Abstract
Live attenuated simian immunodeficiency virus (SIV) is the most efficient vaccine yet developed in monkey models of human immunodeficiency virus infection. In all successful vaccine trials, attenuation was achieved by inactivating at least the nef gene. We investigated some virological and immunological characteristics of five rhesus macaques immunized with a nef-inactivated SIVmac251 molecular clone (SIVmac251Δnef) and challenged 15 months later with the pathogenic SIVmac251 isolate. Three animals were killed 2 weeks postchallenge (p.c.) to search for the challenge virus and to assess immunological changes in various organs. The other two animals have been monitored up for 7 years p.c., with clinical and nef gene changes being noted. The animals killed showed no increase in viral load and no sign of a secondary immune response, although the challenged virus was occasionally detected by PCR. In one of the monkeys being monitored, the vaccine virus persisted and an additional deletion occured in nef. In the other monkey that was monitored, the challenge and the vaccine (Δnef) viruses were both detected by PCR until a virus with a hybrid nef allele was isolated 48 months p.c. This nef hybrid encodes a 245-amino-acid protein. Thus, our results show (i) that monkeys were not totally protected against homologous virus challenge but controlled the challenge very efficiently in the absence of a secondary immune response, and (ii) that the challenge and vaccine viruses may persist in a replication-competent form for long periods after the challenge, possibly resulting in recombination between the two viruses.
Experimental infection of macaque with the simian immunodeficiency virus (SIV) is considered to be the best model of human immunodeficiency virus (HIV) infection in humans. SIV induces an AIDS-like disease in macaques and is therefore a useful tool for the development of AIDS vaccines. Numerous approaches have been tried, including the use of whole inactivated viruses, recombinant proteins, nude DNA, and viral vectors alone or in combination, but vaccines have to date been largely unsuccessful at inducing long-term protection against heterologous challenge viruses in macaques (for a review, see reference 28). Currently, the most successful vaccines tested in the macaque model use a live attenuated SIV. The first demonstration of protection provided by an attenuated virus against challenge with a pathogenic virus (15) has been confirmed and extended by many authors (3, 14, 47, 63, 67, 71). Protection is inversely correlated with the level of attenuation of the vaccine virus and requires several months to establish (39, 71). The attenuated virus induces protection against challenge with virus-infected cells and cell-free virus administered by the intravenous or mucosal route (3, 45). However, the mechanisms underlying this protection are still unclear. All live attenuated viruses that have been shown to induce protection following vaccination carry at least an altered nef gene.
In vitro, the Nef proteins of both HIV and SIV have several different functions. Nef has been reported to down-regulate the surface expression of CD4 (1, 21, 56) and the cell surface expression of class I major histocompatibility complex, preventing the recognition and lysis of infected cells by cytotoxic lymphocytes (11, 60). Nef may also interact with a variety of cell proteins involved in the cellular transduction pathway, although the effect of these interactions on the activation pathway is unclear (5, 25, 57). Nef also increases virion infectivity by acting at the stage of particule production to increase the efficiency of reverse transcription (RT) that immediately follows viral entry (2, 10, 59).
Nef is not essential for in vitro replication. However, nef-inactivated mutants of SIVmac replicate at a much lower rate than the wild type and do not cause an AIDS-like disease in juvenile or adult animals (9, 32), although infection may lead to disease development in newborn macaques (4, 72). In humans, defective nef alleles have been characterized in some long-term nonprogressor subjects (17, 33, 41, 55). However, viruses in most of these subjects carry nef alleles encoding a funtional protein as determined by single-cell infection or CD4 down-regulation assays (27, 44). Moreover, truncations in nef have also been identified in individuals with progressive HIV disease, showing that nef defects are not necessary sufficient to prevent progression toward AIDS (22, 65). Although the use of a live attenuated virus in humans is currently inconceivable, trials of vaccination with such viruses in monkeys may increase our understanding of the mechanisms involved in protection against surperinfection and disease development.
In this study, we assessed the degree of protection against homologous challenge with the pathogenic SIVmac251 isolate in five rhesus monkeys vaccinated 15 months earlier with an attenuated nef-deleted SIVmac251 virus. Three monkeys were killed 2 weeks postchallenge (p.c.). The viral load in various organs and some immune characteristics were evaluated for these monkeys. Two other monkeys were studied for 7 years: we assessed the persistence of vaccine and challenge viruses, changes in their nef genes, and long-term clinical progression. We found that four of the five monkeys were not totally protected but efficiently controlled the SIVmac251 challenge virus in the absence of any signs of a secondary immune response. One of the two monkeys monitored in the long term was protected against challenge, and a new deletion has appeared in the nef region overlapping the U3, long terminal repeat (LTR). In the other animal monitored in the long term, both challenge and vaccine viruses persisted and eventually a new hybrid virus (with a hybrid nef gene) emerged, probably due to a recombination between the two viruses.
MATERIALS AND METHODS
Viruses and animals.
The attenuated virus, SIVmac251Δnef, was provided by M. P. Kieny (Transgene, Strasbourg, France). It was derived from the SIVmac251 BK28 clone (35) by three modifications: (i) the premature stop codon at position 8785 in the env gene was mutated to restore a complete env open reading frame (ORF), (ii) the nef initiator codon ATG was mutated to ACG at position 9059, and (iii) nucleotides 9225 to 9401 in the nef region, which do not overlap either the 3′ end of env or the U3 part of the LTR, were deleted. All viruses were propagated on macaque peripheral blood mononuclear cells (PBMC). The pathogenic SIVmac251 isolate provided by R. Desrosiers (16) was subjected to titer determination in Chineese rhesus macaques (Macaca mulatta) by intravenous inoculation. In a first experiment, 10-fold serial dilutions of the stock (8,000 50% tissue culture infectious doses) were inoculated into groups of three macaques (1 ml of 102 to 106 dilutions). Macaques were determined to be infected after seroconversion and/or virus isolation from PBMC. Since none of the monkeys inoculated with the 105 and 106 dilutions were infected, in a second step macaques were inoculated with 1 ml of 104 and 105 dilutions and two intermediate dilutions to confirm and determine the virus titer more precisely. Again macaques inoculated with the 105 dilution remained uninfected. A 1-ml volume of stock virus contained 4 × 104 50% animal infectious doses (AID50) as determined by the VACMAN program, kindly provided by J. Spouge (62).
Rhesus macaques were maintained in accordance with European guidelines. Before inoculation, they were demonstrated to be seronegative for simian T-cell leukemia virus type 1, simian retrovirus type 1 (type D retrovirus), herpes B virus, and SIVmac. All the animals were inoculated with cell-free virus by the intravenous route. Eight monkeys were included in this study. Five were immunized with the attenuated SIVmac251Δnef molecular clone (corresponding to 100,000 cpm in a reverse transcriptase assay). Fifteen months later, the five immunized monkeys and the three naive monkeys used as controls were inoculated with 10 AID50 of the pathogenic SIVmac251 isolate.
Serologic assays.
Plasma p27 Gag antigen levels were determined using a specific SIVmac antigen capture enzyme-linked immunosorbent assay (ELISA) (Coulter). The antibody response to SIV was monitored by an HIV-2 ELISA (Elavia-II; Sanofi-Pasteur), which is cross-reactive for SIV antibody. Serum samples were tested at serial 10-fold dilutions from 1:10 to 1:100,000.
Flow cytometry.
EDTA-treated blood was incubated for 15 min with antibodies against CD4 (OKT4 [Ortho Diagnostic]) and CD8 (Leu-2a [Becton Dickinson]), added at a 1:20 dilution. Erythrocytes were lysed with Lyse & Fix reagents (Immunotech). The samples were washed three times in phosphate-buffered saline and fixed in phosphate-buffered saline containing 1% paraformaldehyde. They were analyzed by flow cytometry using a FACScan cytometer (Becton Dickinson).
In situ hybridization.
Hybridization was performed as previously described with a 35S-labeled RNA nef probe derived from the nef SIVmac142 sequence (9).
nef amplification and sequencing.
RNA was extracted from serum as follows. First-time-thawed serum was centrifuged at 15,000 rpm (20,627 × g) for 2 h to pellet the virions. The pellet was resuspended in 300 μl of lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 2 mM EDTA, 0.1% sodium dodecyl sulfate, 1 mg of proteinase K per ml) and incubated at 56°C for 30 min. The mixture was extracted twice with phenol and chloroform isoamyl alcohol (24:1), a glycogen carrier (40 μg/ml) (Roche), and a synthetic 7.5-kb RNA (1 μg/ml) (Gibco-BRL) were added. RNA was precipitated with ethanol and pelleted by centrifugation at 15,000 rpm (20,627 × g) for 30 min. Total RNA from organs and total cellular DNA from PBMC were extracted using standard methods. For quantitative RT-PCR, a series of 1:2 dilutions of RNA in diethylpyrocarbonate-treated water was used for RT.
For reverse transcription, 0.25 μg of total RNA was reverse transcribed with 50 U of reverse transcriptase (Moloney murine leukemia virus superscript, Gibco-BRL) in a mixture containing 1× PCR buffer II (Perkin-Elmer), 5 mM MgCl2, 1 mM each deoxynucleoside triphosphate, 2.5 μM oligo-d(T)16 and 20 U of RNase inhibitor (RNAguard; Pharmacia). The reaction was conducted at 42°C for 30 min followed by heat inactivation at 95°C for 5 min.
For quantitative nested PCR, a known amount of DNA competitor was added to the cDNA. The following nef primers were used for detection of the nef sequence: Preco (5′-CAGAGGCTCTCTGCGACCCTAC-3′) and K3 (5′-GACTGAATACAGAGCGAAATGC-3′) in the first amplification, and K1 (5′-TGGAAGATGGATCCTCGCAATCC-3′) and A2 (5′-GGACTAATTTCCATAGCCAGCCA-3′) in the second amplification. DNA was subjected to 35 cycles of amplification in a volume of 100 μl containing 1× PCR buffer II, 0.2 mM each deoxynucleoside triphosphate, 2 mM MgCl2, 0.15 μM each external primer, and 2.5 U of AmpliTaq (Perkin-Elmer). For the second PCR, 5 μl from the first amplification was subjected to 25 cycles of amplification in a total volume of 100 μl of PCR mixture containing the internal primers. Under these conditions, it was possible to detect 25 nef in vitro transcripts. Amplification products were analyzed by electrophoresis in a 2.5% agarose gel stained with ethidium bromide. For quantitative PCR, the amplified competitor was distinguished by its specific size. The competitor-template equivalent point was determined by visual examination of the photograph of the gel.
Amplified fragments were inserted into the pGEM-T Easy vector (Promega) for sequencing by Genome Express (Grenoble, France), or used for coupled transcription-translation with the TNT-coupled reticulocyte lysate system (Promega) as specified by the manufacturer.
Virus recovery from PBMC.
PBMC from heparinized blood were separated on a Ficoll density gradient. The cells were washed twice in RPMI 1640, and CD8+ PBMC cells were removed by immunomagnetic separation with anti-CD8 Dynabeads (Dynabeads M-450 CD8; Dynal) as specified by the manufacturer. CD8+-depleted PBMC were resuspended in RPMI complete medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml) and stimulated by incubation with phytohemagglutinin (5 μg/ml) (Sigma) and recombinant interleukin-2 (20 U/ml) (Roche) for 48 h. CEMx174 cells were then added to the culture. SIV-infected cultures were diagnosed by PCR detection of provirus and assessment of syncytium formation.
RESULTS
Vaccination with SIVmac251Δnef and challenge with the SIVmac251 isolate.
Five monkeys were vaccinated by intravenous injection with a SIVmac251 molecular clone attenuated by inactivation of the nef gene (SIVmac251Δnef). The primary infection of three monkeys (52168, 90141 and 90154) has been previously reported (9). Briefly, the major feature of infection with this attenuated virus was a much lower viral load than obtained in infection with virus containing a full-length nef. During the 15-month vaccination phase, no clinical signs were displayed by the monkeys. Around the time of challenge, anti-SIV immunoglobulin G (IgG) persisted at the level reached 2 months postinfection, with a titer of 1,000 to 10,000, and the virus was sometimes detected in the blood by PCR. Thus, the vaccine virus persisted at a low level, together with a vigorous specific immune response.
Vaccinated monkeys and three naive monkeys used as a control, were challenged intravenously with 10 AID50 of the pathogenic SIVmac251 isolate. Table 1 summarizes the vaccination status and duration of follow-up of the monkeys used in this study. Three vaccinated monkeys (52168, 90141, and 90154) and one naive monkey (92409) were killed 2 weeks p.c. for analysis of the distribution of virus in various organs including the lymph nodes (LN), spleen, and brain. Two other immunized monkeys (49848 and 49851) and two naive challenged monkeys (92418 and 92428) were observed over time, with viral changes and clinical progression being monitored.
TABLE 1.
Vaccination status and duration of follow-up after SIVmac251 challenge
| Groupa | Animal | Vaccination statusb | p.c. follow-upc |
|---|---|---|---|
| I | 52168 | Vaccinated | Killed 2 wk p.c. |
| I | 90141 | Vaccinated | Killed 2 wk p.c. |
| I | 90154 | Vaccinated | Killed 2 wk p.c. |
| I | 92409 | Naive | Killed 2 wk p.c. |
| II | 49848 | Vaccinated | Over 72 mo |
| II | 49851 | Vaccinated | Over 72 mo |
| II | 92418 | Naive | Died 21 mo p.c. |
| II | 92428 | Naive | Died 31 mo p.c. |
The monkeys in group I were killed for LN, spleen, and brain analysis 2 weeks after challenge; the monkeys in group II were monitored and assessed for long-term viral changes and clinical progression.
Vaccination with the attenuated virus, SIVmac251 Δnef, was performed 15 months before challenge with the pathogenic isolate.
Monkeys 92418 and 92428 had wasting syndrome at the time of death.
Viral load and immune response analyses for animals killed 2 weeks p.c.
Viral load in the serum was evaluated after challenge by determining the levels of Gag p27 antigen and virion-associated viral RNA. In all vaccinated monkeys tested, p27 antigen levels, determined by ELISA, were similar to background levels. In contrast, p27 antigen was detectable in the serum of the infected naive monkey (92409) from day 7 onward. Viral RNA was detected on one occasion in the sera of the three vaccinated monkeys killed 2 weeks p.c. (Table 2). Only the deleted form of nef, corresponding to the vaccine virus, was detected and was present at less than 300 copy equivalents per ml. In contrast, the wild-type nef sequence was detected from day 7 onward and reached 2.5 × 105 copy equivalents in the serum of the infected naive monkey at the time when it was killed. Given the sensitivity of this method, these data indicate that particles of the challenge virus did not circulate in the blood of vaccinated monkeys within the 2 weeks p.c. They also show that challenging the vaccinated monkeys with a pathogenic isolate did not stimulate replication of the vaccine virus resident in the blood.
TABLE 2.
Viral RNA level in serum after SIVmac251 challenge
| Time p.c. (days) | Viral RNA level ina:
|
|||
|---|---|---|---|---|
| Vaccinated animals
|
Naive animal (92409) | |||
| 52168 | 90141 | 90154 | ||
| 0 | <300 (Δnef) | ND | ND | ND |
| 4 | <300 (Δnef) | <300 (Δnef) | ND | ND |
| 7 | ND | ND | ND | <300 (Wt) |
| 11 | ND | ND | <300 (Δnef) | 4 × 104(Wt) |
| 14 | ND | ND | ND | 2.5 × 105 (Wt) |
The results are expressed as the number of cDNA reverse transcripts per milliliter of serum. Δnef, nef of the vaccine virus; Wt, nef of the challenge virus; ND, not detected.
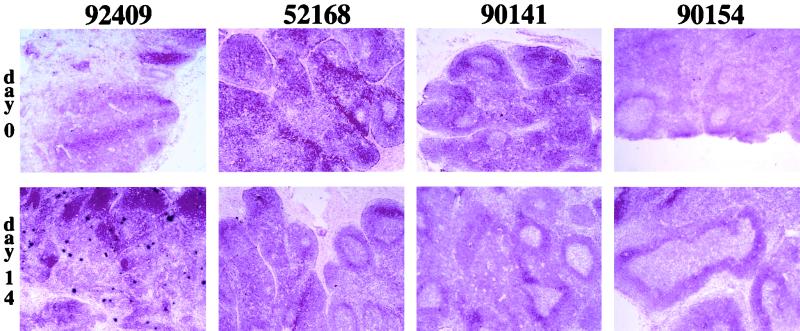
Viral replication was also sequentially investigated in LN from the three vaccinated animals (52168, 90141, and 90154) and the one naive animal (92409) killed 2 weeks p.c. The spleen and brain were also collected at the time of death. We carried out in situ hybridization with a nef probe that detects the transcripts of both the vaccine and challenge viruses. Positive signals were very rarely detected in the LN of vaccinated challenged monkeys collected on days 0, 7, and 14 or in the spleen and brain (Fig. 1). In contrast, in the naive monkey (92409), productively infected cells were detected in the LN from day 7 onward and in the spleen at the time of death, albeit at a lower frequency than in the LN. To increase sensitivity and to discriminate between challenge and vaccine virus mRNA, the viral load in organs was also investigated by RT-PCR, amplifying the nef region (Table 3). As with the in situ approach, no virus was detected in the brain. In the LN and spleen, transcripts of vaccine virus were detected in the three vaccinated monkeys within 2 weeks of challenge. Competitive RT-PCR, performed with 0.25 μg of RNA from these samples, showed that positive signals corresponded to a maximum value of 200 copy equivalents of viral mRNA. In contrast, in the naive monkey (92409), 10,000 copy equivalents were detected 2 weeks p.c. in LN whereas only 700 copy equivalents were detected in the spleen. This is consistent with the lower viral load detected in the spleen by in situ hybridization. Challenge virus transcripts were also detected 2 weeks p.c. in the LN and spleens of two (90141 and 90154) of the three vaccinated monkeys.
FIG. 1.
Comparison between LN collected at the time of challenge and 2 weeks after challenge. LN were obtained at the time of SIVmac251 challenge and 2 weeks p.c. from the three vaccinated monkeys (52168, 90141, and 90154) and the naive monkey (92409) killed for organ analysis. Vaccine and challenge viruses were detected by in situ hybridization with a 35S-labeled nef riboprobe. Sections were stained with hematoxylin and eosin. Magnification, ×40.
TABLE 3.
nef detection in the organs of the monkeys killed
| Animal |
nef detectiona on:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0
|
Day 4 (PBMC)
|
Day 7
|
Day 11 (PBMC)
|
Day 14
|
||||||||||||||
| PBMC
|
LN
|
PBMC
|
LN
|
PBMC
|
LN
|
Spleen
|
Brain
|
|||||||||||
| Δnef | Δnef | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | |
| Vaccinated | ||||||||||||||||||
| 52168 | − | + | − | − | + | + | + | − | − | − | − | − | + | − | + | − | − | − |
| 90141 | − | − | − | − | − | − | + | − | − | − | − | − | + | + | + | + | − | − |
| 90154 | + | + | + | − | + | − | + | − | − | − | − | − | + | + | + | + | − | − |
| Naive | ||||||||||||||||||
| 92409 | − | + | + | + | + | + | + | + | ||||||||||
For PBMC, total DNA corresponding to 2 × 105 PBMC was subjected to nested PCR amplifying a nef gene portion. For organs, 0.25 μg of total RNA was reverse transcribed and subjected to nested PCR amplifying the same nef portion. Except for the naive challenged monkey (92409), less than 200 copy equivalents of viral mRNA were detected in samples from the vaccinated monkeys (52168, 90141, and 90154). Δnef, nef of the vaccine virus; Wt, nef of the challenge virus; +, positive PCR amplification; −, negative PCR amplification.
We also tested for the presence of viral sequences in PBMC by PCR amplification of the nef region. At one time point after challenge, at least, the proviral sequence of the Δnef vaccine virus was detected in two (52168 and 90154) of the three vaccinated monkeys (Table 3). In particular, the SIVmac251 challenge virus was detected in monkey 52168 1 week p.c. At this time point, proviral sequences became detectable in the PBMC of the naive monkey (92409).
The detection of SIVmac251 viral transcripts in the LN and spleen of two vaccinated monkeys (90141 and 90154) and of proviral sequence in the PBMC of the third (52168) indicates that these monkeys were not fully protected. However, viral replication did remain very efficiently controlled. Our data also show that challenge of the vaccinated monkeys was not followed by an increase in Δnef viral load.
We investigated whether the very efficient control of challenge virus involved the development of a secondary immune response by studying some immunological characteristics for 2 weeks p.c. In contrast to what was observed in naive monkeys, the challenge of vaccinated monkeys with the pathogenic SIVmac251 virus did not induce any sign of immune reactivation in the LN according to the following criteria: (i) no extension or decrease of the preexisting germinal centers already well developed before challenge (Fig. 1), (ii) no change in mRNA level for the cytokines or chemokines tested (interleukin-2 [IL-2], IL-10, gamma interferon, RANTES, macrophage inflammatory protein 1α [MIP-1α], or MIP-1β) by RT-PCR (data not shown) and (iii) no secondary IgG response to whole viral antigens during the 2 weeks following challenge (Fig. 2). Therefore, challenging vaccinated monkeys with the pathogenic virus did not seem to induce a secondary immune response and the preexisting immunity was sufficient to control any replication of the pathogenic challenge virus.
FIG. 2.
Antibody response after challenge. Anti-SIV IgG antibody titers were determinated by ELISA in sera of the group I monkeys (see Table 1) challenged with the pathogenic SIVmac251 isolate and killed 2 weeks later. The titer is expressed as the optical density (492 nm) multiplied by the final dilution before extinction of the specific signal.
Long-term persistence of challenge and vaccine viruses in vaccinated monkeys.
We assessed the persistence of vaccine and challenge viruses in the two vaccinated monkeys (49848 and 49851) monitored up for 7 years after being challenged with the pathogenic SIVmac251 isolate. During this period, these monkeys showed no clinical signs or decrease in CD4+ cell counts. As controls, two naive animals were also challenged (92418 and 92428). As expected, the unvaccinated monkeys tested positive for the virus by PCR at all time points after 1 week p.c. Both these monkeys also showed a severe decline in CD4+ cell counts, and they died at 21 and 31 months p.c.
PBMC were regularly collected from vaccinated and challenged monkeys. DNA was isolated from these cells and used for the nested PCR amplification of nef sequences. Proviral sequences were frequently detected during the 62 months of PCR follow-up (Table 4). The only form detected for the PBMC of monkey 49848 was the same size as that of the Δnef amplicon control, suggesting that the vaccine virus had persisted without major modifications in the nef region. In contrast, for monkey 49851, two forms were initially detected, of the same sizes as the vaccinal and challenge nef amplicons, and then at 56 months p.c. an amplicon of intermediate size was detected.
TABLE 4.
Detection of proviral sequence in PBMC of the long-term monitored SIVmac251-challenged monkeys
|
nef detection after a:
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Day 0 (Δnef) | 0.5 mo
|
1 mo
|
2 mo
|
3 mo
|
7 mo
|
12 mo
|
32 mo
|
56 mo
|
62 mo
|
|||||||||||
| Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | Δnef | Wt | ||||
| 49848 | + | + | − | − | − | + | − | − | − | + | − | + | − | + | − | − | − | + | − | ||
| 49851 | + | − | − | + | + | + | + | + | + | + | − | − | − | + | − | HYB | HYB | ||||
Total DNA corresponding to 2 × 105 cells was subjected to nested PCR amplifying a nef gene portion. Δnef, nef of the vaccine virus; Wt, nef of the challenge virus; HYB, nef of intermediate size; +, positive PCR amplification; −, negative PCR amplification.
We investigated whether the proviral sequence amplified from monkeys monitored in the long term corresponded to an efficient replicating virus. We tried to isolate viruses by coculture of activated PBMC with CEMx174 cells. Cocultures starting with PBMC from which CD8+ cells were or were not depleted were established 48, 56, 62, and 76 months p.c. Isolation was successful only from CD8+-depleted PBMC, illustrating the well-known inhibitory role of CD8+ cells in viral replication. For monkey 49851, virus was isolated from cocultures established 48 and 76 months p.c. whereas isolation was successful only 76 months p.c. for monkey 49848. The time from the beginning of coculture to the appearance of the syncytia in CEMx174 culture was 3 weeks for monkey 49848 for coculture 76 months p.c. and 5 and 3 weeks for monkey 49851 for coculture 48 and 76 months p.c., respectively. When the same experiment was conducted with CD8+-depleted PBMC from the two control monkeys (92418 and 92428) in the asymptomatic phase, syncytia were visible within 1 to 3 weeks.
We characterized the nef allele of the viruses isolated from vaccinated and challenged monkeys by PCR amplification of the complete nef gene plus the 3′ end of the LTR U3 region from DNA extracted from the culture (see Fig. 3 for the locations of the primers and the genetic organization of this region). These PCR products, named nef-U3 LTR, were cloned and sequenced.
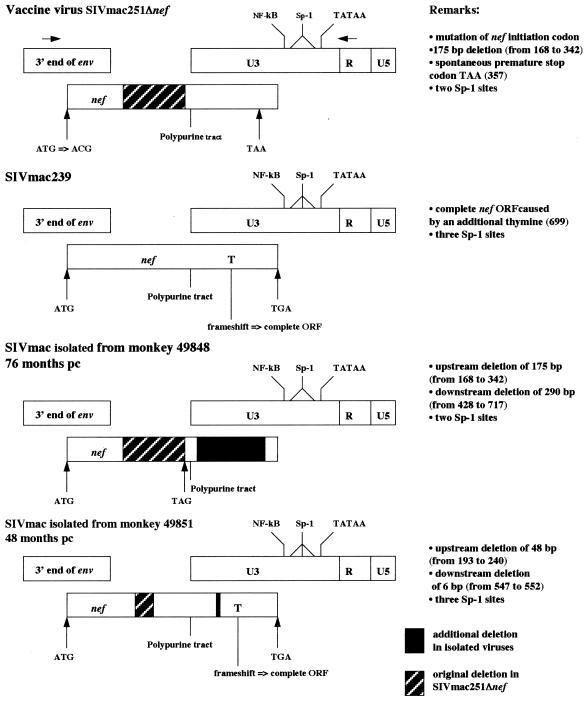
FIG. 3.
Schematic representation of changes in nef and U3 after challenge with the SIVmac251 isolate in macaques vaccinated with the live attenuated virus SIVmac251 Δnef. Nucleotides are numbered according to the SIVmac251 BK28 nef gene from which the vaccine virus was derived (35). Since the challenge virus is an uncloned virus, we have represented the SIVmac239 molecular clone. Arrows at the top indicate the binding sites of the primers used for PCR amplification.
Changes in nef sequences in the vaccinated and challenged monkeys.
Figure 3 shows the nef-U3 LTR region of the virus isolated from the PBMC of monkey 49848, 76 months p.c. The original 175-bp deletion was unaffected, but two important modifications were observed. The first is an additional 291-bp deletion in U3 beginning 43 bp downstream from the polypurine tract and ending 78 bp upstream from the NF-κB site. The second is the presence of a nef initiator codon, inactivated in the original vaccine virus, suggesting a potential ORF encoding the first 56 amino acids of Nef.
The nef-U3 LTR PCR product obtained from the coculture with the PBMC of monkey 49851 collected 48 months p.c. was reproducibly found to have a size intermediate between those of the forms of nef of the vaccine and challenge viruses. Sequence analysis suggested that this nef gene is a hybrid between the vaccine and challenge virus forms of nef. Figure 3 presents a comparison of the nef-U3 LTR region of the hybrid with that of the vaccine virus. This hybrid nef gene has an initiator codon, and the original deletion has been reduced from 175 to 48 bp by insertion of the wild-type sequence and an additional 6-bp deletion in the U3 region. In addition, the insertion of a thymine at position 699 (relative to the nef of SIVmac251 BK28) results in a frameshift, changing the C-terminal 15 amino acids of Nef into a 31-amino-acid extremity identical to that encoded by SIVmac239. Thus, the hybrid ORF potentially encodes a 245-amino-acid Nef protein. Finally, a third Sp-1 site absent from the vaccine virus is also present, as in SIVmac239.
We investigated whether this nef ORF actually encodes a protein by performing a coupled transcription-translation assay using the cloned nef-U3 LTR PCR product. Cloned nef-U3 LTR PCR products from SIVmac251 BK28 and vaccinal SIVmac251Δnef clones were used as controls. The transcription-translation assay resulted in the synthesis of a protein with an apparent molecular mass of 28 kDa, close to that of the SIVmac251 BK28 Nef protein (Fig. 4). This result is consistent with the removal of 16 amino acids created by the 48-bp deletion, compensated by the restoration of a full coding C-terminal extremity generating 16 additional amino acids.
FIG. 4.
Coupled transcription-translation assay of nef alleles. Methionine-labeled Nef protein was analyzed by electrophoresis in a 12% acrylamide denaturing gel. Lanes (from left to right): SIVmac251 Δnef, SIVmac251 BK28 nef, SIVmac nef isolated from monkey 49851, molecular mass markers (M.W.), negative control with T3 and T7 RNA polymerases and no DNA, positive controls from the kit, under the control of the T3 and T7 promoters, respectively.
A nef-U3 LTR PCR product of intermediate size was also detected in PBMC collected 56 and 62 months p.c. (Table 4). The same 48-bp deletion was observed in the PCR product obtained 62 months p.c. and in the virus isolated 76 months p.c. These results suggest that the hybrid virus emerged between month 32 p.c. (i.e., the last point at which vaccine virus was detected in PBMC) and month 48 p.c. (the time point at which the nef hybrid virus was first isolated). After its initial detection, this hybrid form was the only form of nef detected in the PBMC of monkey 49851.
DISCUSSION
This study was designed to assess the degree of protection provided by vaccination with a live attenuated SIVmac251Δnef virus. Five rhesus monkeys vaccinated 15 months previously were challenged with a low dose (10 AID50) of the pathogenic SIVmac251 isolate. Our PCR results indicate that at least four of these monkeys were not totally protected against the challenge virus. In this kind of vaccination approach, protection is greater the more similar the vaccine and challenge viruses, requires the vaccine virus to persist, and takes several months to become fully established (12, 71). Taking into account the duration of vaccination, the persistence of the vaccine virus, and its similarity to the challenge virus, our data suggest that even optimal vaccination conditions do not systematically induce total protection against a homologous challenge virus. Several recent studies have reported that vaccination with a Δnef SIVmac virus may not provide protection against challenge with a heterologous pathogenic virus or against a homologous virus if the duration of vaccination is too short (12, 38, 70). Moreover, superinfection with the pathogenic challenge virus may lead to CD4+ cell depletion. In this study, in contrast, the pathogenic challenge virus was very efficiently controlled, since no increase in the load of the resident vaccine virus and no decrease in the CD4+ cell count were observed in the monkeys observed for 7 years. These data suggest that in our system, vaccination does not protect against superinfection but does protect against disease development.
We observed no signs of a secondary immune response following the pathogenic challenge in the monkeys killed 2 weeks p.c. We therefore conclude that the preexisting immunity was sufficient to control the challenge virus and thus prevented the development of a secondary immune response. Generally, inefficient control of challenge virus is responsible for the development an anaemestic response (47) that may also permit reactivation of the resident vaccine virus (49).
The mechanisms underlying this control are unknown. A nonimmune mechanism such as receptor interference is unlikely, because vaccinated monkeys are also protected against challenge with a SIV pseudotype carrying the amphotropic envelope of the murine leukemia virus (24). However, as far as immune mechanisms are considered, protection against a SIV carrying an amphotropic or HIV-1 envelope excludes a major role for neutralizing antibodies (7, 19, 61). Several studies have implicated the CTL response in the control of SIV infection (31, 42, 51, 58). However, in monkeys vaccinated with the attenuated SIVmac251 32H(C8) virus, the partial depletion of CD8+ cells before challenge did not abolish protection against SIVmac251 32H, the pathogenic isolate corresponding to the vaccine virus (64).
Few studies have focused on the long-term effects of the persitence of vaccine and/or challenge viruses. We therefore monitored two vaccinated monkeys for 6 and 7 years, studying changes in the viruses they carried and their clinical progression. Only vaccine sequences were detected in the PBMC of monkey 49848 throughout the 62 months of follow-up p.c. However, it could not be strictly concluded that this monkey has been totally protected against challenge, because the presence of SIV DNA was not investigated in LN and in two (90141 and 90154) of the three monkeys killed at 2 weeks p.c. the challenge virus was detected in LN but not in PBMC. Moreover, McChesney et al. have recently shown that, in occult infection following inoculation with a low dose of SIVmac251 by the intravaginal route, SIV DNA was detected more frequently in LN than in PBMC years after inoculation (43). Virus isolated 76 months p.c. showed, in addition to the original deletion, a 290-bp deletion in the overlaping nef-U3 region. This deletion did not affect the sequence elements important for viral replication and transcription (i.e., the polypurine tract and its immediate downstream sequences, the enhancer region just upstream from the NF-κB, the NF-κB and Sp-1 binding sites, and the TATAA box). We observed the same features in unchallenged macaques infected with SIVmac251Δnef (data not shown). These data are consistent with previous in vitro and in vivo observations for SIVmac239Δnef-infected monkeys (12, 29, 34, 50). Thus, in the absence of a functional nef, certain regions overlapping nef and U3 are lost, suggesting that these regions are dedicated solely to the encoding of Nef. Since one inner primer bound to the sequence located in the additional deletion, PCR analysis could not be used to detect the virus in PBMC before its isolation.
In the second challenged and monitored monkey (49851), both challenge and vaccine virus sequences were frequently detected. The virus isolated 48 months p.c. carried a hybrid sequence, intermediate between those of the vaccine and challenge viruses in the nef-U3 LTR region, encoding a 245-amino-acid protein. This nef hybrid gene did not result from an in vitro artifact such a recombination during PCR or growth on CEMx174, since it was again directly amplified by PCR from PBMC 62 and 76 months p.c. and reisolated at month 76 p.c. Thus, the nef hybrid virus isolated 48 months p.c. was actually the major form in vivo. The emergence of a SIV strain with a hybrid nef gene raises questions about its origin and the reasons for its positive selection.
As regards its origin, there are two possible hypotheses: a spontaneous 48-bp deletion in the nef gene of the challenge virus or a recombination event between the vaccine and challenge viruses. Many studies have shown that there is strong pressure favoring the maintenance of an intact nef gene in vivo. When first isolated after in vitro amplification, both the SIVmac239 and SIVmac251 BK28 molecular clones showed defects in nef generated by single point mutations (35, 52). However, following the inoculation of monkeys with these viruses, the premature stop codon of SIVmac239 reverted very rapidly and the frameshift in the SIVmac251 BK28 nef tended to disappear by means of a thymine insertion (9, 20, 32, 36). In both cases, the restoration of a full-length nef gene was associated with an increase in virulence. The repair of a 12-bp deletion in the SIVmac251 32H(C8) nef gene was also found to be associated with a reversion to virulence (18, 54, 68). These observations indicate that there is strong selection pressure in vivo, favoring the restoration of an intact nef gene, unless nef is totally inactivated. To our knowledge, Nef alterations have never been associated with positive selection of the virus in vivo. Thus, it is unlikely that a positive advantage is conferred in vivo by the 16-amino-acid nef deletion. Although we cannot formally exclude the possibility of a spontaneous 48-bp deletion in the challenge virus, we believe that the nef hybrid virus results from recombination between the vaccine and challenge viruses, especially since the challenge and vaccine virus forms were simultaneously detected several times before isolation of the hybrid virus.
The high recombination potential of retroviruses has been extensively shown in vitro (26, 30, 73). Until recently, recombinations in vivo between HIV or SIV were rarely described, because for recombination to occur, individuals must be coinfected with divergent strains and because most naturally occurring recombination events would generate nonfunctional viruses, or at least viruses with reduced fitness, which are harder to identify. However, an increasing number of reports based on phylogenetic analysis have strongly suggested that recombination occurs in HIV-infected individuals (6, 8, 13, 40, 46, 48, 53, 66). SIVmac recombination in macaques has been directly demonstrated by Wooley et al., by the simultaneous inoculation of a naive monkey with two SIVmac239 viruses, attenuated by different deletions (69). As early as 2 weeks after infection, the sequence of the full-length pathogenic SIVmac239 was the sequence predominantly detected, demonstrating that recombination is of biological relevance in vivo.
Whatever its origin, the nef hybrid virus became the sole form detected after its first identification, suggesting that it has a selective advantage. The most obvious explanation for this positive selection is that it has a higher intrinsic replication rate. Curiously, in a single-cycle infectivity assay performed on sMAGI cells, the nef hybrid virus was less infectious than even the vaccine SIVmac251Δnef strain (data not shown), suggesting that viral determinants other than nef may be responsible for this in vitro phenotype. In addition, the in vitro assay takes into account only some of the functions mediated by nef during the early stages of the virus cycle in vitro, and the significance of these functions in vivo is unclear. Thus, the data obtained in vitro do not necessarily imply that the nef hybrid allele is not functional in vivo. Indeed, several lines of evidence led us to conclude that this allele has some functions in vivo: (i) the deletion is short, does not map to a region known to have a critical function, and is restricted to a region described as disorder in HIV Nef (23, 37), and (ii) a nef ORF is maintained. However, the 16-amino-acid deletion is unlikely to cause an increase in replication in vivo. An alternative explanation for the predominance of the nef hybrid virus is that it may be able to escape more efficiently from immune control, regardless of replication capacity. It is therefore intriguing that the Δnef vaccine virus, despite its lower replication capacity and greatly impaired potential to maintain a high viral load in naive monkeys, is still codetected with the challenge virus several months p.c. Finally, taking into account the low dose of inoculum and the very efficient immune response, it is possible that defective viruses, which constitute the majority of retroviral particles, are selected after immune clearance. According to this hypothesis, the challenge and vaccine viruses may coexist until a recombination event causes an increase in viral fitness. It would be of value to inoculate monkeys with this nef hybrid virus and to monitor them over time.
In conclusion, our results show (i) that monkeys were not totally protected against homologous virus challenge but controlled the challenge virus very efficiently in the absence of a secondary immune response and (ii) that the challenge and vaccine viruses may persist in a replication-competent form for long periods after the challenge, possibly resulting in recombination between the two viruses.
ACKNOWLEDGMENTS
This work was supported in part by the Agence Nationale de Recherche sur le SIDA. E.K. was awarded a doctoral fellowship from SIDACTION.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 4.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 5.Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart T A. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J Gen Virol. 1998;79:2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 6.Blackard J T, Renjifo B R, Mwakagile D, Montano M A, Fawzi W W, Essex M. Transmission of human immunodeficiency type 1 viruses with intersubtype recombinant long terminal repeat sequences. Virology. 1999;254:220–225. doi: 10.1006/viro.1998.9504. [DOI] [PubMed] [Google Scholar]
- 7.Bogers W M, Niphuis H, ten Haaft P, Laman J D, Koornstra W, Heeney J L. Protection from HIV-1 envelope-bearing chimeric simian immunodeficiency virus (SHIV) in rhesus macaques infected with attenuated SIV: consequences of challenge. AIDS. 1995;9:F13–F18. [PubMed] [Google Scholar]
- 8.Burke D S. Recombination in HIV: an important viral evolutionary strategy. Emerg Infect Dis. 1997;3:253–259. doi: 10.3201/eid0303.970301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti L, Baptiste V, Khatissian E, Cumont M C, Aubertin A M, Montagnier L, Hurtrel B. Limited viral spread and rapid immune response in lymph nodes of macaques inoculated with attenuated simian immunodeficiency virus. Virology. 1995;213:535–548. doi: 10.1006/viro.1995.0026. [DOI] [PubMed] [Google Scholar]
- 10.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. Hiv-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Montefiori D C, Binley J M, Moore J P, Bonhoeffer S, Gettie A, Fenamore E A, Sheridan K E, Ho D D, Dailey P J, Marx P A. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen M, Kampinga G, Zorgdrager F, Goudsmit J. Human immunodeficiency virus type 1 subtypes defined by env show high frequency of recombinant gag genes. The UNAIDS Network for HIV Isolation and Characterization. J Virol. 1996;70:8209–8212. doi: 10.1128/jvi.70.11.8209-8212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cranage M P, Whatmore A M, Sharpe S A, Cook N, Polyanskaya N, Leech S, Smith J D, Rud E W, Dennis M J, Hall G A. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology. 1997;229:143–154. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 15.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 16.Daniel M D, Letvin N L, King N W, Kannagi M, Sehgal P K, Hunt R D, Kanki P J, Essex M, Desrosiers R C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 17.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 18.Dittmer U, Nisslein T, Bodemer W, Petry H, Sauermann U, Stahl-Hennig C, Hunsmann G. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 19.Dunn C S, Hurtrel B, Beyer C, Gloeckler L, Ledger T N, Moog C, Kieny M P, Mehtali M, Schmitt D, Gut J P, Kirn A, Aubertin A M. Protection of SIVmac-infected macaque monkeys against superinfection by a simian immunodeficiency virus expressing envelope glycoproteins of HIV type 1. AIDS Res Hum Retroviruses. 1997;13:913–922. doi: 10.1089/aid.1997.13.913. [DOI] [PubMed] [Google Scholar]
- 20.Edmonson P, Murphey-Corb M, Martin L N, Delahunty C, Heeney J, Kornfeld H, Donahue P R, Learn G H, Hood L, Mullins J I. Evolution of a simian immunodeficiency virus pathogen. J Virol. 1998;72:405–414. doi: 10.1128/jvi.72.1.405-414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by Nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 22.Greenough T C, Sullivan J L, Desrosiers R C. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N Engl J Med. 1999;340:236–237. doi: 10.1056/NEJM199901213400314. [DOI] [PubMed] [Google Scholar]
- 23.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J S, Kaufman J, Palmer I, Stahl S J, Wingfield P T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 24.Gundlach B R, Reiprich S, Sopper S, Means R E, Dittmer U, Matzrensing K, Stahlhennig C, Uberla K. Env-independent protection induced by live, attenuated simian immunodeficiency virus vaccines. J Virol. 1998;72:7846–7851. doi: 10.1128/jvi.72.10.7846-7851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe A Y M, Jung J U, Desrosiers R C. Zeta chain of the T-cell receptor interacts with Nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J Virol. 1998;72:9827–9834. doi: 10.1128/jvi.72.12.9827-9834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W S, Temin H M. Retroviral recombination and reverse transcription. Science. 1990;250:1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Zhang L, Ho D D. Biological characterization of nef in long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1995;69:8142–8146. doi: 10.1128/jvi.69.12.8142-8146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulskotte E G, Geretti A M, Osterhaus A D. Towards an HIV-1 vaccine: lessons from studies in macaque models. Vaccine. 1998;16:904–915. doi: 10.1016/s0264-410x(97)00292-2. [DOI] [PubMed] [Google Scholar]
- 29.Ilyinskii P O, Desrosiers R C. Identification of a sequence element immediately upstream of the polypurine tract that is essential for replication of simian immunodeficiency virus. EMBO J. 1998;17:3766–3774. doi: 10.1093/emboj/17.13.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jetzt A E, Yu H, Klarmann G J, Ron Y, Preston B D, Dougherty J P. High rate of recombination throughout the human immunodeficiency virus type 1 genome. J Virol. 2000;74:1234–1240. doi: 10.1128/jvi.74.3.1234-1240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L Q, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kestler H W D, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 34.Kirchhoff F, Kestler H W R, Desrosiers R C. Upstream U3 sequences in simian immunodeficiency virus are selectively deleted in vivo in the absence of an intact nef gene. J Virol. 1994;68:2031–2037. doi: 10.1128/jvi.68.3.2031-2037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornfeld H, Riedel N, Viglianti G A, Hirsch V, Mullins J I. Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature. 1987;326:610–613. doi: 10.1038/326610a0. [DOI] [PubMed] [Google Scholar]
- 36.Lafont B A P, Rivière Y, Gloeckler L, Beyer C, Hurtrel B, Kieny M P, Kirn A, Aubertin A M. Implication of the C-terminal domain of Nef protein in the reversion to pathogenicity of attenuated SIVmacBK28–41 in macaques. Virology. 2000;266:286–298. doi: 10.1006/viro.1999.9991. [DOI] [PubMed] [Google Scholar]
- 37.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 38.Lewis M G, Yalley-Ogunro J, Greenhouse J J, Brennan T P, Jiang J B, VanCott T C, Lu J C, Eddy G A, Birx D L. Limited protection from a pathogenic chimeric simian-human immunodeficiency virus challenge following immunization with attenuated simian immunodeficiency virus. J Virol. 1999;73:1262–1270. doi: 10.1128/jvi.73.2.1262-1270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lole K S, Bollinger R C, Paranjape R S, Gadkari D, Kulkarni S S, Novak N G, Ingersoll R, Sheppard H W, Ray S C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McChesney M B, Collins J R, Lu D, Lu X, Torten J, Ashley R L, Cloyd M W, Miller C J. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J Virol. 1998;72:10029–10035. doi: 10.1128/jvi.72.12.10029-10035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michael N L, Chang G, d'Arcy L A, Tseng C J, Birx D L, Sheppard H W. Functional characterization of human immunodeficiency virus type 1 nef genes in patients with divergent rates of disease progression. J Virol. 1995;69:6758–6769. doi: 10.1128/jvi.69.11.6758-6769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller C J, McChesney M B, Lu X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris A, Marsden M, Halcrow K, Hughes E S, Brettle R P, Bell J E, Simmonds P. Mosaic structure of the human immunodeficiency virus type 1 genome infecting lymphoid cells and the brain: evidence for frequent in vivo recombination events in the evolution of regional populations. J Virol. 1999;73:8720–8731. doi: 10.1128/jvi.73.10.8720-8731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 48.Peeters M, Liegeois F, Torimiro N, Bourgeois A, Mpoudi E, Vergne L, Saman E, Delaporte E, Saragosti S. Characterization of a highly replicative intergroup M/O human immunodeficiency virus type 1 recombinant isolated from a Cameroonian patient. J Virol. 1999;73:7368–7375. doi: 10.1128/jvi.73.9.7368-7375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petry H, Dittmer U, Stahl-Hennig C, Coulibaly C, Makoschey B, Fuchs D, Wachter H, Tolle T, Morys-Wortmann C, Kaup F J. Reactivation of human immunodeficiency virus type 2 in macaques after simian immunodeficiency virus SIVmac superinfection. J Virol. 1995;69:1564–1574. doi: 10.1128/jvi.69.3.1564-1574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pohlmann S, Floss S, IIyinskii P O, Stamminger T, Kirchhoff F. Sequences just upstream of the simian immunodeficiency virus core enhancer allow efficient replication in the absence of NF-κB and Sp1 binding elements. J Virol. 1998;72:5589–5598. doi: 10.1128/jvi.72.7.5589-5598.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putkonen P, Walther L, Zhang Y J, Li S L, Nilsson C, Albert J, Biberfeld P, Thorstensson R, Biberfeld G. Long-term protection against SIV-induced disease in macaques vaccinated with a live attenuated HIV-2 vaccine. Nat Med. 1995;1:914–918. doi: 10.1038/nm0995-914. [DOI] [PubMed] [Google Scholar]
- 52.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 53.Robertson D L, Hahn B H, Sharp P M. Recombination in AIDS viruses. J Mol Evol. 1995;40:249–259. doi: 10.1007/BF00163230. [DOI] [PubMed] [Google Scholar]
- 54.Rud E W, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke B E. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 55.Salvi R, Garbuglia A R, Dicaro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanfridson A, Cullen B R, Doyle C. The simian immunodeficiency virus Nef protein promotes degradation of CD4 in human T cells. J Biol Chem. 1994;269:3917–3920. [PubMed] [Google Scholar]
- 57.Sawai E T, Khan I H, Montbriand P M, Peterlin B M, Cheng-Mayer C, Luciw P A. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr Biol. 1996;6:1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8(+) lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 61.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, Attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spouge J L. Statistical analysis of sparse infection data and its implications for retroviral treatment trials in primates. Proc Natl Acad Sci USA. 1992;89:7581–7585. doi: 10.1073/pnas.89.16.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stahl-Hennig C, Dittmer U, Nisslein T, Petry H, Jurkiewicz E, Fuchs D, Wachter H, Matz-Rensing K, Kuhn E M, Kaup F J, Rud E W, Hunsmann G. Rapid development of vaccine protection in macaques by live-attenuated simian immunodeficiency virus. J Gen Virol. 1996;77:2969–2981. doi: 10.1099/0022-1317-77-12-2969. [DOI] [PubMed] [Google Scholar]
- 64.Stebbings R, Stott J, Almond N, Hull R, Lines E, Silvera P, Sangster R, Corcoran T, Rose J, Cobbold S, Gotch F, McMichael A, Walker B. Mechanisms of protection induced by attenuated simian immunodeficiency virus I-lymphocyte depletion does not abrogate protection. AIDS Res Hum Retroviruses. 1998;14:1187–1198. doi: 10.1089/aid.1998.14.1187. [DOI] [PubMed] [Google Scholar]
- 65.Switzer W M, Wiktor S, Soriano V, Silva-Graca A, Mansinho K, Coulibaly I M, Ekpini E, Greenberg A E, Folks T M, Heneine W. Evidence of Nef truncation in human immunodeficiency virus type 2 infection. J Infect Dis. 1998;177:65–71. doi: 10.1086/513819. [DOI] [PubMed] [Google Scholar]
- 66.Takehisa J, Osei-Kwasi M, Ayisi N K, Hishida O, Miura T, Igarashi T, Brandful J, Ampofo W, Netty V B, Mensah M, Yamashita M, Ido E, Hayami M. Phylogenetic analysis of HIV type 2 in Ghana and intrasubtype recombination in HIV type 2. AIDS Res Hum Retroviruses. 1997;13:621–623. doi: 10.1089/aid.1997.13.621. [DOI] [PubMed] [Google Scholar]
- 67.Titti F, Sernicola L, Geraci A, Panzini G, Di Fabio S, Belli R, Monardo F, Borsetti A, Maggiorella M T, Koanga-Mogtomo M, Corrias F, Zamarchi R, Amadori A, Chieco-Bianchi L, Verani P. Live attenuated simian immunodeficiency virus prevents super-infection by cloned SIVmac251 in cynomolgus monkeys. J Gen Virol. 1997;78:2529–2539. doi: 10.1099/0022-1317-78-10-2529. [DOI] [PubMed] [Google Scholar]
- 68.Whatmore A M, Cook N, Hall G A, Sharpe S, Rud E W, Cranage M P. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69:5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wooley D P, Smith R A, Czajak S, Desrosiers R C. Direct demonstration of retroviral recombination in a rhesus monkey. J Virol. 1997;71:9650–9653. doi: 10.1128/jvi.71.12.9650-9653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyand M S, Manson K, Montefiori D C, Lifson J D, Johnson R P, Desrosiers R C. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–33. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyand M S, Manson K H, Lackner A A, Desrosiers R C. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat Med. 1997;3:32–6. doi: 10.1038/nm0197-32. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Temin H M. Retrovirus recombination depends on the length of sequence identity and is not error prone. J Virol. 1994;68:2409–2414. doi: 10.1128/jvi.68.4.2409-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]