Abstract
Pediococcus pentosaceus ST65ACC is a bacteriocinogenic lactic acid bacteria (LAB) isolated from Brazilian artisanal cheese that is capable of inhibiting different food pathogens, mainly Listeria monocytogenes. The production of bacteriocins can be influenced by several growth conditions, such as temperature, pH, and medium composition. This study aimed to evaluate the effect of different culture media on the production of bacteriocins and antimicrobial activity of P. pentosaceus ST65ACC on L. monocytogenes Scott A. The strains were inoculated alone and in coculture in four different media: BHI broth, MRS broth, meat broth, and reconstituted skim milk (RSM) 10% (w/v). The culture media were then incubated at 37 °C for 96 h, and count analysis, pH measurement, and bacteriocin production were performed at 0, 24, 48, 72 and 96 h. L. monocytogenes was inhibited to nondetectable levels in coculture with P. pentosaceus ST65ACC in MRS broth within 96 h, consistent with the high production of bacteriocin throughout the analysis period (3,200–12,800 AU/mL). However, lower inhibitory activities of P. pentosaceus ST65ACC on L. monocytogenes Scott A were recorded in BHI, RSM, and meat broth, with low or no production of bacteriocins at the analyzed times. The composition of these culture media may have repressed the production and activity of bacteriocins and, consequently, the antagonist activity of P. pentosaceus ST65ACC on L. monocytogenes Scott A. The results showed that the antimicrobial activity was more effective in MRS broth, presenting greater production of bacteriocins and less variability when compared to the other media analyzed.
Keywords: Pediococcus pentosaceus, Bacteriocin, Pediocin, Antimicrobial activity, Listeria monocytogenes
Introduction
Bacteriocins are antimicrobial proteins or peptides ribosomally encoded by different bacterial species that are capable of controlling the growth of pathogens and spoilage bacteria. Bacteriocins synthesized by lactic acid bacteria (LAB) have great potential as biopreservative agents in food [1–3]. Pediocins, generally cationic hydrophobic molecules and class IIa members of the bacteriocin group, are produced by Pediococcus strains [1, 4], and extensively studied by their bactericidal activity at low concentrations against different pathogenic bacteria [5–8]. Gram-positive bacteria are their main targets and these bacteriocins exert their action against sensitive cells through pore formation and release of intracellular contents [9]. Moreover, pediocins exhibit high activity against Listeria spp., particularly Listeria monocytogenes [10]. In addition to their notable bactericidal activity, they exhibit thermotolerance and maintain their activity in a wide pH range, and their use as a food preservative is allowed in some countries [9].
Pediococcus pentosaceus ST65ACC was isolated from Brazilian artisanal cheese [11], presenting bactericidal and/or bacteriostatic action against different pathogens, mainly L. monocytogenes [11, 12], and can produce the bacteriocins pediocin PA-1 and penocin A [13]. P. pentosaceus ST65ACC was described as able to grow in MRS supplemented with xylo-oligosaccharide as the sole carbon source and produce bacteriocins, but at lower levels than in MRS broth [14].
Optimal bacterial growth and bacteriocin production are strongly dependent on several factors, such as medium composition, pH, temperature, incubation time, and carbon and nitrogen sources [15, 16], and are specific for each producing strain [17]. Thus, for the application of a bacteriocinogenic strain in food, studies that evaluate the effective production of its bacteriocins are very important since the composition of the medium can interfere with its production and/or activity, and the synthesis of low concentrations may be insufficient to inhibit pathogenic and spoilage bacteria [15, 18, 19]. In the present study, we aimed to evaluate the effect of different culture media on bacteriocin production and antimicrobial activity of P. pentosaceus ST65ACC on L. monocytogenes Scott A.
Materials and methods
Bacterial strains and culture conditions
P. pentosaceus ST65ACC was originally isolated from artisanal raw milk cheese and characterized as bacteriocinogenic [11], and through whole-genome sequencing, the presence of the operons of the bacteriocins pediocin PA-1 and penocin-A was identified [13]. P. pentosaceus MLEV8 [20] was selected as a nonbacteriocinogenic strain and used as a negative control. L. monocytogenes Scott A was used as the target. The strains were kept in Tryptone Soya Broth (TSB, Oxoid Ltd., Basingstoke, UK) for L. monocytogenes and de Man, Rogosa and Sharpe broth (MRS, Oxoid) for lactic acid bacteria (LAB) with 20% (v/v) glycerol and stored at -20 °C. At the time of use, the strains were recovered in their respective culture media and incubated at 37 °C for 24 h. Subsequently, the cultures were diluted in NaCl 0.85% (w/v) at a turbidity similar to the McFarland scale 0.5, which corresponds to approximately 1.5 × 108 colony-forming units per milliliter (CFU/mL).
P. Pentosaceus ST65ACC and L. monocytogenes interaction
The antimicrobial activity of P. Pentosaceus ST65ACC against
L. monocytogenes Scott A was performed in brain heart infusion (BHI) broth (Oxoid), MRS broth (Oxoid), meat broth [21], and reconstituted skim milk (RSM; 10%, w/v, Molico, Nestlé Brasil Ltd., São Paulo, SP, Brazil) in different treatments (T): T1, only P. pentosaceus ST65ACC (106 CFU/mL); T2, only P. pentosaceus MLEV8 (nonbacteriocinogenic strain; 106 CFU/mL); T3, coculture of ST65ACC (106 CFU/mL) with L. monocytogenes Scott A (103 CFU/mL); T4, coculture of MLEV8 (106 CFU/mL) with L. monocytogenes Scott A (103 CFU/mL); T5, only L. monocytogenes Scott A (103 CFU/mL); Control, without strain inoculation. Initially, 250 mL of each broth was prepared and sterilized, and the LAB strains and L. monocytogenes Scott A were inoculated alone and in coculture in the broths and incubated at 37 °C for 96 h. The selection of the media incubation temperature was based on previous results [11], in which bacteriocin production was higher at 37 °C. Every 24 h (0, 24, 48, 72 and 96 h), count analysis, pH measurement, and antimicrobial activity were performed. The experiment was conducted in three independent repetitions.
Bacterial count and pH measurement
For enumeration of populations, aliquots of 25 ml of each treatment were obtained under sterile conditions, and serial dilutions were performed in saline solution (NaCl 0.85%, w/v). LAB strains were enumerated on MRS agar (Oxoid) and L. monocytogenes Scott A on Oxford Listeria agar (Oxoid), both by spread plating, in triplicate, and incubated at 37 °C for 24 h, based on official protocols [22, 23]. All results obtained were expressed in log10 CFU/mL. The pH of the samples was determined with a FiveEasy™ bench type 20 pH meter (Mettler Toledo, Greifensee, Switzerland).
Antimicrobial activity
The antimicrobial activity of P. pentosaceus strains against L. monocytogenes Scott A was determined by the critical dilution technique [24], with modifications. Samples of 5 mL from each treatment at the time of analysis were collected, and cell-free supernatants (CFS) were obtained. The samples were centrifuged at 6,800 × g for 20 min at 4 °C, and then the pH of the supernatants was adjusted to 6.5 with 1 M NaOH and heated at 80 °C for 10 min. Subsequently, the CFSs were filter sterilized (0.22 μm pore size filter units, Merck Millipore Ltd., Cork, Ireland), and the supernatants obtained were subjected to the critical dilution method in 10 mM phosphate-buffered saline (PBS) pH 6.5. Aliquots of 10 µL were applied to the surface of TSA agar (Oxoid) containing 106 CFU/mL of L. monocytogenes Scott A, and the plates were incubated at 37 °C for 24 h. The antimicrobial activity was expressed in arbitrary units per milliliter (AU/mL), calculated as 2n x 100, where “2” corresponds to the dilution factor and “n” corresponds to the last dilution that produced an inhibition zone greater than 2 mm in diameter [25].
Data analysis
Statistical analyzes and data visualization were carried out employing the R software version 4.3.0 [26] and RStudio version 2023.03.0 + 386 [27], using the ‘conover.test’, ‘ggplot2’, and ‘stats’ packages [26, 28, 29]. Homogeneity of variance and normality were verified using the Bartlett and Shapiro-Wilk tests, respectively. The Kruskal-Wallis’ test with Conover’s test (using Benjamini-Hochberg method) was used for post-hoc analysis. The statistical significance considered was p < 0.05.
Results
The Fig. 1 depicts the pH variation during bacterial growth over 96 h in different culture media, inoculated with the LAB P. pentosaceus ST65ACC and P. pentosaceus MLEV8, either alone or in coculture with L. monocytogenes Scott A. When using BHI broth, a rapid reduction in pH was observed, as all treatments displaying statistically significant difference compared to the control pH after 24 h of incubation (p < 0.05) (Fig. 1; A). In MRS broth, only the treatment inoculated with L. monocytogenes Scott A (T5) exhibited a delay in pH reduction; after 24 h of incubation, its pH value still did not differ statistically from the control pH (Fig. 1; B). However, T5 was the only treatment that showed a reduction in pH when in meat broth (Fig. 1; C). In RSM, the pH reduction was slower; after 24 h, none of the treatments differed from the control; besides, after 48 h, only T5 still did not statistically differ from the control (Fig. 1; D).
Fig. 1.
Variation of pH values during bacterial growth in BHI broth (A), MRS broth (B), meat broth (C), and reconstituted skim milk (RSM) 10% (w/v) (D). The figure represents means ± standard error, n = 3. The asterisk indicates a significant difference with the control (* p < 0.05). (T1: P. pentosaceus ST65ACC; T2: P. pentosaceus MLEV8; T3: P. pentosaceus ST65ACC with L. monocytogenes Scott A; T4: P. pentosaceus MLEV8 with L. monocytogenes Scott A; T5: L. monocytogenes Scott A alone.)
The populations of P. pentosaceus ST65ACC and P. pentosaceus MLEV8 inoculated alone and in coculture with L. monocytogenes Scott A in different culture media are showed in Fig. 2. In all culture media, it was possible to observe an exponential increase in the population of P. pentosaceus ST65ACC and P. pentosaceus MLEV8 between 0 and 24 h, reaching values from 7.84 to 9.62 log CFU/mL and remaining constant or with a slight decline. For both LAB strains, in all culture media, no statistical difference was observed between the populations when inoculated alone or in coculture with L. monocytogenes Scott A (p > 0.05).
Fig. 2.
Mean counts (± standard error) of P. pentosaceus ST65ACC and P. pentosaceus MLEV8 inoculated in BHI broth (A), MRS broth (B), meat broth (C), and reconstituted skim milk (RSM) 10% (w/v) (D). (T1: ST65ACC alone; T2: MLEV8 alone; T3: ST65ACC in coculture with L. monocytogenes Scott A; T4: MLEV8 in coculture with L. monocytogenes Scott A)
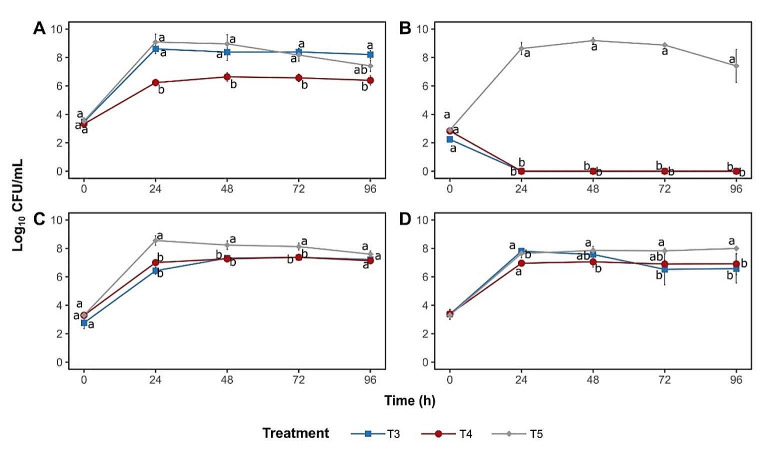
Regarding the antimicrobial activity of P. pentosaceus ST65ACC and P. pentosaceus MLEV8 against L. monocytogenes Scott A in BHI broth, MRS broth, meat broth, and RSM, populations of L. monocytogenes Scott A inoculated alone and in coculture are shown in Fig. 3. The growth of L. monocytogenes strain was affected in all culture media. In BHI broth, P. pentosaceus MLEV8 (nonbacteriocinogenic strain) exhibited a better inhibitory capacity against L. monocytogenes Scott A, compared to ST65ACC at all analyzed times (Fig. 3; A). When using MRS broth, both strains had the same effect in inactivating L. monocytogenes Scott A, with the population reduced to undetectable levels after 24 h of incubation (Fig. 3; B). In meat broth, the population of L. monocytogenes Scott A was lower when in coculture with the BAL strains, but there was no difference after 96 h of incubation (Fig. 3; C). In RSM, the effect of both coculture treatments was significant after 96 h of incubation against the population of L. monocytogenes Scott A (Fig. 3; D).
Fig. 3.
Mean counts (± standard error) of L. monocytogenes Scott A inoculated in BHI broth (A), MRS broth (B), meat broth (C), and reconstituted skim milk (RSM) 10% (w/v) (D). (T3: in coculture with ST65ACC; T4: in coculture with MLEV8; T5: inoculated alone). Different lowercase letter indicates statistical difference (p < 0.05) between treatments, within each time
Inhibition of L. monocytogenes Scott A by the P. pentosaceus ST65ACC strain in MRS broth is consistent with high bacteriocin production throughout the analysis period in all repetitions of the experiment, with values ranging from 3,200 to 12,800 AU/mL (data not shown). However, P. pentosaceus MLEV8 (nonbacteriocinogenic strain) also inhibited the population of L. monocytogenes Scott A in MRS broth, a result that can be attributed to other antimicrobial compounds produced by the strain, such as organic acids.
The production of bacteriocins by P. pentosaceus in BHI broth was not constant between repetitions during the analysis period, with the production of 100 AU/mL being observed in the first repetition only within 24 h (data not shown). In the other repetitions, bacteriocin production was verified at 24, 48 and 72 h, with values varying between 100 and 200 AU/mL. In RSM 10% (w/v), the production of bacteriocins by the strain P. pentosaceus ST65ACC was verified in only one of the experimental repetitions, with values of 200 AU/mL at 24 h and 100 AU/mL in the other periods of analysis. No bacteriocin production was detected when meat broth was used.
Discussion
Bacteriocins, such as pediocins produced by Pediococcus, have been widely investigated, primarily due to the increasing interest of consumers in the use of natural antimicrobial agents [30]. Studies have investigated and demonstrated that pediocins cause inhibition of L. monocytogenes, with experiments conducted in culture media [7, 10], or food matrices [5, 31]. Listeria monocytogenes is a pathogen of significant public health concern worldwide, responsible for causing a foodborne disease called listeriosis [32]. Additionally, Tumbarski et al. [10] demonstrated that the bacteriocin produced by Pediococcus could not only inhibit L. monocytogenes but also other species of Listeria, such as L. innocua and L. ivanovii. Furthermore, pediocin produced by certain strains of Pediococcus can inhibit other pathogens, including Clostridium perfringens, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli [5, 7]. Thus, pediocin is one of the most promising candidates for inhibiting the growth of L. monocytogenes in foods.
The variation in the results obtained in this study regarding the production of bacteriocin in different culture media is directly related to their composition, including the presence of amino acids, carbon/nitrogen ratio, and type of carbohydrate, as they play an essential role in bacterial growth and can also affect the production of enzymes and compounds necessary for the synthesis and expression of bacteriocins [17, 18, 33]. In the MRS broth, a high activity of bacteriocins from P. pentosaceus ST65ACC inoculated alone and in coculture with L. monocytogenes Scott A was observed at all analysis times, despite some variation between replicates. However, lower inhibitory activities of P. pentosaceus ST65ACC were recorded in BHI broth, RSM, and meat broth, with low and/or no bacteriocin production despite relatively good strain growth. MRS broth was described as the best medium for bacteriocin activity produced by Lactobacillus pentosus (recently reclassified as Lactiplantibacillus pentosus) ST151BR (6,400 AU/mL) compared to BHI broth, M17 broth, soy milk, and molasses (200 AU/mL) [34]. Also, a greater inhibitory effect of the bacteriocin produced by Bacillus megaterium 19 strain in MRS broth than in BHI medium, which was not suitable for bacteriocin production [35].
MRS medium is a complex medium rich in different sources of carbon and nitrogen [36], which favors the growth of bacteria and the production of bacteriocins. The amount of glucose in the medium can also regulate the production of bacteriocins [37]. P. pentosaceus NCDC 273 was able to growth and produce bacteriocin at high levels when inoculated in MRS medium supplemented with glucose, in comparison with MRS medium supplemented with lactose [38]. They also verified that the initial pH of 6.0 and 7.0 of MRS supplemented with 20 g/L glucose or lactose, respectively, was ideal for the maximum level of pediocin production. Similarly, P. pentosaceus LB44 presented an enhanced growth and bacteriocin production in the presence of glucose, especially when compared with lactose [17]. Also, an increase in bacteriocin production by Lactobacillus plantarum (recently reclassified as Lactiplantibacillus plantarum) AMA-K was observed in the presence of glucose [24]. This may explain the greater production of bacteriocins in the MRS medium in this study, since it has a higher concentration of glucose than other media. In previous studies, we also verified the low production of bacteriocins by the strain of P. pentosaceus ST65ACC in milk [11], showing no inhibitory effect on L. monocytogenes, a result attributed to the limited metabolism of the strain to ferment lactose, the main sugar in milk [9, 11]. These oscillating results may also be associated with the interaction of bacteriocins with the components of the medium and/or degradation by proteases [33, 39].
A low final pH and a high cell density were needed for a high level of pediocin AcH production by Pediococcus acidilactici H in TGE broth (glucose tryptone extract), since bacteriocin production was negligible when the medium pH was maintained at 5.0 or above, even in the presence of high cell mass [40]. A similar result was observed previously, in which greater pH decrease increased nisin and pediocin production by Lactococcus lactis and Pediococcus acidilactici, respectively [41]. This may also justify the result obtained in this study, since a low pH over 96 h was not observed in BHI broth, meat broth, and RSM.
Conclusion
The results obtained showed that the antimicrobial activity of P. pentosaceus ST65ACC on L. monocytogenes Scott A was more effective in MRS broth, showing greater production of bacteriocins and less variability when compared to the other media under analysis. The composition of the BHI broth, meat broth, and RSM, may have repressed the production and/or activity of bacteriocins and, consequently, the antimicrobial activity of P. pentosaceus ST65ACC on L. monocytogenes Scott A.
Thus, verifying that the composition of the culture medium plays an important role in the production of bacteriocins, some media may not be suitable for this purpose. The evaluation of this parameter, as well as other environmental factors, is important for optimizing the growth and production of bacteriocins and essential for the use of a strain or its bacteriocins as biopreservation agents in food.
Author contributions
Conceptualization, methodology: Nero LA and Oliveira FS; formal analysis, writing and original draft preparation: Oliveira FS; writing, review and editing: Nero LA, Rodrigues RS and Cavicchioli VQ; supervision: Nero LA and de Carvalho AF; funding acquisition: Nero LA. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful for Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, DF, Brazil, Financial code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, DF, Brazil) and Fundação de Amparo à Pesquisa do Estado de Minas gerais (FAPEMIG, Belo Horizonte, MG, Brazil).
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
Authors declare that they have no competing interests. LA Nero is Editor-in-Chief for Brazilian Journal of Microbiology and the peer-review process for this manuscript was independently handled by another member of the journal editorial board.
Footnotes
The original online version of this article was revised: In this article the author name Valéria Quintana Cavicchioli was incorrectly written as Valéria Quintana Caviccholi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/25/2024
A Correction to this paper has been published: 10.1007/s42770-024-01429-4
References
- 1.Papagianni M (2003) Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv 21(6):465–499. 10.1016/S0734-9750(03)00077-6 10.1016/S0734-9750(03)00077-6 [DOI] [PubMed] [Google Scholar]
- 2.Cotter PD, Ross RP, Hill C (2013) Bacteriocins — a viable alternative to antibiotics? Nat Rev Microbiol 11(2):95–105. 10.1038/nrmicro2937 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- 3.Garsa AK, Kumariya R, Sood SK et al (2014) Bacteriocin production and different strategies for their recovery and purification. Probiotics Antimicrob Proteins 6(1):47–58. 10.1007/s12602-013-9153-z 10.1007/s12602-013-9153-z [DOI] [PubMed] [Google Scholar]
- 4.Deegan LH, Cotter PD, Hill C, Ross P (2006) Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int Dairy J 16(9):1058–1071. 10.1016/j.idairyj.2005.10.026 10.1016/j.idairyj.2005.10.026 [DOI] [Google Scholar]
- 5.Nieto-Lozano JC, Reguera-Useros JI, Peláez-Martínez MdelC et al (2010) The effect of the pediocin PA-1 produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish dry-fermented sausages and frankfurters. Food Control 21(5):679–685. 10.1016/j.foodcont.2009.10.007 10.1016/j.foodcont.2009.10.007 [DOI] [Google Scholar]
- 6.Bédard F, Hammami R, Zirah S et al (2018) Synthesis, antimicrobial activity and conformational analysis of the class IIa bacteriocin pediocin PA-1 and analogs thereof. Sci Rep 8(1):9029. 10.1038/s41598-018-27225-3 10.1038/s41598-018-27225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh B, Sukumar G, Ghosh AR (2019) Purification and characterization of pediocin from probiotic Pediococcus pentosaceus GS4, MTCC 12683. Folia Microbiol (Praha) 64(6):765–778. 10.1007/s12223-019-00689-0 10.1007/s12223-019-00689-0 [DOI] [PubMed] [Google Scholar]
- 8.Khorshidian N, Khanniri E, Mohammadi M et al (2021) Antibacterial activity of pediocin and pediocin-producing bacteria against Listeria monocytogenes in meat products. Front Microbiol 12709959. 10.3389/fmicb.2021.709959 [DOI] [PMC free article] [PubMed]
- 9.Papagianni M, Anastasiadou S (2009) Pediocins: the bacteriocins of Pediococci. Sources, production, properties and applications. Microb Cell Fact 83. 10.1186/1475-2859-8-3 [DOI] [PMC free article] [PubMed]
- 10.Tumbarski YD, Pavlova T, Yanakieva V et al (2022) Antimicrobial activity of Pediococcus strains against some Listeria spp. during co-cultivation under static growth conditions. J Microbiol Biotechnol food Sci 12(4). 10.55251/jmbfs.9360
- 11.Cavicchioli VQ, Camargo AC, Todorov SD, Nero LA (2017) Novel bacteriocinogenic Enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J Dairy Sci 100(4):2526–2535. 10.3168/jds.2016-12049 10.3168/jds.2016-12049 [DOI] [PubMed] [Google Scholar]
- 12.Cavicchioli VQ, Camargo AC, Todorov SD, Nero LA (2019) Potential control of Listeria monocytogenes by bacteriocinogenic Enterococcus hirae ST57ACC and Pediococcus pentosaceus ST65ACC strains isolated from artisanal cheese. Probiotics Antimicrob Proteins 11(2):696–704. 10.1007/s12602-018-9449-0 10.1007/s12602-018-9449-0 [DOI] [PubMed] [Google Scholar]
- 13.Oliveira FS, da Silva Rodrigues R, de Carvalho AF, Nero LA (2023) Genomic analyses of Pediococcus pentosaceus ST65ACC, a bacteriocinogenic strain isolated from artisanal raw-milk cheese. Probiotics Antimicrob Proteins 15(3):630–645. 10.1007/s12602-021-09894-1 10.1007/s12602-021-09894-1 [DOI] [PubMed] [Google Scholar]
- 14.Todorov SD, Cavicchioli VQ, Ananieva M et al (2020) Expression of coagulin A with low cytotoxic activity by Pediococcus pentosaceus ST65ACC isolated from raw milk cheese. J Appl Microbiol 128(2):458–472. 10.1111/jam.14492 10.1111/jam.14492 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang Y, Liu S et al (2012) Modelling growth and bacteriocin production by Pediococcus acidilactici PA003 as a function of temperature and pH value. Appl Biochem Biotechnol 166(6):1388–1400. 10.1007/s12010-011-9532-4 10.1007/s12010-011-9532-4 [DOI] [PubMed] [Google Scholar]
- 16.Todorov SD, Oliveira RPS, Vaz-velho M (2012) Media optimization of bacteriocin ST22Ch production by Lactobacillus sakei ST22Ch isolated from Salpicão, a traditional meat-product from Portugal. Chem Eng Trans 27283–27288. 10.3303/CET1227048
- 17.Kaur R, Kumar Tiwari S (2017) Optimization of culture conditions for bacteriocin production by soil isolates Pediococcus pentosaceus LB44 and Weissella confusa LM85. Int J Infect 4(3):e15842. 10.5812/iji.15842 10.5812/iji.15842 [DOI] [Google Scholar]
- 18.Gänzle M (1999) Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int J Food Microbiol 46(3):207–217. 10.1016/S0168-1605(98)00205-0 10.1016/S0168-1605(98)00205-0 [DOI] [PubMed] [Google Scholar]
- 19.Perin LM, Dal Bello B, Belviso S et al (2015) Microbiota of Minas cheese as influenced by the nisin producer Lactococcus lactis subsp. lactis GLc05. Int J Food Microbiol 214159–214167. 10.1016/j.ijfoodmicro.2015.08.006 [DOI] [PubMed]
- 20.Colombo M, Castilho NPA, Todorov SD, Nero LA (2018) Beneficial properties of lactic acid bacteria naturally present in dairy production. BMC Microbiol 18(1):219. 10.1186/s12866-018-1356-8 10.1186/s12866-018-1356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freney J, Kloos WE, Hajek V et al (1999) Recommended minimal standards for description of new staphylococcal species. Int J Syst Evol Microbiol 49(2):489–502. 10.1099/00207713-49-2-489 10.1099/00207713-49-2-489 [DOI] [PubMed] [Google Scholar]
- 22.Frank J, Yousef A (2004) Chap. 08 tests for groups of microorganisms. In: Standard Methods for the Examination of Dairy Products
- 23.ISO (2017) ISO 11290-1:2017 - Microbiology of the food chain - Horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp. - Part 1: Detection method
- 24.Todorov SD (2008) Bacteriocin production by Lactobacillus plantarum AMA-K isolated from Amasi, a Zimbabwean fermented milk product and study of the adsorption of bacteriocin AMA-K to Listeria Sp. Brazilian J Microbiol 39(1):178–187. 10.1590/S1517-838220080001000035 10.1590/S1517-838220080001000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavicchioli VQ, Dornellas W, dos S, Perin LM et al (2015) Genetic diversity and some aspects of antimicrobial activity of lactic acid bacteria isolated from goat milk. Appl Biochem Biotechnol 175(6):2806–2822. 10.1007/s12010-015-1511-8 10.1007/s12010-015-1511-8 [DOI] [PubMed] [Google Scholar]
- 26.R Core Team (2023) R: a Language and. Environment for Statistical Computing
- 27.RStudio T (2020) RStudio: Integrated Development Environment for R
- 28.Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis
- 29.Dinno A (2017) conover.test: Conover-Iman Test of multiple comparisons using Rank sums. 1–7
- 30.Silva CCG, Silva SPM, Ribeiro SC (2018) Application of bacteriocins and protective cultures in dairy food preservation. Front Microbiol 9594. 10.3389/fmicb.2018.00594 [DOI] [PMC free article] [PubMed]
- 31.Ramos B, Brandão TRS, Teixeira P, Silva CLM (2020) Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol 85103282. 10.1016/j.fm.2019.103282 [DOI] [PubMed]
- 32.de Noordhout CM, Devleesschauwer B, Angulo FJ et al (2014) The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis 14(11):1073–1082. 10.1016/S1473-3099(14)70870-9 10.1016/S1473-3099(14)70870-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lajis AFB (2020) Biomanufacturing process for the production of bacteriocins from Bacillaceae family. Bioresour Bioprocess 7(1):8. 10.1186/s40643-020-0295-z 10.1186/s40643-020-0295-z [DOI] [Google Scholar]
- 34.Todorov SD, Dicks LMT (2004) Effect of medium components on bacteriocin production by Lactobacillus pentosus ST151BR, a strain isolated from beer produced by the fermentation of maize, barley and soy flour. World J Microbiol Biotechnol 20(6):643–650. 10.1023/B:WIBI.0000043196.09610.de 10.1023/B:WIBI.0000043196.09610.de [DOI] [Google Scholar]
- 35.Khalil R, Elbahloul Y, Djadouni F, Omar S (2009) Isolation and partial characterization of a bacteriocin produced by a newly isolated Bacillus megaterium 19 strain. Pakistan J Nutr 8(3):242–250. 10.3923/pjn.2009.242.250 10.3923/pjn.2009.242.250 [DOI] [Google Scholar]
- 36.Yang E, Fan L, Yan J et al (2018) Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Express 8(1):10. 10.1186/s13568-018-0536-0 10.1186/s13568-018-0536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malheiros PS, Sant’Anna V, Todorov SD, Franco BDGM (2015) Optimization of growth and bacteriocin production by Lactobacillus sakei subsp. sakei 2a. Brazilian J Microbiol 46(1):825–834. 10.1590/S1517-838246320140279 [DOI] [PMC free article] [PubMed]
- 38.Vijay Simha B, Sood SK, Kumariya R, Garsa AK (2012) Simple and rapid purification of pediocin PA-1 from Pediococcus pentosaceous NCDC 273 suitable for industrial application. Microbiol Res 167(9):544–549. 10.1016/j.micres.2012.01.001 10.1016/j.micres.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 39.Aasen IM, Møretrø T, Katla T et al (2000) Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biotechnol 53(2):159–166. 10.1007/s002530050003 10.1007/s002530050003 [DOI] [PubMed] [Google Scholar]
- 40.Biswas SR, Ray P, Johnson MC, Ray B (1991) Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl Environ Microbiol 57(4):1265–1267. 10.1128/aem.57.4.1265-1267.1991 10.1128/aem.57.4.1265-1267.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerra NP, Pastrana L (2003) Influence of pH drop on both nisin and pediocin production by Lactococcus lactis and Pediococcus acidilactici. Lett Appl Microbiol 37(1):51–55. 10.1046/j.1472-765X.2003.01346.x 10.1046/j.1472-765X.2003.01346.x [DOI] [PubMed] [Google Scholar]