Abstract
The purpose of this study was to characterize the antiviral activity, cytotoxicity, and mechanism of action of TMC114, a novel human immunodeficiency virus type 1 (HIV-1) protease inhibitor (PI). TMC114 exhibited potent anti-HIV activity with a 50% effective concentration (EC50) of 1 to 5 nM and a 90% effective concentration of 2.7 to 13 nM. TMC114 exhibited no cytotoxicity at concentrations up to 100 μM (selectivity index, >20,000). All viruses in a panel of 19 recombinant clinical isolates carrying multiple protease mutations and demonstrating resistance to an average of five other PIs, were susceptible to TMC114, defined as a fold change in EC50 of <4. TMC114 was also effective against the majority of 1,501 PI-resistant recombinant viruses derived from recent clinical samples, with EC50s of <10 nM for 75% of the samples. In sequential passage experiments using HIV-1 LAI, two mutations (R41T and K70E) were selected. One selected virus showed a 10-fold reduction in susceptibility to TMC114, but <10-fold reductions in susceptibility to the current PIs (atazanavir was not assessed), except saquinavir. However, when the selected mutations were introduced into a laboratory strain by site-directed mutagenesis, they had no effect on susceptibility to TMC114 or other PIs. There was no evidence of antagonism between TMC114 and any currently available PIs or reverse transcriptase inhibitors. Combinations with ritonavir, nelfinavir, and amprenavir showed some evidence of synergy. These results suggest that TMC114 is a potential candidate for the treatment of both naïve and PI-experienced patients with HIV.
The human immunodeficiency virus type 1 (HIV-1) protease is essential for the correct processing of viral precursor proteins and the maturation of infectious virus and is therefore an important target for antiretroviral therapy (17). The use of inhibitors of HIV-1 protease as a component of highly active antiretroviral therapy in patients with HIV infection has been shown to achieve durable virological suppression as well as appreciably reducing the considerable morbidity and mortality associated with HIV disease (12, 21, 27). As a result, protease inhibitors (PIs) have become cornerstones in the treatment of HIV infection, particularly in patients with a long history of antiretroviral therapy. However, the emergence of drug-resistant HIV-1 strains continues to jeopardize the efficacy of existing anti-HIV medications, and the need to develop novel therapies with activity against resistant virus is of increasing importance. Toxicity and lack of adherence to therapy are also important barriers to effective treatment and are fast becoming the most common reasons for treatment failure in HIV-infected patients (2, 6). New compounds should therefore be highly selective and potent to limit both toxicity and pill burden and thus promote patient compliance.
A lead optimization program initially produced a series of bis-tetrahydrofuranyl compounds of which TMC126 was studied as the prototype. TMC126 (also known as UIC-94003) is a PI containing a 3(R),3a(S),6a(R)-bis-tetrahydrofuranyl moiety and a sulfonamide isostere (10). This compound has been shown to be extremely potent against a wide spectrum of HIV (50% effective concentration [EC50] of 0.3 to 0.5 nM), including multi-PI-resistant HIV-1 strains isolated from patients (EC50, 0.5 to 5.5 nM) (29). However, sequential passage of HIV-1 NL4-3 in the presence of increasing concentrations of TMC126 resulted in the selection of virus carrying multiple mutations in the protease that have been previously associated with resistance to other PIs (L10F, M46I, I50V, A71V, and N88D) in addition to the novel mutation A28S (29). Preliminary pharmacokinetics studies with rats and dogs showed that plasma levels observed after oral administration of TMC126 were too low to warrant further clinical development.
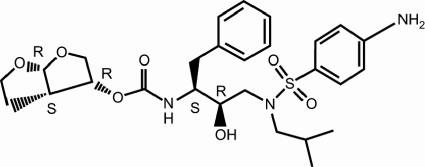
Further optimization of the series resulted in the selection of TMC114 (also described by Ghosh et al. as UIC-94017 [10]) (Fig. 1) due to its superior pharmacokinetics and antiviral profile in comparison with all the other compounds of the series.
FIG. 1.
Structure of TMC114.
The present study characterizes the antiviral activity (against both wild-type and PI-resistant HIV) and cytotoxicity of TMC114, as well as the high genetic barrier to resistance observed with the inhibitor. High-throughput cell-based screening assays allowed the assessment of activity against a very large panel of PI-resistant recombinant viruses derived from recent clinical samples. The results of this extensive screening suggest that TMC114 is a potential drug candidate for the treatment of both naïve and PI-experienced HIV patients.
MATERIALS AND METHODS
Compounds.
TMC114 and analogues were synthesized by the medicinal chemists at Tibotec (unpublished data). Currently approved PIs were purified from commercially available formulations.
Cells and viruses.
MT-4 cells are human lymphoblastoic T cells that are highly sensitive to HIV infection, producing a rapid and strong cytopathic effect (CPE). MT-4-LTR-EGFP (designated MT-4-LTR-EGFP) cells were obtained by transfection of MT-4 cells with a selectable construct encompassing the coding sequences for the HIV long terminal repeat (LTR) as a promoter for the expression of enhanced green fluorescent protein (EGFP) and subsequent selection of permanently transfected cells. Peripheral blood mononuclear cells (PBMCs) from HIV-negative donors for use in drug susceptibility assays were purified and stimulated with the mitogen phytohemagglutinin (1.5 μg/ml) in the presence of human interleukin 2 (20 U/ml) for 72 h, as previously described (8). Mature monocyte/macrophages (M/Ms) were separated out from freshly isolated PBMCs by adhesion as described by Perno et al. (24). All cells were cultured in RPMI 1640 medium supplemented with fetal calf serum and antibiotics in a humidified incubator with a 5% CO2 atmosphere at 37°C.
Virus stocks of HIV-1 and HIV-2 strains were produced in MT-4 cells, except for HIV-1 Ba-L, which was cultured in M/Ms.
Recombinant clinical isolates.
Recombinant viruses, derived from clinical samples, were constructed as previously described by cotransfection of MT-4 cells with sample derived viral protease (PR) and reverse transcriptase (RT) coding sequences and an HIV-1 HXB2-derived proviral clone deleted in the protease and RT coding region (13).
Site-directed mutants.
Mutant PR coding sequences were generated from a pGEM vector containing the HIV-1 LAI (clone HXB2) PR and RT coding sequence, using the QuikChange Site-Directed Mutagenesis kit (Stratagene) and high-performance liquid chromatography-purified primers (Genset Oligos). Genotyping of the HIV coding sequences in the plasmids upon site-directed mutagenesis confirmed the presence of the desired change(s). Mutant viruses were obtained by recombination of the mutant PR-RT sequence with a PR-RT-deleted HIV-1 HXB2 proviral clone (13). The presence of the desired mutation(s) in the recombinant viruses was confirmed by genotyping.
Time-of-addition assay.
For the time-of-addition assay, MT-4-LTR-EGFP cells were infected with HIV-1 LAI at a high multiplicity of infection (MOI) (>0.2 50% cell culture infectious doses [CCID50]/cell) and then centrifuged for 10 min at 1,200 × g. Two washing steps were carried out at 4°C to remove nonadsorbed virus and to synchronize the infection before cells were transferred to 37°C. From 30 min postinfection onwards, the compounds to be tested were added to parallel cultures at different time points. At 24 h postinfection, the cultures were scored microscopically for fluorescence, and supernatants were tested for p24 concentration.
Antiviral assays.
The antiviral activity of compounds against laboratory-adapted HIV strains and clinical sample-derived recombinant viruses was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay method as previously described (23). Briefly, various concentrations of the test compounds were added to wells of a flat-bottom microtiter plate. Subsequently, virus and MT-4 cells were added to a final concentration of 200 to 250 CCID50/well and 30,000 cells/well, respectively. Cytotoxicity of the test compound was determined in parallel using mock-infected cell cultures containing an identical compound concentration range but no virus. After 5 days of incubation at 37°C with 5% CO2, the CPE of the replicating virus was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method.
The reporter gene assay was similar to the CPE assay but used MT-4-LTR-EGFP cells. Upon infection by HIV-1, expression of the viral tat product increases transcription from the HIV-1 LTR promoter, leading to high-level expression of the reporter gene product. End reading of the assay was carried out after 3 days of incubation.
PBMC-based antiviral assays were carried out as previously described (8). Briefly, phytohemagglutinin-stimulated PBMCs from HIV-negative donors were incubated with serial dilutions of the test compounds and infected with virus at a multiplicity of infection (MOI) of 0.001 CCID50 per cell. Infected cells were washed and incubated in fresh medium containing the same concentration of drug every 3 to 4 days. New virus production was quantified after 7 to 11 days using a p24 enzyme-linked immunosorbent assay (NEN Life Sciences).
To test the compound's antiviral activity against HIV-1 Ba-L, adherent M/Ms were exposed to various concentrations of drugs for 1 h before infection with HIV-1 Ba-L (300 CCID50/ml) for 2 h. Cells were then washed and cultured for 14 days, with fresh medium containing the appropriate drug concentrations supplied every 5 days. At day 14 after infection, the production of infectious virus was quantified using a p24 enzyme-linked immunosorbent assay as described above (24).
The results of antiviral assays were expressed as an EC50 value, defined as the concentration of a compound achieving 50% inhibition of infection compared with the drug-free control or as an EC90 value defined as the dose achieving 90% inhibition of infection. In some cases, a fold change in susceptibility was calculated by dividing the EC50 for the tested virus by the EC50 for the wild-type virus (HIV-1 LAI) tested in parallel. Toxicity results were expressed as CC50, defined as the concentration of a compound that resulted in a 50% reduction in mock-infected cell viability compared to the drug-free control.
Genotyping and subtype determination.
Genotypic analysis was performed by automated population-based full-sequence analysis (ABI PRISM BigDye Terminator cycle sequencing). Sequencing results are reported as amino acid changes compared to the wild-type (HXB2) reference sequence (18). Subtypes were determined by heteroduplex mobility assay (7) or sequencing.
Antiviral assay in the presence of human serum and human serum proteins.
MT-4-LTR-EGFP cells were infected with HIV-1 LAI at a MOI of 0.001 to 0.01 CCID50/cell. Following 1 h of incubation, cells were washed and plated into a 96-well plate containing serial dilutions of compound in the presence of 10% fetal calf serum (FCS), 10% FCS plus 1 mg/ml α1-acid glycoprotein (AAG) or 50% human serum. After 5 to 6 days incubation, the EC50 was determined by a cell viability assay using resazurin (9). Briefly, 1/10 volume of resazurin solution [0.1 mg/ml resazurin, 1 mM K4Fe(CN)6, 1 mM K3Fe(CN)6 in 0.1 mM potassium phosphate buffer, pH 7.4] was added, and cultures were incubated at 37°C. After 5 or 25 h of incubation, the fluorescence of the formed resofurin was quantified.
In vitro selection of resistant strains.
MT-4-LTR-EGFP cells were infected at a MOI of 0.01 to 0.001 CCID50/cell in the presence of the inhibitor compound at a starting concentration that was two to three times the EC50. The cultures were subcultivated and scored microscopically on virus-induced fluorescence and cytopathicity every 3 to 4 days. Cultures were subcultivated in the presence of the same concentration of compound until full virus CPE was observed and subsequently at a higher compound concentration, to select for variants able to grow in the presence of the highest possible inhibitor concentration.
Combination with other antiretrovirals.
The antiviral activity of TMC114 in combination with current HIV inhibitors was determined against HIV-1 LAI in MT-4 cells at three different molar ratios: 3×, 1×, and 1/3× the estimated EC50 ratio of the compounds. A combination index (CI) at 50% protection was determined by using the classical isobologram model for combinations (11). In this model, a CI is calculated by the expression CI = d1/D1 + d2/D2, where d1 and d2 are the EC50s of drug 1 and drug 2 in the combination and D1 and D2 are the EC50s of each drug when used alone. Each combination was tested three times in separate experiments. A drug combination was scored synergistic when the mean CI was <0.8 and antagonistic when the mean CI was >1.2.
RESULTS
Activity of TMC114 against wild-type HIV-1 and HIV-2.
The in vitro antiviral activity of TMC114 against laboratory HIV strains was evaluated in acutely infected MT-4 cells, in PBMCs, and in M/Ms. The results are presented in Table 1. The EC50 of TMC114 against different HIV-1 laboratory strains ranged from 1 to 5 nM and the corresponding EC90 ranged from 2.7 to 12 nM. Moreover, TMC114 inhibited replication of HIV-2 ROD with a median EC50 of 4.2 nM and a median EC90 of 13 nM, which was comparable with the activity observed against HIV-1. The CC50 of TMC114 was found to be >100 μM (data not shown), from which the selectivity index (CC50/EC50) was calculated to be >20,000 for wild-type HIV.
TABLE 1.
Activity of TMC114 against wild-type HIVa
| Virus strain | Cell type | Assay | n | EC50 (nM)
|
EC90 (nM)
|
||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||||
| HIV-1 LAI | MT-4 | CPEb | 229 | 4.6 | 2.6-6.2 | 10 | 5.5-13 |
| HIV-1 LAI | MT-4 | RGAc | 685 | 3.7 | 2.9-4.3 | 8.0 | 5.8-11 |
| HIV-1 LAI | PBMC | p24d | 2 | 1.0 | 1.0-1.1 | 2.7 | 2.1-3.3 |
| HIV-1 SF2 | PBMC | p24d | 2 | 1.6 | 1.3-1.9 | 10 | 7.2-14 |
| HIV-1 BaL | M/M | p24d | 2 | 5.0 | 3.6-6.5 | 12 | 8.4-16 |
| HIV-2 ROD | MT-4 | CPEb | 5 | 4.2 | 3.6-5.2 | 13 | 11-13 |
PBMC, peripheral blood mononuclear cell; M/M, monocyte/macrophage; IQR, interquartile range, defined as the distance between the 25th and 75th percentiles; n, number of determinations.
CPE, inhibition of viral cytopathic effect.
RGA, inhibition of HIV LTR driven reporter gene expression, measured in a reporter gene assay.
p24, inhibition of p24 accumulation.
In addition, a panel of 32 recombinant strains derived from clinical isolates classified as group M clades A to H, group M circulating recombinant forms (CRF) CRF01_AE, CRF02_AG, CRF05_DF, and group O was assembled (M.-P. de Béthune, K. Hertogs, L. Hendrickx, J. Vingerhoets, K. Fransen, H. Azijn, L. Michiels, W. Janssens, A. Scholliers, B. Larder, S. Bloor, R. Pauwels, and G. van der Groen, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 446, 1999). The phenotypic susceptibility of these strains to TMC114 is given in Table 2. TMC114 was equally active against all of the isolates.
TABLE 2.
Activity of TMC114 against recombinant viruses of different subtypesa
| Subtype | n | EC50 (nM)
|
Fold change in EC50 relative to HIV-1 LAI
|
||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| CRF01_AE | 5 | 1.1 | 1.0-2.2 | 0.4 | 0.3-0.7 |
| CRF02_AG | 4 | 1.6 | 0.7-2.4 | 0.5 | 0.2-0.8 |
| B | 8 | 2.4 | 2.0-3.0 | 0.8 | 0.6-0.9 |
| C | 4 | 1.2 | 1.0-1.4 | 0.4 | 0.3-0.5 |
| D | 3 | 2.5 | 1.8-2.8 | 0.8 | 0.6-0.9 |
| CRF05_DF | 3 | 1.1 | 0.9-2.1 | 0.4 | 0.4-0.7 |
| F | 1 | 2.4 | NA | 0.8 | NA |
| H | 3 | 1.3 | 1.2-2.6 | 0.4 | 0.4-0.7 |
| O | 1 | 2.2 | NA | 0.8 | NA |
n, number of isolates; IQR, interquartile range, defined as the distance between the 25th and 75th percentiles; NA, not applicable.
Time-of-addition.
A time-of-addition experiment was carried out to identify the stage in the HIV life cycle at which TMC114 acts. This experiment revealed that effective inhibition of virus replication could be achieved by the addition of TMC114 up to 19 h after initial infection, a pattern similar to that seen with the PI saquinavir (data not shown).
Activity in the presence of human serum and human serum proteins.
The influence of human serum and AAG on the anti-HIV activity of TMC114 and currently approved PIs was assessed by determining the ratio between the EC50 observed in the presence of 10% FCS alone and the EC50 observed in the presence of 50% human serum or 10% FCS plus 1 mg/ml AAG (Table 3). With the exception of indinavir, all tested PIs showed a decrease in potency against wild-type HIV-1 in the presence of 50% human serum. The median EC50 of TMC114 was increased 20 fold. This was within the range of increases in EC50 seen for the other PIs (3 to 30 fold). Further characterization revealed that this shift in activity is mainly due to the binding of the inhibitors to AAG. The results in Table 3 show that generally higher shifts in EC50 were observed in the presence of AAG than in the presence of human serum, when antiviral activity was assessed against wild type virus, i.e., at EC50s of <50 nM. However, when virus strains with decreased susceptibility to TMC114 and other PIs, i.e., with EC50s ranging from 50 nM to >5 μM, were used, the ratios of EC50 in the presence of AAG to EC50 in the absence of AAG decreased in a manner inversely proportional to EC50 levels in the absence of proteins. At micromolar concentrations (0.5 to 5 μM), TMC114 and the other PIs showed only a <7-fold decrease in potency, pointing to a saturable binding of PIs to AAG.
TABLE 3.
Effect of human serum and AAG on the antiviral activity of TMC114 and approved protease inhibitorsa
| Protease inhibitor | Ratio of EC50 with 50% human serum/ EC50 with 10% FCS | Ratio of EC50 with AAG (1 mg/ml) plus 10% FCS/EC50 with 10% FCS at inhibitor EC50 range of:
|
||||
|---|---|---|---|---|---|---|
| >5 μM | 500 nM to 5 μM | 50-500 nM | 5-50 nM | <5 nM | ||
| TMC114 | 20 | 4 | 7 | 10 | 20 | 80 |
| IDV | 1* | 2 | 1 | 2 | ||
| RTV | 20 | 1 | 4 | 8 | 7 | |
| NFV | 30 | 5 | 9 | 10 | ||
| SQV | 3 | 2 | 3 | 4 | 5 | 20* |
| APV | 10 | 5 | 10 | 40* | ||
| LPV | 5 | 4* | 20 | 60 | ||
The effect of human serum on the antiviral activity of TMC114 and other protease inhibitors was assessed with the wild-type HIV-1/LAI strain. The effect of AAG on the antiviral activity of TMC114 and other protease inhibitors was assessed with the wild-type HIV-1/LAI strain and PI-resistant strains with different levels of resistance to the different PIs. The results were clustered in the indicated concentration ranges according to the EC50 values obtained for the various inhibitors on the different strains, in the absence of human serum proteins. FCS, fetal calf serum; AAG, α1-acid glycoprotein; IDV, indinavir; RTV, ritonavir; NFV, nelfinavir; SQV, saquinavir; APV, amprenavir; LPV, lopinavir. Median values of at least three determinations are presented, except for values labeled with * (two determinations).
Activity against PI-resistant viruses.
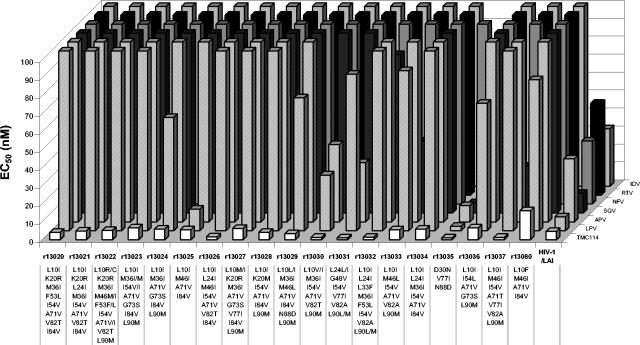
For the purpose of lead optimization, a panel of 19 recombinant clinical isolates was assembled to evaluate the newly synthesized compounds. This panel encompassed viruses resistant to an average of 5 PIs, defined as a fold change in EC50 of ≥4. More than 1,000 compounds were tested during the lead optimization process. With the exception of one strain (r13080), TMC114 showed EC50s of <10 nM for all isolates of the lead optimization panel, as illustrated in Fig. 2, whereas EC50s of >100 nM were shown by nelfinavir (NFV) for all 19 isolates, by indinavir and ritonavir for 18 strains, by amprenavir (APV) for 16 strains, by saquinavir for 15 strains, and by lopinavir (LPV) for 10 strains, respectively.
FIG. 2.
Activity of TMC114 and currently approved protease inhibitors against PI-resistant viruses. Differences from the subtype B consensus at amino acid positions implicated in PI resistance according to the International AIDS Society-USA drug resistance mutation figures are presented (14). IDV, indinavir; RTV, ritonavir; NFV, nelfinavir; SQV, saquinavir; APV, amprenavir; LPV, lopinavir. Median values of at least three determinations are presented, except for RTV and strains r13022, r13023, r13026, r13032, and r13036; for SQV and strain r13032; and for LPV and strains r130230 to r13024, r13026 to r13033, and r13035 to r13037, where only two determinations were available.
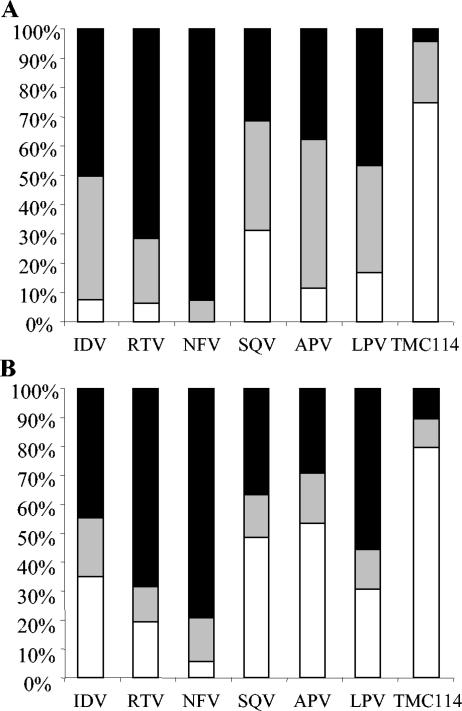
To further evaluate the antiviral activity of TMC114 against the virus populations currently present in patients, drug sensitivity assays were performed on a broad, random selection of recombinant clinical isolates (Fig. 3). Among the 4,024 samples analyzed, 1,666 (41%) exhibited a fourfold or greater change in EC50 for one or more PIs. Phenotypic data were available for TMC114 and all six first approved PIs from 1,501 of these 1,666 samples; these were used for further analysis. Among the 1,501 PI-resistant samples, 75% could be inhibited by TMC114 with an EC50 of <10 nM, while 21% showed an EC50 between 10 and 100 nM, and only 4% showed an EC50 of >100 nM (Fig. 3A). Analysis of fold changes in EC50 showed a similar pattern of results, with 80% of samples yielding a <4-fold change in EC50 to TMC114 and only 10% with >10-fold change in EC50 to TMC114. In contrast, 29% of samples had a >10-fold reduction in susceptibility to amprenavir (Fig. 3B). TMC114 was more potent against these PI-resistant samples than any of the other tested PIs, and this confirms the results obtained by testing TMC114 against the selected panel of PI-resistant strains (Fig. 2).
FIG. 3.
Antiviral activity of different protease inhibitors tested against a panel of 1,501 recent recombinant clinical isolates resistant to at least one protease inhibitor. (A) EC50; black shading, EC50 > 100 nM; grey shading, EC50 < 100 nM > 10 nM; white, EC50 < 10 nM. (B) Fold change in EC50 compared to the wild type; black shading, fold change > 10; grey shading, fold change < 10 > 4; white, fold change < 4.
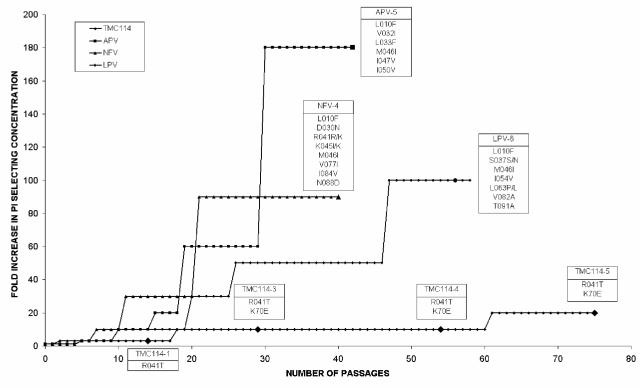
In vitro selection experiments starting from wild-type HIV-1.
In vitro selection experiments were performed with nelfinavir, amprenavir, lopinavir, or TMC114, starting with wild-type HIV-1 LAI. Selection of resistant HIV with the current PIs was easy and resulted in the emergence of strains carrying PI resistance-associated mutations (Fig. 4). Those mutations are also among the ones observed in the clinic in patients treated with these PIs. The concentrations of the current PIs could readily be increased to >1 μM, still allowing for virus replication. On the contrary, TMC114 concentration could not be increased rapidly, and it was not possible to observe virus replication at concentrations above 200 nM. This type of experiment was conducted three times with the HIV-1 LAI strain and once with the HIV-1 NL4-3 strain; results were very comparable, i.e., the concentration of TMC114 could not be increased above 200 nM even after prolonged exposure. Results of a typical experiment are presented here (Fig. 4). In the presence of 100 nM TMC114, virus strains harboring the R41T and K70E mutations were isolated. However, it was difficult to grow infectious stocks of these selected viruses in MT-4 cells, even in the absence of the inhibitor, which made us conclude that these virus strains replicated poorly. One of the viruses isolated at 100 nM (TMC114-4) showed a 10-fold reduction in susceptibility to TMC114 (Table 4). When this virus was tested against the current PIs (except for atazanavir), this strain showed reductions in susceptibility of <10 against all the current PIs except saquinavir. Site-directed mutagenesis was used to construct HIV-1 HXB2 viruses containing the R41T and K70E substitutions individually and in combination. These viruses showed no reduction in susceptibility to either TMC114 or the other PIs tested (Table 4). Further research is ongoing to explain the decreased sensitivity to TMC114 of the in vitro-selected viruses.
FIG. 4.
In vitro selection of resistant HIV in the presence of nelfinavir, amprenavir, lopinavir, or TMC114. Selection curves have been normalized, and starting selection concentrations were 10, 20, 100, and 100 nM for TMC114, lopinavir, amprenavir, and nelfinavir, respectively. Each passage corresponds to 3 to 4 days. Passage 75 corresponds to 260 days. Genotypes of virus strains selected at defined time points (indicated by enlarged symbols) list all changes from the starting strain, HIV-1 LAI.
TABLE 4.
Characterization of strains selected from HIV-1 LAI in the presence of TMC114 and comparison with site-directed mutantsa
| Virus strain | Virus selection condition(s)
|
Protease mutation(s) | Median EC50 (nM) (median fold change in EC50 relative to HIV-1 LAI) for phenotype
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concn | No. of days (passage no.) | TMC114 | IDV | RTV | NFV | SQV | APV | LPV | ||
| HIV-1 LAI | NA | NA | NA | 3.2 (1) | 28 (1) | 31 (1) | 30 (1) | 7.8 (1) | 36 (1) | .9 (1) |
| TMC114-1 | 30 | 45 (14) | R41T | 7.7 (2) | 33 (1) | 32 (1) | 32 (1) | 30* (4) | 38 (1) | 27 (3) |
| TMC114-3 | 100 | 97 (29) | R41T, K70E | 26 (8) | 98 (4) | 21 (1) | 18 (1) | 35 (4) | 29 (1) | 32 (4) |
| TMC114-4 | 100 | 188 (54) | R41T, K70E | 44 (10) | 140* (5) | 46* (1) | 37* (1) | 150 (20) | 39 (1) | 47 (6) |
| TMC114-5c | 200 | 260 (75) | R41T, K70E | NT | NT | NT | NT | NT | NT | NT |
| HIV-1 LAI | NA | NA | NA | 3.2 (1) | 18 (1) | 20 (1) | 21 (1) | 7.1 (1) | 21 (1) | 5.8 (1) |
| SDM020007b | K70E | 2.5 (0.8) | 13 (0.7) | 22 (1) | 19 (0.9) | 3.4 (0.5) | 25 (1) | 4.6 (0.8) | ||
| SDM020006b | R41T | 0.9 (0.3) | 4.6 (0.3) | 8.5 (0.4) | 6.3 (0.3) | 3.2 (0.5) | 13 (0.6) | 2.6 (0.4) | ||
| SDM020039b | R41T, K70E | 0.6 (0.2) | 3.5 (0.2) | 6.7 (0.3) | 6.9 (0.3) | 1.3 (0.2) | 9.0 (0.4) | 2.3 (0.4) | ||
PI, protease inhibitor; IDV, indinavir; RTV, ritonavir; NFV, nelfinavir; SQV, saquinavir; APV, amprenavir; LPV, lopinavir; NT, not tested; NA, not applicable. Median values of at least three median EC50 determinations are presented, except for values labeled with * (two determinations).
The phenotypic susceptibility of site-directed mutants to TMC114 and current PIs was characterized in a separate experiment.
Due to the low replication rate of strain TMC114-5, it was not possible to further characterize this strain phenotypically
Activity in combination with current HIV inhibitors.
Combinations of TMC114 with current HIV inhibitors were studied by an anti-HIV-1 LAI assay, and the results were evaluated by the classical isobologram approach. Within the range of tested concentrations, there was no evidence of antagonism (CI > 1.2) between TMC114 and any of the nucleoside reverse transcriptase inhibitors (abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine, and tenofovir), nonnucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, and nevirapine), PIs (indinavir, ritonavir, nelfinavir, saquinavir, amprenavir, lopinavir, and atazanavir) or the fusion inhibitor T-20. Some evidence of synergy (CI < 0.8) was observed between TMC114 and the PIs ritonavir (CI = 0.66 to 0.81), nelfinavir (CI = 0.61 to 0.80), and amprenavir (CI = 0.65 to 0.77).
DISCUSSION
We identified a new PI, TMC114, that is highly active against both wild-type and PI-resistant HIV and appears to have a very high genetic barrier to the development of resistance. The EC50 of TMC114 against both HIV-1 and HIV-2 is in the low nanomolar range and compares favorably with EC50s usually observed for the most active of the currently available PIs, saquinavir (5). No cellular toxicity was observed at the highest concentrations of TMC114 used during this study (100 μM), indicating a selectivity index of >20,000.
Previous studies have demonstrated the strong binding of TMC114 to the wild-type HIV-1 protease (15, 16, 28). The binding affinity of TMC114 to wild type HIV-1 protease was very high (Kd = 0.0045 nM) and approximately 1,000 times higher than the Kd of indinavir, nelfinavir, and saquinavir. Moreover, although the structure of TMC114 is related to amprenavir, the Kd for amprenavir was much higher (0.39 nM) (15). In the present study, it was demonstrated that TMC114 acted at the same stage of replication as saquinavir in a time-of-addition experiment. This is in line with the inhibition of the viral protease as the mechanism of action of TMC114.
Binding of PIs to human serum proteins is known to inhibit their entry into cells and to reduce their activity in vivo (3, 4). The increase in EC50 seen with TMC114 in the presence of human serum was within the range seen for other PIs. This effect of human serum on the anti-HIV activity of TMC114 has been associated with a saturable binding to AAG, as was shown by assessing the EC50s of the different inhibitors against wild-type HIV-1 in the presence of 1 mg/ml AAG. It has been previously shown that the binding of protease inhibitors to AAG is a saturable reaction (W. Cao, H. Azijn, M.-P. de Béthune, S. Gulnik, J. Erickson, and D. Xie, Abstr., 5th Int. Workshop on HIV Drug Resist. Treat. Strat., abstr. 83, 2001) and hence that the influence of AAG in vivo may be overestimated when measured in vitro at low concentrations (wild-type EC50) where the free and active fraction is low. To investigate this and to assess whether the same magnitude of effect is to be expected at clinically relevant concentrations, i.e., micromolar concentrations of the inhibitors, resistant viruses with higher EC50s for the different PIs were used. The data shown in Table 3 demonstrated that the decrease in potency observed in the presence of AAG for the tested PIs is inversely proportional to the EC50 in the absence of protein. This indicates that the free fraction of the inhibitor is expected to be higher at in vivo micromolar plasma concentrations. Hence, at physiologically achieved, low-micromolar plasma drug concentrations, binding to AAG might be a far less relevant factor to make extrapolations from in vitro findings to the in vivo situation.
Though HIV-1 group M subtype B currently predominates in Europe and the United States, recent studies indicate that the prevalence of non-B subtypes and circulating recombinant forms may soon reach significant levels, particularly in immigrants and heterosexually infected individuals (1, 25). TMC114 showed comparable potent activity against a range of clinically derived recombinant viruses representing HIV-1 group M subtypes A through H, including several circulating recombinant forms, and HIV-1 group O.
A major challenge faced by clinicians today is overcoming the increasing levels of resistance and cross-resistance that develop at each successive therapeutic failure. The extensive cross-resistance that exists between currently approved PIs limits the sequential use of these agents. We have assessed the potential activity of TMC114 against a very large range of PI-resistant viruses. TMC114 showed good activity against a selected panel of 19 recombinant clinical isolates carrying multiple protease mutations and demonstrating resistance to an average of five other PIs. In all cases, the change in TMC114 EC50 compared to wild-type virus was less than fourfold. High-throughput cell-based screening assays allowed the testing of TMC114 against a panel of 1,501 PI-resistant recombinant viruses derived from recent clinical samples. In contrast with the current PIs, TMC114 showed a high level of activity against this panel of resistant viruses, with EC50s of <10 nM for 75% of the samples.
Isothermal titration calorimetry experiments have shown a very tight binding of TMC114 to HIV-1 protease as evidenced by an extremely low Kd of 0.0045 nM (15). In addition, X-ray crystallography of crystals of TMC114 bound to the wild-type enzyme indicated that it occupies a volume that is contained within the substrate envelope, unlike most of the currently approved PIs, which extend beyond the envelope (15). Crystallographic analysis reported by Koh et al. (16), as well as by King et al. (15), further suggest that TMC114 forms strong hydrogen bonds with residues in the main chains of the protease active site (Asp-29 and Asp-30). These observations may serve to explain the high potency of TMC114 against viruses resistant to other PIs as well as the difficulty in selecting viruses with reduced susceptibility to TMC114.
In a standardized protocol, in vitro selection of resistant HIV was much slower with TMC114 than with amprenavir, nelfinavir, or lopinavir. While virus capable of replicating in micromolar levels of APV, NFV, or LPV was readily selected, the selection concentration of TMC114 could not be raised above 200 nM, even after prolonged exposure to this concentration, enabling for enrichment in minor variants of the virus population. Moreover, it was difficult to grow infectious stocks of the viruses isolated in the presence of 100 nM TMC114, even in the absence of the inhibitor. This observation led us to conclude that these virus strains have a lower replication rate, compared to wild type and other strains resistant to the current PIs. Experiments are ongoing to further quantify the replication rate of those viruses. Viruses isolated in the presence of 100 nM TMC114 showed only a 10-fold change in susceptibility to the inhibitor. Little or no cross-resistance to current PIs, except to SQV, was observed with these viruses. These results suggest an increased genetic barrier to the development of resistance to TMC114. Good correlation was found between the in vitro and in vivo selected mutations for APV, NFV and LPV (88). In sequential passage experiments with TMC114 starting from the wild type, only two mutations (R41T and K70E), both far from the active site, were selected. These mutations had no effect on susceptibility to TMC114 or other PIs when they were introduced into a laboratory strain by site-directed mutagenesis. Moreover, these mutations have so far not been associated with resistance to PIs (14). This suggests that regions beyond the protease, potentially in the gag cleavage sites, may play a key role in determining susceptibility to TMC114. Alternatively, population sequencing did not allow the identification of minor variants in the selected virus populations, which might help explain the decreased susceptibility to TMC114. Further sequencing to explain the decreased sensitivity to TMC114 of these in vitro selected viruses is ongoing.
Combination therapy is the current standard of care for antiretroviral therapy. Since combinations of antiretroviral agents may be synergistic, additive, or antagonistic, it is important to test developmental compounds in combination with all the currently prescribed antiretrovirals. Within the range of tested concentrations, there was no evidence of antagonism between TMC114 and any of the antiviral agents tested, and combinations with ritonavir, nelfinavir, and amprenavir showed some evidence of synergy. Other groups have also observed apparent synergy between some PIs, for example, lopinavir and saquinavir (20) and atazanavir and saquinavir (26), while Merrill et al. (19) and Patick et al. (22) reported antagonism between indinavir and saquinavir and indinavir and nelfinavir, respectively. Since all these PIs are competitive inhibitors of HIV protease, an enzyme with only one single active site, the mechanisms for such interactions is unknown. It is possible that interactions affecting cellular penetration play a role (20).
The excellent activity of TMC114 against PI-resistant HIV indicates that this new antiretroviral could be particularly useful in treating patients failing a PI-containing regimen. This potential has been confirmed in the clinical study TMC114-C207, where multiple PI-experienced subjects who were currently failing a PI-containing regimen had their PI(s) replaced by TMC114 boosted with low-dose ritonavir (TMC114/r). Treatment with TMC114/r for 14 days was associated with a maximum and median drop in viral load of −2.49 and −1.35 log10 copies/ml, respectively (K. Arasteh, N. Clumeck, A. Pozniak, H. Jaeger, M. De Pauw, H. Muller, M. Peeters, R. Hoetelmans, S. De Meyer, I van der Sandt, S. Comhaire, and R. van der Geest, Abstr. 9th Conf. Retrovir. Opport. Infect., abstr. 4, 2002).
Overall, these results suggest that TMC114 possesses at least three distinct advantages over currently approved PIs. (i) It has extremely potent antiretroviral activity. (ii) It is able to maintain this activity against HIV variants that are highly cross-resistant to current PIs. (iii) It appears to have a very high genetic barrier to the development of resistance, possibly due to its tight binding to the HIV-1 protease through extra hydrogen bonds with the main-chain atoms of the enzyme. We conclude that TMC114 may be a potential drug candidate for the treatment of both naïve and PI-experienced patients with HIV.
Acknowledgments
We thank Carlo-Federico Perno for carrying out the monocyte/macrophage drug susceptibility assays and Liesbet Smeulders, Rudy Strijbos, and Els Fransen for skillful technical assistance.
REFERENCES
- 1.Balotta, C., G. Facchi, M. Violin, S. Van Dooren, A. Cozzi-Lepri, F. Forbici, A. Bertoli, C. Riva, D. Senese, P. Caramello, G. Carnevale, G. Rizzardini, L. Cremonini, L. Monno, G. Rezza, C. F. Perno, G. Ippolito, A. d'Arminio-Monforte, A. M. Vandamme, and M. Moroni. 2001. Increasing prevalence of non-clade B HIV-1 strains in heterosexual men and women, as monitored by analysis of reverse transcriptase and protease sequences. J Acquir. Immune Defic. Syndr. 27:499-505. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. A., R. DeMasi, J. Quinn, C. Moxham, and F. Rousseau. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS 15:1369-1377. [DOI] [PubMed] [Google Scholar]
- 3.Bilello, J. A., P. A. Bilello, K. Stellrecht, J. Leonard, D. W. Norbeck, D. J. Kempf, T. Robins, and G. L. Drusano. 1996. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilello, J. A., and G. L. Drusano. 1996. Relevance of plasma protein binding to antiviral activity and clinical efficacy of inhibitors of human immunodeficiency virus protease. J. Infect. Dis. 173:1524-1526. [DOI] [PubMed] [Google Scholar]
- 5.Craig, J. C., I. B. Duncan, D. Hockley, C. Grief, N. A. Roberts, and J. S. Mills. 1991. Antiviral properties of Ro 31-8959, an inhibitor of human immunodeficiency virus (HIV) proteinase. Antiviral Res. 16:295-305. [DOI] [PubMed] [Google Scholar]
- 6.d'Arminio, M. A., A. C. Lepri, G. Rezza, P. Pezzotti, A. Antinori, A. N. Phillips, G. Angarano, V. Colangeli, A. De Luca, G. Ippolito, L. Caggese, F. Soscia, G. Filice, F. Gritti, P. Narciso, U. Tirelli, M. Moroni, et al. 2000. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS 14:499-507. [DOI] [PubMed] [Google Scholar]
- 7.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rubsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262:1257-1261. [DOI] [PubMed] [Google Scholar]
- 8.Division of AIDS, National Institute of Allergy and Infectious Diseases. 1997. DAIDS Virology Manual for HIV Laboratories. Publication NIH-97-3828. U.S. Department of Health and Human Services, Washington, D.C.
- 9.Fields, R. D., and M. V. Lancaster. 1993. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 11:48-50. [PubMed] [Google Scholar]
- 10.Ghosh, A. K., J. F. Kincaid, W. Cho, D. E. Walters, K. Krishnan, K. A. Hussain, Y. Koo, H. Cho, C. Rudall, L. Holland, and J. Buthod. 1998. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg. Med. Chem. Lett. 8:687-690. [DOI] [PubMed] [Google Scholar]
- 11.Greco, W. R., G. Bravo, J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 12.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, A. Meibohm, J. H. Condra, F. T. Valentine, D. McMahon, C. Gonzalez, L. Jonas, E. A. Emini, J. A. Chodakewitz, R. Isaacs, and D. D. Richman. 2000. 3-Year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann. Intern. Med. 133:35-39. [DOI] [PubMed] [Google Scholar]
- 13.Hertogs, K., M. P. de Béthune, V. Miller, T. Ivens, P. Schel, A. Van Cauwenberge, C. Van Den Eynde, V. Van Gerwen, H. Azijn, M. Van Houtte, F. Peeters, S. Staszewski, M. Conant, S. Bloor, S. Kemp, B. Larder, and R. Pauwels. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transciptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob. Agents Chemother. 42:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, V. A., F. Brun-Vézinet, B. Clotet, B. Conway, R. D'Aquila, L. Demeter, D. Kuritzkes, D. Pillay, J. Shapiro, A. Telenti, and D. Richman. 2004. Update of the drug resistance mutations in HIV-1: 2004. Top. HIV Med. 12:119-124. [PubMed] [Google Scholar]
- 15.King, N., M. Prabu-Jeyalaban, P. Wigerinck, M.-P. de Béthune, and C. Schiffer. 2004. The structural and thermodynamic basis for the binding of TMC114, a next-generation HIV-1 protease inhibitor. J. Virol. 78:12012-12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh, Y., H. Nakata, K. Maeda, H. Ogata, G. Bilcer, T. Devasamudram, J. F. Kincaid, P. Boross, Y. F. Wang, Y. Tie, P. Volarath, L. Gaddis, R. W. Harrison, I. T. Weber, A. K. Ghosh, and H. Mitsuya. 2003. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 47:3123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohl, N. E., E. A. Emini, W. A. Schleif, L. J. Davis, J. C. Heimbach, R. A. Dixon, E. M. Scolnick, and I. S. Sigal. 1988. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. USA 85:4686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larder, B. A., A. Kohli, P. Kellam, S. D. Kemp, M. Kronick, and R. D. Henfrey. 1993. Quantitative detection of HIV-1 drug resistance mutations by automated DNA sequencing. Nature 365:671-673. [DOI] [PubMed] [Google Scholar]
- 19.Merrill, D. P., D. J. Manion, T. C. Chou, and M. S. Hirsch. 1997. Antagonism between human immunodeficiency virus type 1 protease inhibitors indinavir and saquinavir in vitro. J. Infect. Dis. 176:265-268. [DOI] [PubMed] [Google Scholar]
- 20.Molla, A., H. Mo, S. Vasavanonda, L. Han, C. T. Lin, A. Hsu, and D. J. Kempf. 2002. In vitro antiviral interaction of lopinavir with other protease inhibitors. Antimicrob. Agents Chemother. 46:2249-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 22.Patick, A. K., T. J. Boritzki, and L. A. Bloom. 1997. Activities of the human immunodeficiency virus type 1 (HIV-1) protease inhibitor nelfinavir mesylate in combination with reverse transcriptase and protease inhibitors against acute HIV-1 infection in vitro. Antimicrob. Agents Chemother. 41:2159-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 24.Perno, C. F., and R. Yarchoan. 1993. Culture of HIV in monocytes and macrophages, p. 12.4.1-12.4.11. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, vol 3. John Wiley & Sons, New York, N.Y.
- 25.Perrin, L., L. Kaiser, and S. Yerly. 2003. Travel and the spread of HIV-1 genetic variants. Lancet Infect. Dis. 3:22-27. [DOI] [PubMed] [Google Scholar]
- 26.Robinson, B. S., K. A. Riccardi, Y. F. Gong, Q. Guo, D. A. Stock, W. S. Blair, B. J. Terry, C. A. Deminie, F. Djang, R. J. Colonno, and P. F. Lin. 2000. BMS-232632, a highly potent human immunodeficiency virus protease inhibitor that can be used in combination with other available antiretroviral agents. Antimicrob. Agents Chemother. 44:2093-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanstrom, R., and J. Erona. 2000. Human immunodeficiency virus type-1 protease inhibitors: therapeutic successes and failures, suppression and resistance. Pharmacol. Ther. 86:145-170. [DOI] [PubMed] [Google Scholar]
- 28.Tie, Y., P. Boross, Y. Wang, L. Gaddis, A. Hussain, S. Leshchenko, A. Ghosh, J. Louis, R. Harrison, and I. Weber. 2004. High resolution crystal structures of HIV-1 protease with a potent non-peptide inhibitor (UIC-94017) active against multi-drug resistant clinical strains. J. Mol. Biol. 338:341-352. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura, K., R. Kato, M. F. Kavlick, A. Nguyen, V. Maroun, K. Maeda, K. A. Hussain, A. K. Ghosh, S. V. Gulnik, J. W. Erickson, and H. Mitsuya. 2002. A potent human immunodeficiency virus type 1 protease inhibitor, UIC-94003 (TMC126), and selection of a novel (A28S) mutation in the protease active site. J. Virol. 76:1349-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]