Abstract
Neutralizing antiviral antibodies are typically detected on the basis of inhibition of viral function, such as propagation of a viral infection or inhibition of viral gene expression. Evidence is presented that anti-adenovirus neutralizing antibodies can be evaluated by analysis of cell-associated capsids or by analysis of intracellular trafficking of the capsids within 1 h after infection. Quantitative analyses of these morphologic parameters represent rapid, broadly applicable, functional assays for the detection of anti-adenovirus neutralizing antibodies.
Neutralizing antibodies are one of the primary determinants governing the efficacy of adenovirus (Ad)-mediated gene transfer in vivo (12, 22). Historically, anti-Ad neutralizing antibodies have been characterized through their ability to inhibit the propagation of a viral infection (11). Given the high efficiency with which subgroup C adenovirus vectors bind to cells and traffic to the nucleus (8), we hypothesized that the number of Ad particles bound to cells would be directly proportional to transgene expression, allowing the development of assays based strictly on quantitative morphological criteria. To test this hypothesis, digital image analysis was used to measure the inhibition of cell association and intracellular trafficking by fluorophore-conjugated virions in the presence of anti-Ad neutralizing serum. A549 lung epithelial cells were infected briefly with a high concentration of fluorophore-conjugated Ad in the presence of a serial dilution of human sera. Quantitative digital image analysis of total-cell-associated vector and the percentage of vector colocalized with nuclei were performed and compared with titers of sera determined using an assay of transgene expression. Both morphometric assays provided quantita- tive data characterizing the neutralizing titer of four human sera. A comparision of morphological and gene expression assays demonstrated that morphological criteria of Ad infection can predict the neutralizing titer of human sera with accuracy equal to that of assays that rely on gene expression. The data also confirm that the number of Ad particles that enter the cell and traffic to the nucleus correlates with the infectious titer of the virus.
(A preliminary description of these observations was previously reported as part of a Master's thesis [18].)
MATERIALS AND METHODS
Cells, Ad vectors, and human neutralizing sera.
A549 lung epithelial carcinoma cells (American Type Culture Collection, Rockville, Md) were infected with replication-deficient, recombinant Ad gene transfer vectors (E1−, E3−) with an expression cassette inserted in the E1 position. Ad vectors were propagated and maintained as previously described (16, 17). The expression cassette included a promoter-enhancer from cytomegalovirus, the β-galactosidase cDNA, and a simian virus 40 poly(A) termination site (7). Human neutralizing sera were obtained in the course of gene therapy clinical trials that were approved by the Food and Drug Administration and the local Institutional Review Board (Rockefeller University and/or the Weill Medical College of Cornell University) and reviewed by the National Institutes of Health Recombinant DNA Advisory Committee (6). Sera were previously assayed for neutralizing anti-Ad titer by using propagation of a wild-type Ad5 infection in a monolayer culture of A549 cells (6).
Western blot analysis.
Ad capsid proteins (5 × 1010 wild-type Ad5 particles/lane) were denatured for 10 min at 95°C in Laemmli sample buffer containing 6 M urea, separated on a 4 to 20% polyacrylamide gradient gel, transferred to nitrocellulose, and probed with human sera (1:1,000 dilution). Anti-Ad antibodies were detected using horseradish peroxidase-conjugated anti-human antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) with chemiluminescent evaluation (ECL detection kit; Amersham/Pharmacia, Little Chalfont, England).
Morphological assays for evaluating the neutralizing antibody content of sera.
The morphological titer determination assay was performed using a modification of a previously described infection protocol (8, 14). Cy3 fluorescent dye conjugated to Ad (Cy3-Ad) (1011 particles/ml) was added to cell cultures in the presence of serial dilutions of neutralizing sera (dilutions from 1:3 to 1:104). The serum dilutions were mixed with Cy3-Ad for 5 min at 23°C, with the balance of the volume made up by binding buffer (modified Eagle medium [Life Technologies, Gaithersburg, Md.], 1% bovine serum albumin [Sigma, St. Louis, Mo.], 10mM HEPES [pH 7.3] [Sigma]). A 30-μl volume of the serum-Ad mixture was added to 104 cells in the well of a 35-mm coverslip dish (8). After 10 min, unbound Ad was washed away (three washes each with 2 ml of binding buffer) and the culture was incubated in binding buffer for an additional 60 min to permit trafficking of Ad within the cell. After 60 min, the cells were washed (three washes, each with 2 ml of phosphate-buffered saline, fixed with 2 ml of 4% paraformaldehyde in phosphate-buffered saline for 20 min at 23°C, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, Oreg.) to identify the positions of nuclei within cells. The cells were viewed with epifluorescence illumination from a 100-W Hg arc using a 60× N.A. 1.4 PlanApo objective lens, and images were captured and analyzed using a cooled charge-coupled device camera (Princeton Instruments, Inc., Trenton, N.J.) with imaging software (Universal Imaging, West Chester, Pa.). Five fields per slide were analyzed. Determination of total cell-associated fluorescence and percent nuclear localization have been described previously (8). Briefly, following background subtraction, a uniform threshold was applied to images of Cy3-Ad-infected cells to mark Cy3 fluorescence, software was used to integrate the gray value of pixels within the threshold area, and the total gray value was divided by the number cells in the image identified by nuclear staining with DAPI. The percent nuclear localization was based on the total cell-associated fluorescence, but it incorporated an additional step using the image of nuclei to mark and digitally subtract areas of the Cy3-Ad image that coincided with nuclei. A comparision of the total cell-associated fluorescence with and without subtraction of fluorescence in nuclear areas provided the percent nuclear localization of Cy3-Ad.
β-Galactosidase assay.
β-Galactosidase transgene expression was evaluated in cell lysates 24 h following infection by the method described above. Preparation of cell lysates and evaluation of β-galactosidase expression were done using quantitative chemiluminescent detection (Tropix, Bedford, Mass.). The protein concentration of the lysate was evaluated using the bicinchoninic acid reagent (Bio-Rad, Hercules, Calif.).
RESULTS AND DISCUSSION
The morphometric assays of Ad infectivity used a novel viral infection procedure (8). Cells were treated with a high concentration of viral capsids (1011 particles/ml; 3 × 106 particles per cell) to saturate cell surface Ad receptors. After 10 min, unbound virions were removed by washing. Bound virions were permitted to traffic as a wave through the cell for an additional 60 min before fixation. Prior to infection, the vectors were conjugated with Cy3, a red carbocyanine fluorophore, to permit the detection of Ad without postfixation labeling proce- dures. The fluorophore-conjugated virus (Cy3-Ad) faithfully recapitulates each step of the viral infection pathway (8, 9, 14). Human sera containing different levels of anti-Ad neutralizing antibodies were characterized by a viral replication assay (plaque assay) (6), as well as by Western blot analysis against viral capsid proteins (Fig. 1). By the plaque assay, the four sera analyzed had anti-Ad neutralizing antibody titers of <10 (nonneutralizing), 640, 2,560, and 49,000. A Western blot analysis using four human sera confirmed that anti-Ad antibodies could be detected in all four sera, although one of the sera had no neutralizing titer (Fig. 1). Overall, the amount of anti-Ad antibody in the sera correlated with the amount of neutralizing antibody, similar to results of previous studies (1).
FIG. 1.
Western blot of human sera containing anti-Ad antibodies. Sera were collected from patients enrolled in clinical Ad gene transfer protocols. Following heat inactivation of complement, anti-Ad neutralizing titers were assessed using a plaque assay (6). Ad proteins were separated on sodium dodecyl sulfate–10% polyacrylamide gels, transferred to nitrocellulose, and probed with 1,000-fold dilutions of serum. Bound antibodies were detected with peroxidase-conjugated anti-human antisera and a peroxidase-based chemiluminescence assay. All sera contained anti-Ad antibodies as assessed by Western analysis.
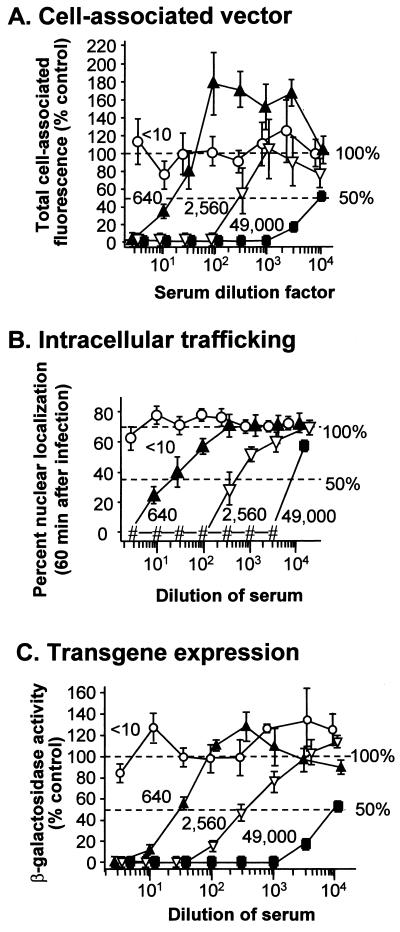
The morphological assays were based on fluorescence microscopy analysis of cell cultures 60 min after infection. At high concentrations of serum containing anti-Ad neutralizing antibodies, no virus was able to bind to cells, whereas the highest concentration of nonneutralizing serum (titer, <10) had no effect on viral binding or trafficking to the nucleus 60 min after infection (Fig. 2A). As the neutralizing serum was diluted, an increasing association of Cy3-Ad with cells was observed. The number of dilutions required to achieve cell association correlated with the amounts of anti-Ad neutralizing antibodies determined by the plaque assay. As the neutralizing sera were diluted, cell association was initially observed without subsequent trafficking to the nucleus (Fig. 2). With greater dilution, Cy3-Ad was observed in association with the nucleus at 60 min. The intermediate cell-associated, nonnuclear localization of Ad was less apparent in the serum with the highest neutralizing titer (titer, 49,000). The data clearly indicated that extracellular neutralization was the primary form of neutralization at serum concentrations approaching that found in vivo.
FIG. 2.
Morphological titer determination assay. A549 cells were infected with Cy3-Ad5 (1011 particles/ml) for 10 min at 37°C in the presence of human sera at different dilutions (1:3 to 1:104). After the 10-min infection, the cells were washed to remove unbound virus, and cell-associated virus was permitted to traffic in cells for 60 min. Fixed cells were counterstained with DAPI to show the position of the nucleus. (A) Representative micrographs showing cell nuclei (blue) and cell-associated Cy3-Ad (red). Bar, 10 μm. (B) Qualitative assessment of micrographs. For each con- dition, Cy3-Ad infection was scored 60 min after infection as either non-cell-associated (indicating extracellular neutralization), cell-associated but nonnuclear (indicating intracellular neutralization), or nuclear (indicating efficient trafficking of Ad to the nucleus). The neutralizing titer of each serum sample is listed on the corresponding curves. Sera evaluated had neutralizing titers of <10, 640, 2,560, and 49,000.
The qualitative observations in the morphological assay were quantitatively analyzed using digital image analysis. Both total cell-associated fluorescence and percent nucleus-localized fluorescence were quantified. Total cell-associated fluorescence was evaluated using digital image analysis to integrate the gray level (a measure of fluorescence intensity) in single optical planes of images containing 4 to 13 cells per field (8). To adjust for the variation between the number of cells per field, the total fluorescence intensity of the field was divided by the number of nuclei in the field determined by staining with the DNA-binding dye, DAPI. Data were compared to the results of control experiment in which no serum was added during infection. The cell-associated fluorescence in the presence of nonneutralizing serum (titer, <10) was comparable to control values at all dilutions tested, while sera containing neutralizing titers of anti-Ad antibodies (titers, 640, 2,560, and 49,000) inhibited Cy3-Ad binding completely at the highest concentrations, and with dilution, a steadily increasing association of Cy3-Ad with cells was observed (Fig. 3A). Comparison of 50% inhibitory concentrations (IC50) for each serum sample in the total cell-associated fluorescence intensity assay indicated that the relative order of neutralizing effect corresponded to the neutralizing titers previously determined by plaque assay. The analysis of total cell-associated fluorescence was subject to variability resulting from differences in the absolute amount of Cy3-Ad bound by individual cells. In addition, some values for total cell-associated fluorescence intensity exceeded the value for the control sample, probably due to the binding of small antibody-Ad aggregates to the cell surface (reference 19 and data not shown).
FIG. 3.
Quantitative assays of infectivity. A549 cells were infected in a manner identical to that described in the legend to Fig. 2. Quantitative assessments included analyses of cell-associated fluorescence, percent nuclear -localization of vector, and transgene expression. In each case, data are presented as the mean and standard error of triplicate samples in a representative experiment. Each experiment was repeated two to four times. On each plot, the value for the control samples (infection in the absence of serum) is shown (100% dotted line). The point at which the curves reach 50% of the control value (50% dotted line) defines the IC50. Sera evaluated had neutralizing titers of <10, 640, 2,560, and 49,000. (A) Cell-associated fluorescence determined by digital image analysis of fluorescence derived from capsid-bound Cy3 and divided by the number of cells in the field, as indicated by DAPI staining of nuclei (8). To facilitate comparisons among experiments, data are presented as a percentage of the cell-associated fluorescence in the absence of serum. Five fields per condition were analyzed in each experiment. (B) Percent nuclear localization of vector. The fluorescence intensity that was coincident with the position of nuclei (provided by an image of the DAPI stain) was digitally subtracted from the total cell-associated fluorescence (from panel A), giving the nonnuclear fluorescence intensity. The difference between the total fluorescence intensity and the nonnuclear fluorescence intensity gave the value for nuclear fluorescence for each field. The ratio of nuclear fluorescence to total fluorescence for each field gave the percent nuclear localization (8). Five fields per condition were analyzed in each experiment. (C) Transgene expression. At 24 h after infection with Adβgal, cell lysates were analyzed for transgene activity and protein concentration. Data were calculated as β-galactosidase activity per milligram of protein. To facilitate comparisons among experiments, data are presented as a percentage of the β-galactosidase activity in the absence of serum. Triplicate samples were analyzed for each condition.
To overcome these sources of variability, a second morphological analysis was performed in which the percentage of vector that trafficked to the nucleus was determined. This value was independent of the absolute amount of vector on the cell and incorporated intracellular trafficking as a functional evaluation of the capsid. Using the same infection protocol and analysis of total fluorescence intensity, the percent nuclear localization was determined by using the image of nuclei as a mask to locate the capsid fluorescence that overlapped the nucleus (8). In agreement with previously published data, the control value representing the percentage of Ad capsid coincidently localized with the nucleus was 71% at 60 min after infection (8, 14). The percent nuclear localization of cell-associated vector increased as the dilution of neutralizing sera increased (Fig. 3B). As observed for total cell-associated fluorescence intensity, the IC50 for inhibition of trafficking to the nucleus corresponded to the previously determined anti-Ad neutralizing antibody titer (6). The data generated in the analysis of vector trafficking exhibited a higher degree of precision than did the data from the analysis of total cell-associated fluorescence.
To compare these assays to a standard assay of gene transfer using a similar infection protocol (1011 particles/ml for 10 min), transgene expression was evaluated following infection of A549 cells with an Ad encoding β-galactosidase (Adβgal). Nonneutralizing serum (titer, <10) did not change the β-galactosidase expression relative to control, while sera with neutralizing titers (640, 2,560, and 49,000) blocked transgene expression at high serum concentrations and permitted transgene expression at low serum concentrations (Fig. 3C).
A comparison of the three methods of analysis (total cell-associated fluorescence, percent nuclear localization of vector, and transgene expression) showed that the assays were comparable (Table 1). While the morphological and gene expression data agree, the IC50 for these three analyses differed from titers determined for the same sera in a conventional plaque assay (Table 1). The titers determined by the plaque assay indicate dilutions 5- to 20-fold higher than inhibitory dilutions in the other assays. The difference probably resulted from differences in assay design. Whereas the infection protocols for assays presented in this paper (morphologic and transgene expression analyses) involved an infection with a high concentration of Ad (1011 particles per ml) for a short period (10 min), the infection for the plaque assay utilized a low concentration of virus (2 × 107 particles per ml) for a longer period (1 h) (12). In addition, sera were incubated with Ad for 1 h prior to infection of A549 cells in the plaque assay while vectors were mixed with sera immediately prior to infection in the morphological and transgene expression assays. The lower viral concentration and longer preincubation with serum in the plaque assay probably contributed to more efficacious neutralization (i.e., a higher dilution factor of the sera) compared with the morphologic and transgene expression assays. This comparison highlights the variability that may arise among different neutralizing antibody assays and suggests that standardization of this assay be contemplated if neutralizing titer is to be used as a parameter in clinical gene transfer protocols.
TABLE 1.
Comparison of assays for the determination of anti-Ad neutralizing titers
| Reference serum titera | Titer in:
|
|||
|---|---|---|---|---|
| Plaque assay (IC90)b | Cell-associated vector assay (IC50)c | Intracellular trafficking assay (IC50)c | Transgene expression assay (IC50)c | |
| <10 | NDd | ND | ND | ND |
| 640 | 640 | 10 | 30 | 30 |
| 2,560 | 2,560 | 300 | 300 | 300 |
| 49,000 | 49,000 | 10,000 | 10,000 | 10,000 |
Human sera are referenced by the original neutralizing titer determined in a plaque assay.
Plaque assay evaluated the dilution of sera that provided 90% inhibition of plaque formation.
IC50s for the cell-associated vector assay, intracellular trafficking assay, and transgene expression assay were taken as the dilution with an arithmetic mean assay value closest to 50% inhibition compared with control samples (Fig. 3).
ND, inhibitory concentration was not determined because sera did not inhibit infection.
The data indicate that quantitative, morphometric analyses of Ad vector binding to cells and trafficking within cells provide a useful surrogate marker for Ad infection. The assay requires the generation of fluorophore-conjugated Ad, a relatively simple technique that has been successfully used in several laboratories (2, 5, 8, 10, 15, 20). The morphologic assay offers dis- tinct advantages over existing assays for neutralizing titers. First, the assay can be completed more rapidly than any other standard titer determination assay, requiring only 1 h following infection before the samples can be processed. Second, it requires fewer steps in sample manipulation, since the cells do not have to be removed from the well in which the infection occurs. Third, it does not rely on the availability of a particular transgene that has been engineered into a vector or on specific detection of any capsid component, and so it can potentially be extended to analyzing the neutralizing titer for any Ad gene transfer vector and for any serotype of wild-type Ad. Finally, the assay has potential for automation. The level of fluorescence that associates with the cells is sufficient for analysis by flow cytometry (21), and recent advances in automated fluorescence microscopy in a 96- or 386-well format suggest that a plate-based assay could be developed. Compared with other assays of neutralizing titer, including gene expression and plaque assays, the fluorescence assay is likely to achieve similar or superior sensitivity. Fluorescence techniques are generally regarded to have high signal-to-noise ratios, permitting direct detection of vector without employing amplification steps such as enzymatic activity (used to quantify transgene expression) or viral replication (used to quantify plaque formation).
The data presented also bear on an important debate in the field of viral gene therapy vectors: the relationship of particles to PFU (13). Preparations of viruses and viral vectors are currently evaluated based on physical and biological standards (4). The physical standard commonly used is the number of particles per milliliter of vehicle. The biological standard is often the PFU per milliliter of vehicle. For Ad, the number of particles per milliliter often exceeds the PFU per milliliter by a factor of 10 to 100. This has been interpreted to mean that up to 99 of 100 Ad particles are not infectious. However, detailed optimization of biological infectivity assays has revealed that the assay is subject to variability depending on the precise protocol used, and particle-to-PFU ratios as low as 3 have been reported (13). The present data indicate that the particles bound to infected cells and trafficking of those particles through infected cells correlate well with the resulting level of infection. Morphological assessments (cell-associated vector and percent nucleus-targeted vector) largely agreed with a functional measure of infectivity (transgene expression) when the IC50 for the neutralizing sera was evaluated. In other words, any inhibition in the infectivity observed by gene ex- pression was matched by an inhibition of viral binding to cells and intracellular trafficking. The most reasonable explanation for the correlation between morphological and gene expression data is that nearly all Ad particles that bind to cells are infectious and that a morphological assessment of a population of Ad inside cells accurately reflects infectivity.
A further implication of this work concerns the predictions of clinical outcome when Ad vectors are administered by different routes of administration to patients with preexisting neutralizing titers. Gene transfer via intravascular administration is likely to correspond to infection at high serum concentration (i.e., 100% serum). At the highest concentration of neutralizing serum tested (threefold dilution of serum, equal to 33% serum), the 640, 2,560, and 49,000 serum titers completely neutralized Ad. in all three assays tested. Thus, intravascular administration of these serum titers to an individual may be futile. Local injection of vector into a tissue may have different results. Tissue injection offers direct access of the vector to target cells with minimal exposure to serum. Therefore, the increased dose may partially overcome the limited, immediately available neutralizing antibody in the extracellular space at the site of injection. Data supporting different dose-response curves for tissue administration and intravenous administration in the presence of different levels of neutralizing antibody show that tissue administration is proportionately less affected by neutralizing antibody than is systemic administration (3).
ACKNOWLEDGMENTS
We thank N. Mohamed for help in preparing the manuscript.
These studies were supported, in part, by grant P01 HL59312; the Will Rogers Memorial Fund, Los Angeles, Calif.; and GenVec, Inc., Gaithersburg Md. PLL. is also supported, in part, by NIH grant R29AI 42250. C.J.B. is supported, in part, by NIH grant T32HL-07423-21.
REFERENCES
- 1.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 2.Fasbender A, Lee J H, Walters R W, Moninger T O, Zabner J, Welsh M J. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Investig. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackett N R, Crystal R G. Adenovirus vectors for gene therapy. In: Lasic D, Templeton N S, editors. Gene therapy: therapeutic mechanisms and strategies. New York, N.Y: Marcel Dekker, Inc.; 2000. pp. 17–40. [Google Scholar]
- 5.Harrod K S, Trapnell B C, Otake K, Korfhagen T R, Whitsett J A. SP-A enhances viral clearance and inhibits inflammation after pulmonary adenoviral infection. Am J Physiol. 1999;277:L580–L588. doi: 10.1152/ajplung.1999.277.3.L580. [DOI] [PubMed] [Google Scholar]
- 6.Harvey B G, Hackett N R, El-Sawy T, Rosengart T K, Hirschowitz E A, Lieberman M D, Lesser M L, Crystal R G. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hersh J, Crystal R G, Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 1995;2:124–131. [PubMed] [Google Scholar]
- 8.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 9.Leopold P L, Kreitzer G, Miyazawa N, Rempel S, Pfister K K, Rodriguez-Boulan E, Crystal R G. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 10.Linette G P, Shankara S, Longerich S, Yang S, Doll R, Nicolette C, Preffer F I, Roberts B L, Haluska F G. In vitro priming with adenovirus/gp 100 antigen-transduced dendritic cells reveals the epitope specificity of HLA-A∗0201-restricted CD8+ T cells in patients with melanoma. J Immunol. 2000;164:3402–3412. doi: 10.4049/jimmunol.164.6.3402. [DOI] [PubMed] [Google Scholar]
- 11.Mandel B. Neutralization of animal viruses. Adv Virus Res. 1978;23:205–268. doi: 10.1016/S0065-3527(08)60101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastrangeli A, Harvey B G, Yao J, Wolff G, Kovesdi I, Crystal R G, Falck-Pedersen E. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 13.Mittereder N, March K L, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazawa N, Leopold P L, Hackett N R, Ferris B, Worgall S, Falck-Pedersen E, Crystal R G. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J Virol. 1999;73:6056–6065. doi: 10.1128/jvi.73.7.6056-6065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld M A, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L E, Paakko P K, Gilardi P, Stratford-Perricaudet L D, Perricaudet M, Jallat S, Pavirani A, Lecocq J-P, Crystal R G. Adenovirus-mediated transfer of a recombinant alpha 1- antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, Perricaudet M, Guggino W B, Pavirani A, Lecocq J-P, Crystal R G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 18.Vincent T. Quantitative, morphometric assays of adenoviral infection in the presence of anti-adenovirus neutralizing serum. M.S. thesis. Stockholm, Sweden: Karolinska Institute; 2000. [Google Scholar]
- 19.Wallis C, Melnick J L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J Virol. 1967;1:478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 21.Worgall S, Worgall T, Kostarelos K, Singh R, Leopold P L, Hackett N R, Crystal R G. Free cholesterol enhances adenoviral vector gene transfer and expression in CAR-deficient cells. Mol Ther. 2000;1:39–48. doi: 10.1006/mthe.1999.0013. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune response to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]