Abstract
The effects of caspofungin combined with amphotericin B were investigated with Candida glabrata. Although in vitro experiments showed an indifferent interaction, the combination regimen was the only therapeutic approach yielding organ sterilization in a murine candidemia model.
Candida glabrata has emerged as a significant pathogen, representing the most common cause of fungemia after Candida albicans (2, 3, 9). Infections due to C. glabrata are characterized by a high mortality rate, and they are difficult to treat due to a reduced susceptibility of this species to azole drugs, especially to fluconazole (2, 3, 9).
Although amphotericin B (AMB) MICs for C. glabrata are higher than those seen for C. albicans, the polyene is generally effective in infections caused by this yeast species (3, 7). Similarly, caspofungin (CAS) is active against C. glabrata (1). Its innovative mechanism of action makes this drug a suitable candidate for antifungal combination therapy (1, 4, 5).
In this study, we hypothesized that the combination of CAS and AMB could be advantageous over each monotherapy against C. glabrata. To investigate this interaction, we applied in vitro methods and an experimental mouse model of systemic infection.
Two clinical bloodstream isolates of C. glabrata (4205 and 4293) were used in this study. Stock solutions of CAS (Merck Sharp & Dohme) and AMB (Fungizone; Bristol-Myers Squibb) were prepared in sterile distilled water. Stock solutions of AMB (Sigma) were prepared in dimethyl sulfoxide for in vitro studies. Drug activity was assessed by a checkerboard method derived from the standardized procedure established by the NCCLS (6). MICs of AMB ranged from 0.015 to 8.0 μg/ml, while those of CAS ranged from 0.06 to 4.0 μg/ml. Readings were performed spectrophotometrically with an automatic plate reader (Dynatech MR 700) set at 490 nm. Two MIC endpoints were considered: the first concentration of the antifungal agent tested alone or in combination at which the turbidity in the well was either 50% (inhibitory concentration) or 90% less than that in the control well. According to the fractional inhibitory concentration (FIC) index, the interaction was defined as synergistic (FIC index of ≤0.50), indifferent (>0.50 to ≤4.0), or antagonistic (>4.0) (5). Experiments were conducted in quintuplicate.
Time-kill studies were performed using C. glabrata 4293. Drugs alone and in combination were used at 1× MIC (1.0 μg/ml for both drugs). The numbers of CFU were determined at 0, 2, 6, and 24 h. The limit of detection was 20 CFU/ml. Fungicidal activity was considered to have been achieved when the number of CFU per milliliter was <99.9% compared with the initial inoculum size. Synergy and antagonism were defined, respectively, as a ≥100-fold increase or decrease in killing compared with that achieved with the most active single agent. If less than a 100-fold change, the interaction was considered indifferent (8). Experiments were conducted in triplicate.
For in vivo studies, CD1 male mice (Charles River) weighing 25 g were rendered neutropenic by intraperitoneal administration of cyclophosphamide (200 mg/kg of body weight/day) on days −4, +1, and +4 postinfection. They were infected intravenously with C. glabrata 4293 given in a 0.2-ml volume. Both drugs were administered intraperitoneally in a 0.2-ml volume starting after 24 h postchallenge. Drug efficacy was assessed by determining the number of CFU per kidney pair. Briefly, the mice were sacrificed, the kidneys were homogenized, and diluted and undiluted aliquots, including the entire organ, were grown on Sabouraud dextrose agar for colony count determination. Two studies were performed. In the first study, the mice were challenged with 2.6 × 108 CFU/mouse, treated for six consecutive days with CAS at 0.3 or 1 mg/kg/day (CAS-0.3 and CAS-1), with AMB at 0.3 or at 1 mg/kg/day (AMB-0.3 and AMB-1), or with their respective combinations (COMBO-0.3 and COMBO-1). Tissue burden studies were performed on day 7 postinfection. In the second study, the mice were challenged with 1.2 × 108 CFU/mouse and treated daily with CAS at 3 mg/kg/day (CAS-3), with AMB at 3 mg/kg/day (AMB-3), or with their combination (COMBO-3). Tissue burden experiments were performed on days 3, 5, and 7 postinfection, which corresponded to a total of 2, 4, and 6 days of therapy, respectively. There were from 7 to 10 animals in each group. Animal experiments were conducted with the approval of University of Ancona Ethics Committee.
The Mann-Whitney U test was used to compare tissue burden counts. Due to multiple comparisons, a P value of <0.016 was considered statistically significant.
The interactions between CAS and AMB measured by the checkerboard method are reported in Table 1. Although MICs of both drugs given in combination were generally lower than those observed when the drugs were tested alone, indifference was the only type of interaction observed by this method.
TABLE 1.
In vitro activities of CAS and AMB, alone and in combination, determined by the checkerboard method, against two clinical isolates of C. glabrataa
| Isolate no. | Reading criterionb | Median (range) CAS MIC (μg/ml) tested:
|
Median (range), AMB MIC (μg/ml) tested:
|
Type of interaction by:
|
|||
|---|---|---|---|---|---|---|---|
| Alone | In combination | Alone | In combination | FIC rangec | Definition | ||
| 4205 | IC50 | 1.0 (1.0-2.0) | 0.25 (0.125-0.5) | 1.0 (1.0-2.0) | 1.0 (1.0) | 1.06-1.25 | Indifference |
| IC90 | 4.0 (4.0) | 1.0 (0.5-1.0) | 1.0 (1.0-2.0) | 1.0 (1.0) | 0.75-1.12 | Indifference | |
| 4293 | IC50 | 1.0 (1.0) | 0.25 (0.125-0.5) | 1.0 (1.0-2.0) | 0.5 (0.25-1.0) | 0.625-1.06 | Indifference |
| IC90 | 4.0 (2.0->4.0) | 1.0 (0.25-2.0) | 2.0 (2.0) | 1.0 (0.125-2.0) | 1.03-1.09 | Indifference | |
Each isolate was tested in quintuplicate.
Readings were performed spectrophotometrically with an automatic plate reader; two endpoints were considered: the first concentration of the antifungal agent tested alone or in combination which the turbidity in the well was either 50% (inhibitory concentration [IC50]) or 90% (IC90) less than that in the control well.
FIC index. The interaction was defined as synergistic if the FIC index was less than or equal to 0.50, indifferent if the FIC index was greater than 0.50 and less than or equal to 4.0, and antagonistic if the FIC index was greater than 4.0 (5).
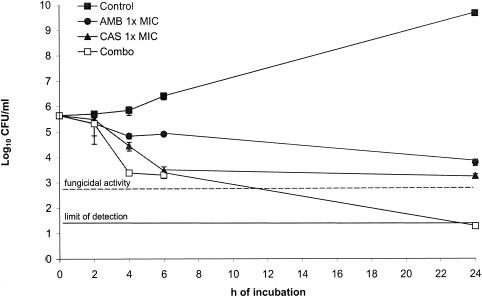
Similarly to that reported by the checkerboard method, an indifferent effect was observed in time-kill studies with C. glabrata 4293 (Fig. 1). However, the combination approach was the only regimen able to reach a fungicidal activity.
FIG. 1.
Time-kill studies conducted with C. glabrata 4293. The dashed line represents a >99.9% growth reduction compared with the initial inoculum size. The limit of detection was 20 CFU/ml. Each data point represents the mean ± standard deviation for three independent experiments.
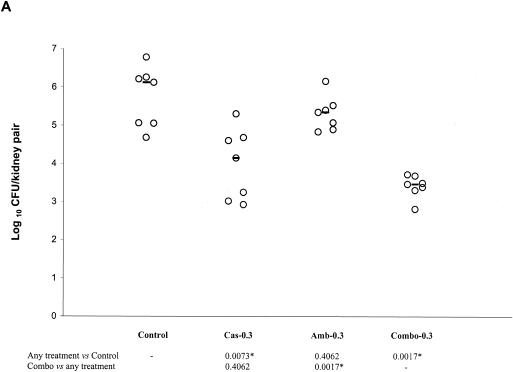
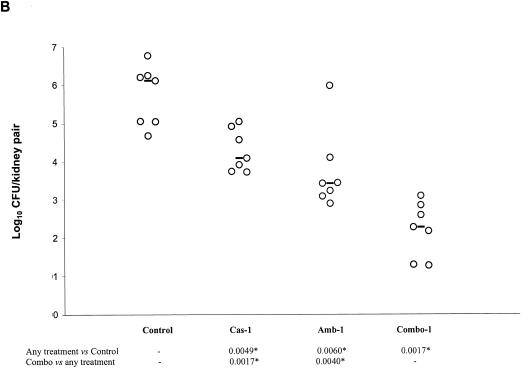
The results of the first in vivo study are presented in Fig. 2A and B. CAS-0.3, but not AMB-0.3, was effective at reducing the fungal burden against the controls. COMBO-0.3 was effective against the controls, but it was not superior to CAS-0.3 (Fig. 2A). Both CAS-1 and AMB-1 were effective against the controls. COMBO-1 significantly reduced the tissue burden counts with respect to controls, CAS-1, and AMB-1 (Fig. 2B).
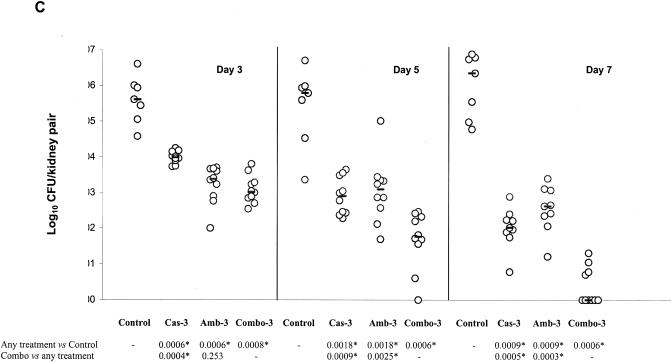
FIG. 2.
Kidney tissue burden of neutropenic CD1 mice. Study no. 1: panels A and B, the mice were infected intravenously with 2.6 × 108 CFU/mouse of C. glabrata 4293, treated for six consecutive days with CAS at 0.3 or 1 mg/kg/day (Cas-0.3 and Cas-1), with AMB at 0.3 or at 1 mg/kg/day (Amb-0.3 and Amb-1), or with their respective combinations (Combo-0.3 and Combo-1). The mice were sacrificed on day 7 postinfection. Study no. 2: panel C, the mice were infected intravenously with 1.2 × 108 CFU/mouse, treated daily with CAS at 3 mg/kg/day (Cas-3), with AMB at 3 mg/kg/day (Amb-3), or with their combination (Combo-3). Tissue burden experiments were performed on days 3, 5, and 7 postinfection, which corresponded to a total of 2, 4, and 6 days of therapy, respectively. The bars represent the medians. The asterisks at the bottom of panels A, B, and C indicate P values of <0.016.
The results of the second in vivo study are presented in Fig. 2C. All drug regimens were effective against the controls at each time interval. On day 3 postinfection, COMBO-3 was more effective than CAS-3 but not than AMB-3 at reducing tissue burden counts. However, on day 5 postinfection, COMBO-3 was superior to each single therapy. At this time interval, 1 out of 10 animals in the COMBO group (10%) showed complete organ clearance. On day 7 postinfection, COMBO-3 was significantly more effective than either CAS-3 or AMB-3 at reducing colony counts, with 6 out of 10 (60%) animals showing organ sterilization.
In this study, we investigated the effects of combination therapy with CAS and AMB against C. glabrata. Although the in vitro results did not show a synergistic effect upon the combination, both methods clearly excluded antagonism. Moreover, time-kill studies showed that the combination was the only regimen able to yield a fungicidal effect.
Our in vivo results mirrored those obtained in vitro. Actually, combination therapies with both drugs given at 0.3 mg/kg/day confirmed the lack of antagonism, while when they were associated at 1 mg/kg/day, the combination was more effective than each single therapy. Moreover, when the drugs were administered at 3 mg/kg/day, the combination regimen not only confirmed this positive interaction but was the only therapeutic approach yielding organ sterilization.
Although the present study was limited to one isolate of C. glabrata, our results demonstrate that this therapeutic approach might be useful in infections due to this emerging and difficult-to-treat fungal pathogen.
Acknowledgments
This work was supported in part by a grant from Istituto Superiore di Sanità, Rome, Italy (IV AIDS project, grant no. 50D.29).
REFERENCES
- 1.Deresinski, S. C., and D. A. Stevens. Caspofungin. 2003. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 2.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 3.Fidel, P. L., Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain, M. A., G. H. Reyes, L. A. Long, P. K. Mukherjee, and M. A. Ghannoum. 2003. Efficacy of caspofungin combined with amphotericin B against azole-resistant Candida albicans. J. Antimicrob. Chemother. 51:1427-1429. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS antifungal surveillance program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]