepare the bacteria for MIC testing, frozen cultures were either diluted in 7H9 liquid medium (Difco, Detroit, MI) to a concentration of 106 CFU/ml (strains CSU 25, CSU 36, CSU 45, and H37Rv) or, alternatively, were cultured in 7H9 medium to an OD600 of 0.3 to 0.6 (mid-log phase) and then diluted in broth to an OD600 of 0.1 (strains CSU 19, CSU 21, CSU 31, CSU 34, and CSU 40).
Drug compounds.
Gatifloxacin (GAT) and moxifloxacin (MXF) were obtained through a contract with the National Institute of Allergy and Infectious Diseases, National Institutes of Health, known as the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) (18). The fluoroquinolones were extracted from tablets by Robert Reynolds and Jerry Rose (Southern Research Institute, Birmingham, AL) through TAACF. GAT was purified from 400-mg film-coated Tequin tablets (Bristol-Myers Squibb), and MXF hydrochloride was purified from 400-mg Avelox tablets (Bayer). The identities and purities of the quinolones were verified by mass spectrometry, nuclear magnetic resonance imaging, and elemental analysis. PA-824 was obtained from the Global Alliance for TB Drug Development. INH, PZA, streptomycin (STR), RIF, and metronidazole were obtained from Sigma-Aldrich.
Microdilution MIC plate assay.
A method described by Wallace et al. (24) was used to determine the MICs by a microdilution plate assay by using M. tuberculosis H37Rv. INH was dissolved in sterile, double-distilled water at a stock concentration of 500 μg/ml. PA-824 was dissolved in 100% dimethyl sulfoxide (DMSO; Sigma-Aldrich) to a stock concentration of 100 μg/ml. A 1:2 dilution series of both compounds was made in a separate 96-well microtiter plate by using the same diluents. The interior 60 wells of a 96-well round-bottom microtiter assay plate were seeded with 98 μl of bacterial suspension (as described above). Two microliters of each drug was transferred to the assay plate wells containing bacteria. The final concentrations of INH in the wells ranged from 10.0 to 0.039 μg/ml; the final concentrations of PA-824 ranged from 2.0 μg/ml to 8.0 pg/ml. The assay plates were incubated at 37°C for at least 21 days and were observed every 3 to 4 days to evaluate changes in growth. Inhibition of growth was determined both by visual examination and with a spectrophotometer at an OD600 (Bio-Rad Benchmark Plus spectrophotometer and Bio-Rad MicroPlate Manager 5.2 software; Bio-Rad, Hercules, CA).
M. tuberculosis in vitro oxygen depletion assay.
The protocol used for the M. tuberculosis in vitro oxygen depletion assay was a slight modification of the method described first by Wayne and Hayes (26) and the model described by Murugasu-Oei and Dick (16). Briefly, mid-log-phase aerobic M. tuberculosis H37Rv cultures were diluted 100-fold in Dubos medium and transferred to tubes (150 by 20 mm) in 21-ml volumes. The tubes were closed with sterile 8.0-mm silicone rubber septa (Aldrich, Milwaukee, WI). The cultures were grown at 37°C with slow stirring for 24 days. Control tubes contained methylene blue dye (1.5 μg/ml) as an indicator of oxygen depletion. The blue dye fades and finally disappears under anaerobic conditions, as described by Wayne and Hayes (26). The drugs were injected at final concentrations ranging from 2 to 50 μg/ml through the septa of 24-day-old cultures. Culture tubes were prepared in duplicate for each concentration of drug. Control tubes received DMSO without drugs, with a final concentration of DMSO in the cultures of 1%. After drug treatment, the cultures were incubated at 37°C with slow stirring for 4 days. After 96 h of drug exposure, the septa were removed and 1-ml samples were drawn from the middle of the culture tubes. The cultures were serially diluted in saline and plated onto 7H11/OADC agar plates (Difco). The plates were incubated at 37°C under normal atmospheric conditions for 21 days, at which time the colonies were counted.
Drug preparation for in vivo models.
RIF was dissolved in 100% DMSO, with subsequent dilution in sterile water prior to administration. The final concentration of DMSO in the drug preparation was 5%. INH, PZA, STR, GAT, and MXF were dissolved in water. PA-824 was formulated either in 0.5% methylcellulose (MC; Sigma Chemical Co.) or in cyclodextrin/lecithin (CM2). The CM2 formulation for PA-824 was developed by PathoGenesis (22), and that formulation was exactly copied for the in vivo experiments. Briefly, for the preparation of the 100-mg/kg dose, 10 mg of PA-824 was added to 1 ml of a 10% solution of hydroxypropyl-β-cyclodextrin (Sigma Chemical Co.), and the mixture was stirred gently for 24 h at room temperature. The resulting suspension was sonicated with a Vibra Cell probe sonicator (model VC-130; Sonics and Materials, Inc., Newtown, CT) for 10 min at 25% amplitude. Frozen lecithin (Sigma Chemical Co.) was added at a final concentration of 10%; the suspension was stirred for 10 min at room temperature, cooled in an ice-water bath, and sonicated at 30% amplitude for 15 min while the solution temperature was kept at less than 50°C. For the preparation of the lower and higher doses (50 and 300 mg/kg of PA-824, respectively, in CM2), the amount of drug was adjusted. The concentration of cyclodextrin/lecithin remained the same as that described above, as was the volume administered to each mouse (200 μl).
Rapid in vivo screen.
Eight- to 10-week-old female specific-pathogen-free C57BL/6-Ifngtm1ts mice (gamma interferon gene-disrupted [GKO] mice) (9) were purchased from Jackson Laboratories, Bar Harbor, ME. The mice were infected via a low-dose aerosol exposure to M. tuberculosis Erdman in a Middlebrook aerosol generation device (Glas-Col Inc., Terre Haute, IN), and the short-course mouse model was performed as described previously (13). One day postinfection, three mice were killed to verify the uptake of 50 to 100 CFU of bacteria per mouse. Following infection, the mice were randomly divided into 11 treatment groups. Negative control mice remained untreated. Positive control mice received INH (at 25 mg/kg of body weight), RIF (at 20 mg/kg), or MXF (at 100 or 300 mg/kg). Six groups received PA-824 formulated in either MC or CM2 (at 50, 100, or 300 mg/kg). Each treatment group consisted of five mice. Treatment was started 19 days postinfection and continued for nine consecutive days. Three infected mice were killed at the start of treatment as pretreatment controls. Drugs were administered daily by oral gavage.
Long-term in vivo screen.
Six- to 8-week-old female specific-pathogen-free immunocompetent C57BL/6 mice (Charles River, Wilmington, MA) were infected via a low-dose aerosol exposure to M. tuberculosis Erdman as described before (5, 6, 11). Two successive aerosol runs were performed with 90 mice in each round. One day postinfection, three mice from each run were killed to verify the uptake of 50 to 100 CFU of bacteria per mouse. Following infection, the mice were randomly divided into 10 treatment groups. Negative control mice remained untreated. Positive control mice received INH (at 25 mg/kg of body weight), GAT (at 100 mg/kg), or MXF (at 100 mg/kg). The other treatment groups received PA-824 (at 100 mg/kg) in the CM2 formulation. Each group consisted of five to six mice at each time point. Treatment was started 3 weeks postinfection and continued for 12 weeks. Five infected mice were killed at the start of treatment as pretreatment controls. Drugs were administered 5 days per week by oral gavage. To determine drug efficacies at intermediate time points, a group of mice from each treatment group was killed at weeks 2, 6, and 12 after the start of treatment.
Enumeration of viable M. tuberculosis in mouse organs.
The mice were killed by CO2 inhalation. The spleens and left lung lobes were aseptically removed and disrupted in a tissue homogenizer. The number of viable organisms was determined by serial dilution of the homogenates on nutrient Middlebrook 7H11 agar plates (GIBCO BRL, Gaithersburg, MD). The plates were incubated at 37°C in ambient air for 4 weeks prior to the counting of viable M. tuberculosis colonies (CFU). After long-term treatment, the entire volume of each organ homogenate was plated to determine the total number of culturable mycobacteria per organ.
Statistical analysis.
The viable counts were converted to logarithms, which were then evaluated by a one-way analysis of variance, followed by a multiple-comparison analysis of variance by a one-way Tukey test (SigmaStat software program). Differences were considered significant at the 95% level of confidence.
RESULTS
In vitro activity against drug-resistant M. tuberculosis.
The activity of PA-824 against multidrug-resistant clinical isolates from Asia (India and South Korea) and from throughout the United States was determined by the “microdilution” 96-well plate assay (24). On every microtiter plate containing one MDR M. tuberculosis strain, a dilution series of INH was included as a control compound. The results were scored by taking multiple visual readings as well as by taking a final spectrophotometric reading of the microtiter plates at 600 nm. Both data sets gave the same results for PA-824 and INH, and these are presented in Table 1. PA-824 was highly active against all the isolates tested (MIC < 1 μg/ml) and was equally active against the drug-sensitive and multidrug-resistant isolates of M. tuberculosis (MICs range, 0.039 to 0.531 μg/ml).
In vitro activity against M. tuberculosis cultured under oxygen depletion conditions.
The activity of PA-824 was tested in an in vitro model in which M. tuberculosis was grown under conditions of transient oxygen depletion. We incubated 28-day-old anaerobic cultures for 96 h with 2, 10, or 50 μg/ml of INH, RIF, metronidazole, GAT, MXF, or PA-824. The results are shown in Table 2 and are presented as the percent growth of the drug-treated culture compared to the growth of the untreated control cultures. The lower that the percentage of growth for the drug-treated culture was, the more active the compound was against bacteria grown under conditions of low oxygen tension. The results are represented as the means of two duplicate cultures for every time point and for every drug concentration, unless mentioned otherwise. For the untreated controls, the results are the means of triplicate cultures. As expected, INH had very little activity against nonreplicating bacteria; only at the highest concentrations some activity could be observed (20 and 40% killing at 10 and 50 μg/ml, respectively). RIF, GAT, and MXF were very effective at all concentrations tested and were effective in a dose-dependent manner; the bactericidal activities of concentrations of 10 and 50 μg/ml of all three compounds ranged from 98.6% to 100%. PA-824 showed high levels of activity at all doses tested and was active in a dose-dependent manner; with 85%, 89.6%, and 93.5% killing at 2, 10, and 50 μg/ml, respectively. Metronidazole had activity very similar to that of PA-824, with 85.4% and 89.9% killing at 10 and 50 μg/ml, respectively.
TABLE 2.
Growth of M. tuberculosis cultures grown under conditions of oxygen depletion for 28 days after addition of drugs
| Compound | Concn (μg/ml) | Log10 CFU ± SEM (per ml) | % Growth compared with that of untreated controls at day 28a |
|---|---|---|---|
| None | NAb | 6.42 ± 0.05 | 100.0 |
| Isoniazid | 2 | 6.38 ± 0.14 | 92.8 |
| 10 | 6.32 ± 0.11 | 80.4 | |
| 50 | 6.20 ± 0.10 | 60.6 | |
| Rifampin | 2 | 5.15 ± 0.01 | 5.2 |
| 10 | 4.10 ± 0.14 | 0.5 | |
| 50 | 1.40 ± 0.00 | 0.0 | |
| Gatifloxacin | 2 | 5.27 ± 0.38 | 9.7 |
| 10 | 4.29 ± 0.27 | 0.9 | |
| 50 | 3.85c | 0.3 | |
| Moxifloxacin | 2 | 5.72 ± 0.48 | 32.8 |
| 10 | 4.13 ± 0.22 | 0.6 | |
| 50 | 4.56 ± 0.14 | 1.4 | |
| Metronidazole | 2 | NDd | ND |
| 10 | 5.58 ± 0.10 | 14.6 | |
| 50 | 5.38 ± 0.28 | 10.9 | |
| PA-824 | 2 | 5.61 ± 0.00 | 15.0 |
| 10 | 5.43 ± 0.12 | 10.4 | |
| 50 | 5.24 ± 0.01 | 6.5 |
Percent of bacterial growth of the drug-treated cultures is compared with the growth of the untreated controls (represented as 100% growth).
NA, not applicable.
Data are based on these for a single culture tube.
ND, not done.
Efficacy of PA-824 in the rapid mouse model.
In an initial in vivo experiment, the efficacy of PA-824 was tested in the rapid mouse model. Drug treatment was started 18 days postinfection and was given for nine consecutive treatment days. Mice were treated daily by oral gavage with PA-824 (at 50, 100, or 300 mg/kg) formulated in either MC or CM2. The efficacy of PA-824 in mice was compared to those of INH (at 25 mg/kg), RIF (at 20 mg/kg), and MXF (at 100 or 300 mg/kg), which were administered as parallel treatments.
PA-824 showed significant activity in the GKO model at all concentrations used and was active in a dose-dependent manner (P < 0.001): at 50 mg/kg, PA-824 in MC produced a more than 1-log10 reduction of the CFU in the lungs compared to the growth in the untreated control group (7.06 versus 8.28 log10 CFU), at 100 mg/kg it produced about a 2-log reduction (6.25 log10 CFU in the PA-824 group), and at 300 mg/kg it produced about a 3-log reduction (5.31 log10 CFU) (Table 3).
TABLE 3.
Bacterial numbers in whole lungs and spleens of GKO mice after a 9-day drug treatment regimen
| Treatment regimena | Log10 CFU ± SEM
|
|
|---|---|---|
| Lungs | Spleens | |
| D18, untreated controls | 7.16 ± 0.06 | 5.61 ± 0.38 |
| D28, untreated controls | 8.28 ± 0.10 | 7.14 ± 0.02 |
| D28, INH (25) | 5.76 ± 0.11 | 3.59 ± 0.31 |
| D28, RIF (20) | 6.36 ± 0.14 | 5.14 ± 0.12 |
| D28, moxifloxacin (100) | 5.54 ± 0.09 | 3.25 ± 0.35 |
| D28, moxifloxacin (300) | 3.02 ± 0.19 | 0.90 ± 0.20 |
| D28, PA-824 (50), MC | 7.09 ± 0.09 | 5.35 ± 0.14 |
| D28, PA-824 (100), MC | 6.25 ± 0.06 | 4.89 ± 0.25 |
| D28, PA-824 (300), MC | 5.31 ± 0.25 | 4.24 ± 0.28 |
| D28, PA-824 (50), CM2 | 6.68 ± 0.11 | 4.98 ± 0.25 |
| D28, PA-824 (100), CM2 | 5.70 ± 0.14 | 3.92 ± 0.10 |
| D28, PA-824 (300), CM2 | 6.01 ± 0.11 | 5.54 ± 0.11 |
D18 and D28, treatment for 18 and 28 days, respectively; values in parentheses are doses (in milligrams per kilogram).
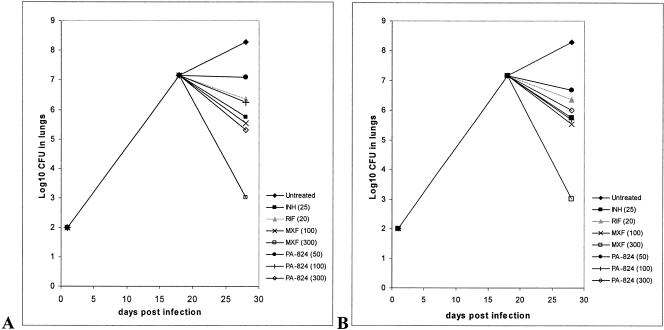
PA-824 at 50 or 100 mg/kg reduced the bacterial load to a similar extent in the lungs when it was formulated in either MC or CM2 (P > 0.05) (Table 3 and Fig. 1). In the spleens, PA-824 was slightly more effective when it was administered at 100 mg/kg in CM2 than when it was administered at the same dose in MC (P < 0.05). In the lungs, PA-824 formulated in CM2 at 300 mg/kg showed only similar activity as that at 100 mg/kg (P > 0.05). In the spleens this effect was even more pronounced; PA-824 in CM2 at 300 mg/kg was only as effective as PA-824 at 50 mg/kg in CM2 (P > 0.05) and was significantly less effective than PA-824 at 100 mg/kg in CM2 (P < 0.05) (Table 3 and Fig. 1).
FIG. 1.
Bacterial numbers in lungs of GKO mice after a 9-day drug treatment regimen. (A) PA-824 formulated in MC; (B) PA-824 formulated in CM2.
In the same experiment, the activity of PA-824 was compared to those of clinically available compounds. The activity of PA-824 at 100 mg/kg formulated in MC was similar to that of RIF at 20 mg/kg and INH at 25 mg/kg (P > 0.05). At the same dose, but with the dose formulated in CM2, the activity of PA-824 was not different from that of MXF at 100 mg/kg or INH at 25 mg/kg (P > 0.05) but was slightly better than that of RIF (P < 0.05) (Table 3 and Fig. 1).
Efficacy of PA-824 after long-term treatment.
C57BL/6 mice were infected via a low-dose aerosol with M. tuberculosis Erdman, which resulted in a lung burden of approximately 100 CFU per mouse. Three weeks after infection, the mice were randomly divided into 10 groups and treatment by oral gavage was started for 12 weeks. Mice received either the clinically available drugs INH (at 25 mg/kg of body weight), RIF (at 10 mg/kg), GAT (at 100 mg/kg), or MXF (at 100 mg/kg) or PA-824 formulated in CM2 (at 100 mg/kg). The mice were killed after 2, 6, or 12 weeks of drug treatment; and the bacterial numbers in the lungs and spleens of the mice were determined.
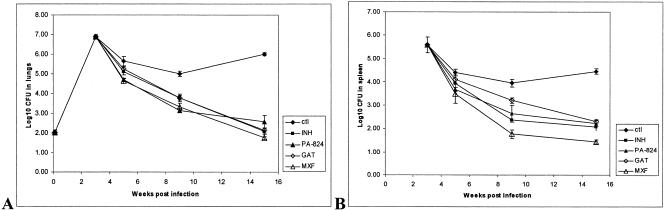
The results show that all drug treatments reduced the numbers of CFU in the lungs and spleens significantly at every time point compared with the numbers in the untreated controls (P < 0.001) (Table 4 and Fig. 2). After 2 weeks of treatment, PA-824 reduced the bacterial loads in the lungs by 0.94 log10 CFU (4.71 versus 5.65 log10 CFU for the untreated controls). The activity of PA-824 in the lungs was not statistically different from those of INH and MXF (P > 0.05) and was slightly better than that of GAT after 2 weeks of treatment (P < 0.05). In the spleens, no significant difference in the counts could be observed between the different treatment groups (P > 0.05). After 6 weeks of treatment, PA-824 reduced the bacterial numbers in the lungs by another 1.85 log units (3.15 versus 5.00 log10 CFU in the untreated controls). No significant difference in the reduction in the numbers of CFU in the lungs could be observed between the different treatment groups (P > 0.05). PA-824 reduced the bacterial load in the spleens to a similar extent as INH and GAT and was slightly less effective than MXF (P < 0.05). After 12 weeks of treatment the bacterial counts in the lungs were reduced to very low numbers in all treatment groups (range, 1.75 to 2.56 versus 6.00 log10 CFU in the untreated controls), as was the case for the spleens (Fig. 2). Complete eradication of the bacterial load was not achieved in any of the mice after 12 weeks of treatment with single compounds. No statistically significant differences in activity between PA-824 and the other single-drug treatments could be observed for the spleens or the lungs (P > 0.05). PA-824 formulated in CM2 was able to reduce the bacterial load to less than 500 CFU in the lungs (a reduction of 3.44 log10 CFU compared with the counts in the untreated controls) after 12 weeks of treatment (Table 4).
TABLE 4.
Bacterial numbers in lungs and spleens of C57BL/6 mice after 2, 6, and 12 weeks of drug treatment
| Treatment regimena | Lungs
|
Spleens
|
||
|---|---|---|---|---|
| Log10 CFU ± SEM | nb | Log10 CFU ± SEM | nb | |
| Start of treatment (untreated controls) | 6.88 ± 0.11 | 5 | 5.59 ± 0.33 | 5 |
| Wk 2, untreated controls | 5.65 ± 0.24 | 5 | 4.40 ± 0.14 | 5 |
| Wk 2, INH (25) | 5.12 ± 0.17 | 5 | 3.93 ± 0.20 | 5 |
| Wk 2, PA-824 (100) | 4.71 ± 0.08 | 5 | 3.68 ± 0.09 | 5 |
| Wk 2, gatifloxacin (100) | 5.25 ± 0.17 | 5 | 4.11 ± 0.18 | 5 |
| Wk 2, moxifloxacin (100) | 4.64 ± 0.13 | 5 | 3.50 ± 0.40 | 5 |
| Wk 6, untreated controls | 5.00 ± 0.11 | 5 | 3.95 ± 0.15 | 5 |
| Wk 6, INH (25) | 3.79 ± 0.19 | 5 | 2.36 ± 0.08 | 5 |
| Wk 6, PA-824 (100) | 3.15 ± 0.09 | 5 | 2.64 ± 0.36 | 5 |
| Wk 6, gatifloxacin (100) | 3.79 ± 0.11 | 5 | 3.21 ± 0.12 | 5 |
| Wk 6, moxifloxacin (100) | 3.33 ± 0.18 | 5 | 1.77 ± 0.18 | 5 |
| Wk 12, untreated controls | 6.00 ± 0.07 | 6 | 4.54 ± 0.12 | 6 |
| Wk 12, INH (25) | 2.08 ± 0.16 | 5c | 2.07 ± 0.15 | 5c |
| Wk 12, PA-824 (100) | 2.56 ± 0.33 | 6 | 2.23 ± 0.11 | 6 |
| Wk 12, gatifloxacin (100) | 2.14 ± 0.13 | 6 | 2.31 ± 0.07 | 6 |
| Wk 12, moxifloxacin (100) | 1.75 ± 0.07 | 6 | 1.44 ± 0.09 | 6 |
Values in parentheses are doses (in milligrams per kilogram).
n, number of mice killed per time point.
One mouse died during the treatment regimen due to a drug- or disease-unrelated event.
FIG. 2.
Bacterial numbers determined in lungs (A) and spleens (B) of C57BL/6 mice after 2, 6, and 12 weeks of drug treatment with single drugs (with standard errors of the mean log10 CFU). ctl, untreated control.
At the times of killing, the weights of the organs (lungs, spleens, and livers) and the whole body weights of the drug-treated and the control mice were determined. The body weights at the 2- and 6-week time points were similar for all treatment groups (P > 0.05). After 12 weeks of treatment the only significant difference could be observed in the gatifloxacin group, in which the mice had a slightly lower mean body weight compared with that of the PA-824 treatment group but not compared with that of the untreated controls (P < 0.05) (data not shown). The spleen weights and the liver weights between the different treatment groups were not significantly different from those for the untreated controls (P > 0.05) (data not shown).
DISCUSSION
The activity of the nitroimidazopyran PA-824 against M. tuberculosis in vitro and in vivo was reported before (22). The present study confirms and extends these earlier results for PA-824 in our in vitro and mouse M. tuberculosis infection models. PA-824 was found to exert comparable activities against mono- and multidrug-resistant strains of M. tuberculosis, indicating that there was no cross-resistance to current anti-TB drugs. Here we tested the PA-824 compound against a panel of MDR M. tuberculosis strains retrieved from a wide geographical region (strains from India and Korea as well as a diverse panel of strains from the United States). Our results showed that PA-824 was highly active against all the isolates tested, with an MIC <1 μg/ml, and was equally active against the drug-sensitive and multidrug-resistant isolates of M. tuberculosis, with MICs ranging from 0.039 to 0.531 μg/ml. This confirms the findings of earlier reports, in which MICs for PA-824 against a panel of pansensitive, mono- and multiresistant M. tuberculosis clinical isolates ranged from 0.015 to 0.25 μg/ml (22). The activity of PA-824 against this broad panel of multidrug-resistant M. tuberculosis isolates indicates that the compound acts by a novel mechanism. Studies of the macromolecular effects of PA-824 on M. tuberculosis have revealed that both protein synthesis and lipid synthesis are substantially inhibited (22). Genetic studies have demonstrated that PA-824 is activated by a process that requires the unique deazaflavin F420 and depends on electron transport (7, 8).
We also evaluated the activity of PA-824 in an in vitro model in which M. tuberculosis was grown under conditions of transient oxygen depletion by use of a slightly modified version of the protocol first developed by Wayne and Hayes (26). In this model, M. tuberculosis cultures were grown under closed caps with a limited headspace. After 28 days most oxygen in the headspace is consumed, and the bacteria are not replicating and appear to be in a low metabolic state. This reversible transition in the physiology of the bacteria was extensively studied and was described by Wayne and Hayes (26) as nonreplicating phase 2 (NRP-2) state. We incubated these 28-day-old anaerobic cultures for 96 h with 2, 10, or 50 μg/ml of INH, RIF, metronidazole, MXF, or PA-824. INH showed little activity against the nonreplicating bacteria in the NRP-2 state. On the other hand, RIF, GAT, and MXF were very effective at all concentrations tested against M. tuberculosis grown under conditions of oxygen depletion. These results with the clinical drugs were as expected, as INH targets mycobacterial cell wall synthesis (4, 19, 20) and is therefore mainly effective against actively replicating bacteria. On the other hand, RIF, GAT, and MXF, which target protein synthesis or DNA synthesis (10, 12), are active against metabolically active bacteria (14, 15). Importantly, PA-824 also showed strong activity against these persistent bacteria in a dose-dependent manner. The activity of PA-824 was only slightly less than those of RIF, GAT, and MXF. Metronidazole, an antibiotic that is structurally related to PA-824 and that in the clinic is used to treat infections caused by anaerobic bacteria, was described to possess activity against M. tuberculosis isolates that survive under anaerobic conditions (26). In our assay, PA-824 showed activity very similar to that of metronidazole.
The activity of PA-824 against static M. tuberculosis isolates maintained in microaerophilic culture was described earlier (22), but an alternate method was used. In the alternate method, log-phase recombinant M. tuberculosis lux bacilli expressing the luciferase gene were mixed with the indicated drugs (at 10 μg/ml) and placed inside an activated GasPak chamber. After 7 days of microaerophilic incubation, the cells were centrifuged and resuspended in fresh medium, and recovery was monitored by determination of the relative light units and enumeration of the CFU. The main difference in the two approaches is that the earlier study monitored the cultures for the ability to regrow (recovery), whereas the currently described model determines the bactericidal activity (% killing) by the drug against the persistent bacterial population. Even though these different methods were used to test activity against persistent bacteria, very similar results were found for the drugs tested in both models. INH had little activity against nonreplicating bacteria and at 10 μg/ml produced about 20% less growth compared with that for the untreated controls in both models, whereas PA-824 showed high levels of activity at all doses tested in a dose-dependent manner and had activity very similar to that of metronidazole in both models. In summary, PA-824 exhibited bactericidal activity comparable to that of the related compound metronidazole, whereas the frontline antituberculosis drug INH was far less active against nonreplicating M. tuberculosis isolates in this model. After adaptation to microaerophilic culture, M. tuberculosis does not multiply, and therefore, any drug activity under these conditions would suggest a mode of killing that is independent of cell replication.
The solubility of PA-824 is poor in an aqueous solution, and therefore, in previously described animal models (22), PA-824 was administered in a complex CM2 formulation. In a first in vivo experiment with PA-824, the efficiency of PA-824 at 50, 100, and 300 mg/kg formulated in either MC or CM2 was tested in the GKO mouse model; and its activity was compared to those of INH at 25 mg/kg, RIF at 20 mg/kg, and MXF at 100 or 300 mg/kg. PA-824 showed significant activity in this model at all concentrations used and was active in a dose-dependent manner, with a more than 1-log10 reduction in CFU at 50 mg/kg, about a 2-log10 reduction at 100 mg/kg, and about a 3-log10 reduction at 300 mg/kg compared with the counts for the untreated controls. The activity of PA-824 at 50 or 100 mg/kg was not significantly different when it was formulated in either MC or CM2. In the spleen, PA-824 was slightly more effective when it was administered at 100 mg/kg in CM2 compared with its activity when it was administered at the same dose in MC. It is noteworthy here that PA-824 formulated in MC had to be administered as a drug suspension due to the partial insolubility of the drug. Administration of the full dose could not be guaranteed every time, since a fraction of the insoluble compound would remain in the preparation tube. This might explain, at least in part, the slightly lower activity of PA-824 in MC compared with that of the drug in the CM2 formulation. The difficulty with the administration of the drug suspension, however, did not influence the animal-to-animal variation, since the standard error for the PA-824 (MC) group was similar to those for the other treatment groups.
Unexpectedly, PA-824 formulated in CM2 showed less activity at 300 mg/kg than at 100 mg/kg in the lungs. In the spleens this effect was even more pronounced: PA-824 in CM2 at 300 mg/kg was less effective than the drug in CM2 at 50 mg/kg. The reason for this finding is not completely clear. One likely explanation is the preparation of the CM2 formulation itself. As described above, the protocol for the formulation of CM2 was optimized for PA-824 to be administered at 100 mg/kg (as 10 mg of PA-824 in 1 ml of cyclodextrin/lecithin suspension) (22). For the 300-mg/kg treatment group, the drug concentration was increased threefold, but the cyclodextrin/lecithin concentrations were kept the same due to increasing solubility issues. Further optimization of the CM2 formulation would be required for use of the higher doses in order to reach an optimal molar concentration of cyclodextrin/lecithin versus that of PA-824. No additional formulation optimization steps were initiated, since in the long-term animal trials a dose of 100 mg/kg was used. When the activity of PA-824 was compared to those of the clinically available drugs, the results obtained with PA-824 look promising. The activity of PA-824 at 100 mg/kg formulated in MC was very similar to those of RIF at 20 mg/kg and INH at 25 mg/kg; and at that same dose but formulated in CM2, PA-824 was as active as MXF at 100 mg/kg and INH at 25 mg/kg and was slightly more active than RIF at 20 mg/kg. In conclusion, PA-824 showed very potent antituberculosis activity as a single agent in our rapid, acute tuberculosis model and had activities very similar to these achieved by known anti-TB drugs. In this model, the efficacy of PA-824 was not greatly affected by the formulation method used for administration of the drug.
The long-term treatment experiment with PA-824 was performed to determine its sterilizing capacity as a single agent compared with those of known anti-TB drugs, i.e., INH at 25 mg/kg, RIF at 10 mg/kg, GAT at 100 mg/kg, and MXF at 100 mg/kg, and compared with that of PA-824 formulated in CM2 at 100 mg/kg in a 12-week study. In addition, the safety of PA-824 was assessed in the low-dose aerosol M. tuberculosis infection model after long-term administration of these agents. C57BL/6 mice were infected with the Erdman strain via a low-dose aerosol. Three weeks after infection, treatment was initiated for 12 weeks, with two intermittent killing points at 2 and 6 weeks. After 2 weeks of treatment, PA-824 reduced the bacterial loads in the lungs by about 1 log10 CFU compared with the counts in the untreated controls, which was similar to the results obtained with INH and MXF and which was slightly better than the results obtained with GAT. In the spleens, no significant difference could be observed between the results for the different treatment groups and those for the untreated controls. After 6 weeks of treatment, PA-824 reduced the bacterial numbers in the lungs by another 1.85 log units, a result that, again, was not significantly different from those for the other treatments. In the spleens, PA-824 reduced the bacterial load to a similar extent as INH and GAT but was slightly less effective than MXF. After 12 weeks of treatment, the bacterial counts in the lungs as well as in the spleens were reduced to very low numbers in all treatment groups (range, 20 to 500 CFU). A complete eradication of the bacterial load was not achieved in any of the mice. No statistically significant differences in activities between PA-824 and the other single-drug treatments could be observed in the spleens or the lungs (P > 0.05). PA-824 formulated in CM2 was very active in this long-term model, with a reduction of 3.44 log10 CFU compared with the counts for the untreated controls after 12 weeks of treatment. PA-824 was able to reduce the bacterial numbers to an average of 360 CFU in the lungs and an average of 170 CFU in the spleen. The weights of the organs (lungs, spleens, and livers) and whole body weights were determined when the mice were killed. Both the body weights and the organ weights of mice treated with PA-824 were not statistically different from the weights of the untreated control mice at all time points. PA-824 administered for 12 weeks showed no adverse effects in terms of behavioral changes or the findings on gross necropsy after the mice were killed. In summary, the data from our in vivo animal infection models indicate that there is significant potential for the oral delivery of PA-824 for the treatment of M. tuberculosis infection to be effective.
Even though we used a different set of animal models to test the efficacy of PA-824 in vivo, our results confirm previous data from in vivo studies of PA-824 (22). Several differences between the animal tests described by Stover et al. (22 and our animal models can be noted: (i) in the study by Stover et al., the mice were inoculated by the intravenous route, while in our models the mice were infected by a low-dose aerosol; (ii) in the study of Stover et al., PA-824 was formulated in CM2, while in this study we compared the efficacy of PA-824 administered in CM2 with that of PA-824 administered in MC; (iii) in the study of Stover et al., the mice were infected with the H37Rv reporter strain, which expresses the lux gene, whereas in this report our standard laboratory strain, M. tuberculosis Erdman, was used; and (iv) in this study (which is the first study to do so) the activity of PA-824 was compared to those of a broad panel of clinically available drugs.
As demonstrated by culture with PA-824 and models of animal infection, the NAPs have been found to possess bactericidal activities against M. tuberculosis comparable to those of INH, RIF, and GAT and close to that of MXF at doses that appear to be safe in mice. Its activity against multidrug-resistant M. tuberculosis isolates and considerable activity against M. tuberculosis in a potentially latent state make PA-824 an attractive drug candidate for the therapy of tuberculosis. Overall, our in vivo infection models indicate that there is significant potential for effective oral delivery of PA-824 for the treatment of tuberculosis.
Acknowledgments
We thank the staff of the Laboratory Animal Resources (Colorado State University) for animal care. We acknowledge the Global Alliance for TB Drug Development for the provision of the PA-824 compound for conducting these studies, and we thank Doris Rouse and Rick Satcher for their kind assistance.
Support was provided by TAACF and a research and development contract (grant NO1 AI-95385) with the U.S. National Institute of Allergy and Infectious Diseases (Barbara Laughon and Karen Near, project officer).
REFERENCES
- 1.Agrawal, K. C., K. B. Bears, R. K. Sehgal, J. N. Brown, P. E. Rist, and W. D. Rupp. 1979. Potential radiosensitizing agents. Dinitroimidazoles. J. Med. Chem. 22:583-586. [DOI] [PubMed] [Google Scholar]
- 2.Allen, B. W. 1998. Mycobacteria. General culture methodology and safety considerations. Methods Mol. Biol. 101:15-30. [DOI] [PubMed] [Google Scholar]
- 3.Ashtekar, D. R., R. Costa-Perira, K. Nagrajan, N. Vishvanathan, A. D. Bhatt, and W. Rittel. 1993. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 37:183-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, P. J. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 83:91-97. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, J. V., S. K. Furney, and I. M. Orme. 1999. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 43:1285-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, J. V., and I. M. Orme. 1998. Evaluation of once-weekly therapy for tuberculosis using isoniazid plus rifamycins in the mouse aerosol infection model. Antimicrob. Agents Chemother. 42:3047-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, K. P., T. B. Bair, Y. M. Bae, and L. Daniels. 2001. Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F(420) biosynthesis by Mycobacterium bovis BCG. J. Bacteriol. 183:7058-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, K. P., N. Kendrick, and L. Daniels. 2002. Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F(420) and FO biosynthesis. J. Bacteriol. 184:2420-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs, M. R. 1999. Activity of quinolones against mycobacteria. Drugs 58(Suppl. 2):19-22. [DOI] [PubMed] [Google Scholar]
- 11.Kelly, B. P., S. K. Furney, M. T. Jessen, and I. M. Orme. 1996. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2809-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konno, K., K. Oizumi, and S. Oka. 1973. Mode of action of rifampin on mycobacteria. II. Biosynthetic studies on the inhibition of ribonucleic acid polymerase of Mycobacterium bovis BCG by rifampin and uptake of rifampin-14 C by Mycobacterium phlei. Am. Rev. Respir. Dis. 107:1006-1012. [DOI] [PubMed] [Google Scholar]
- 13.Lenaerts, A. J., V. Gruppo, J. V. Brooks, and I. M. Orme. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchison, D. A. 2004. The search for new sterilizing anti-tuberculosis drugs. Front Biosci. 9:1059-1072. [DOI] [PubMed] [Google Scholar]
- 15.Mitchison, D. A., and A. R. Coates. 2004. Predictive in vitro models of the sterilizing activity of anti-tuberculosis drugs. Curr. Pharm. Design 10:3285-3295. [DOI] [PubMed] [Google Scholar]
- 16.Murugasu-Oei, B., and T. Dick. 2000. Bactericidal activity of nitrofurans against growing and dormant Mycobacterium bovis BCG. J. Antimicrob. Chemother. 46:917-919. [DOI] [PubMed] [Google Scholar]
- 17.Nagarajan, K., R. Gowrishankar, V. P. Arya, T. George, M. D. Nair, S. J. Shenoy, and V. Sudarsanam. 1992. Nitroimidazoles. Part XXIII. Activity of satranidazole series against anaerobic infections. Indian J. Exp. Biol. 30:193-200. [PubMed] [Google Scholar]
- 18.Orme, I. M. 2001. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 45:1943-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder, E. K., N. de Souza, D. S. Santos, J. S. Blanchard, and L. A. Basso. 2002. Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis. Curr. Pharm. Biotechnol. 3:197-225. [DOI] [PubMed] [Google Scholar]
- 20.Scior, T., I. Meneses Morales, S. J. Garces Eisele, D. Domeyer, and S. Laufer. 2002. Antitubercular isoniazid and drug resistance of Mycobacterium tuberculosis—a review. Arch. Pharm. (Weinheim) 335:511-525. [DOI] [PubMed] [Google Scholar]
- 21.Stead, W. W. 1967. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am. Rev. Respir. Dis. 95:729-745. [DOI] [PubMed] [Google Scholar]
- 22.Stover, C. K., P. Warrener, D. R. VanDevanter, et al. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 23.Tead, W. W., G. R. Kerby, D. P. Schlueter, and C. W. Jordahl. 1968. The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann. Intern. Med. 68:731-745. [DOI] [PubMed] [Google Scholar]
- 24.Wallace, R. J., D. R. Nash, L. C. Steele, and V. Steingrube. 1986. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J. Clin. Microbiol. 24:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, J. S., R. Wang, E. Bagan, C. C. Wang, P. Wislocki, and G. T. Miwa. 1987. Structural alterations that differentially affect the mutagenic and antitrichomonal activities of 5-nitroimidazoles. J. Med. Chem. 30:150-156. [DOI] [PubMed] [Google Scholar]
- 26.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 2002. Controlling multi-drug-resistant tuberculosis and access to expensive drugs: a rational framework. Bull. W. H. O. 80:489-501. [PMC free article] [PubMed] [Google Scholar]