Abstract
Background
Chronic pain after breast cancer surgery, affecting 25%-60% of patients, significantly impacts the survivors’ quality of life. With improved survival rates, more individuals are experiencing this long-term complication. It is often overlooked that this chronic pain may stem from peripheral nerve injury, resulting in neuropathic pain characterized by burning sensations, electric shocks, and heightened sensitivity. Although neuropathic pain prevalence is reported at 24%-36% post-mastectomy, the data following breast-conserving surgery remain limited. This systematic review aimed to investigate the prevalence of neuropathic pain after breast-conserving surgery and its potential association with axillary procedures.
Methods
The electronic databases, Medline, Embase, Web of Science and Cochrane Central, were searched. Inclusion criteria were defined to include studies reporting on the prevalence of neuropathic pain following breast-conserving surgery and exploring associations with axillary procedures. A meta-analysis was performed to compute a pooled prevalence rate.
Results
Eight studies, covering 1,469 patients post-breast-conserving surgery, met the inclusion criteria. The meta-analysis revealed a pooled prevalence of 31% (95% confidence intervals [CI] 0.14-0.56) neuropathic pain among patients who underwent breast-conserving surgery. Six studies explored associations with axillary procedures; however, none suggested a correlation between axillary procedures and neuropathic pain after breast-conserving surgery.
Conclusion
This systematic review and meta-analysis indicated a pooled prevalence of 31% neuropathic pain following breast-conserving surgery of, with confidence interval ranging from 14% to 56%. The review did not provide conclusive evidence to suggest correlations between axillary procedures and neuropathic pain after breast-conserving surgery.
Key words: Breast cancer surgery, Breast-conserving surgery, Lumpectomy, Chronic pain, Neuropathic pain
Introduction
Chronic pain following surgical procedures for breast cancer presents a significant concern for breast cancer survivors.1,2 With the advancements in breast cancer screening and treatment, improved survival rates have led to more individuals living with chronic pain after treatment.2,3 This pain frequently results in reduced quality of life, marked by functional impairments and psychological distress.3,4 Studies indicate that 25% to 60% of individuals experience chronic pain following breast cancer surgery, including mastectomy and breast-conserving surgery.5, 6, 7, 8, 9
It is often overlooked that this chronic pain may be the result of a peripheral nerve injury, potentially leading to neuropathic pain.5,10,11 Within the context of breast cancer surgery, neuropathic pain is considered to be the result of direct surgical injury to the intercostal and intercostobrachial nerves or as a consequence of indirect nerve damage through excessive stretching or compression through scar formation.12, 13, 14, 15, 16 Neuropathic pain is typically characterized by a burning or shooting pain and frequently presents with altered skin sensations in the surgically treated axilla or breast.3,13,17 What sets neuropathic pain apart is its potential to improve with tailored interventions. Therefore, understanding how much of the post-surgical chronic pain is neuropathic and indeed nerve-related is crucial.
Neuropathic pain can occur after mastectomy and breast-conserving surgery, with studies indicating a prevalence of 24%-36% following mastectomy.18, 19, 20 However, comprehensive prevalence data on breast-conserving surgery remain underexplored, despite its increasing predominance within breast cancer surgery.21 This systematic review aimed to fill this knowledge gap by examining neuropathic pain prevalence after breast-conserving surgery. Additionally, the review intended to evaluate whether axillary procedures, commonly performed in addition to breast-conserving surgery, represent a potential risk factor for the development of neuropathic pain. Studying the prevalence of neuropathic pain post-breast-conserving surgery is essential to gain better understanding of the various types of pain, enabling us to identify targeted pain management strategies and improve patient outcomes among breast cancer survivors.
Methods
Literature search
The methods and results of this systematic review are written following the preferred reporting items of systematic reviews and meta-analyses (PRISMA) guidelines.22 The electronic bibliographic databases of Medline, Embase, Web of Science, and Cochrane Central were searched from inception until September 18, 2023. The full electronic search strategy, including the search terms, is detailed in the Appendix (Appendix 1).
Study selection
Four authors (EK, LG, KK, and PR) independently screened relevant studies based on titles and abstracts and reviewed the full-text articles according to predetermined inclusion criteria. These criteria required clinical studies reporting the prevalence of neuropathic pain after lumpectomy or breast-conserving surgery, with the pain persisting for at least 3 months post-surgery. Studies that did not distinguish neuropathic pain outcomes between different types of breast cancer surgery were excluded. Additionally, reviews, case reports, animal studies, conference abstracts, poster presentations, and non-English articles were excluded. Any discrepancies were resolved by consulting the last author (MZ).
Data extraction and quality scoring
During the data collection process, 2 authors (EK and LG) analyzed the included articles in detail and extracted data using a standardized data collection form. The following data were extracted: year of publication, publication classification, sample size, breast cancer surgeries, neuropathic pain assessment tool, proportion and percentage of patients with neuropathic pain after breast-conserving surgery, and the time to follow-up. The primary outcome was the prevalence of neuropathic pain following breast-conserving surgery, persisting for at least 3 months post-surgery. The secondary outcome focused on investigating any correlations between neuropathic pain after breast-conserving surgery and sentinel lymph node or axillary lymph node procedures.
Articles were classified according to the strength of evidence using the Jovell and Narvarro–Rubio classification (Appendix 2).23 Quality assessment was performed using the study quality assessment tools of the National Institutes of Health for observational cohort and cross-sectional studies (Appendix 3).24 The Jadad Scale was employed to evaluate the quality of randomized controlled trials (Appendix 4).25
Statistical analysis
The calculated prevalence rates of neuropathic pain following breast-conserving surgery in each study, along with their 95% confidence intervals (CIs), were visually depicted in a forest plot. The pooled prevalence of neuropathic pain among patients undergoing breast-conserving surgery in each included study was estimated using the “metaprop” function in R. Notably, studies were not weighted in the analysis. Heterogeneity among the included studies was assessed using the chi-squared test. A p-value <0.05 was considered statistically significant. I² values were interpreted in accordance with the Cochrane guidelines.26 A random effects model was employed when substantial heterogeneity between studies was suspected, based on the I² values and methodological differences between studies.
Results
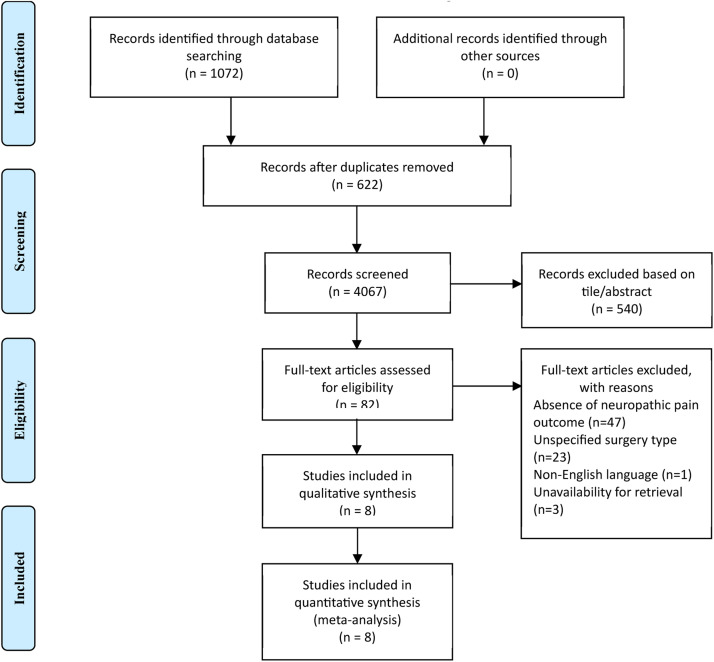
The literature search initially identified 1072 articles, among which 622 remained after duplicate removal following title and abstract screening. A detailed full-text review of 82 studies resulted in the inclusion of 8 articles meeting the predefined criteria (Figure 1).27, 28, 29, 30, 31, 32, 33, 34
Figure 1.
Flowchart depicting the selection of articles according to the PRISMA guidelines.
The overview of the included studies is detailed within Table 1, Table 2, primarily compromising observational cohort and cross-sectional studies. Four studies were specifically designed to assess pain prevalence following breast cancer surgery, while the remaining studies focused on investigating risk factors and treatment effects.27,28,31,33 In total, the 8 included studies encompassed 1,469 patients who underwent breast-conserving surgery.
Table 1.
Prevalence of chronic neuropathic pain following breast-conserving surgery.
| Author (year), LoE | Patients after BCS | Mean follow-up months (range) | Neuropathic pain assessment tool | Patients after BCS with neuropathic pain | % Neuropathic pain patients after BCS of total |
|---|---|---|---|---|---|
| Fuzier et al. (2022), III | 137 | 3 (0-4) | DN4 | 25 | 18.3% |
| Abdallah et al. (2021), II | 3 | 3 (0-3) | S-LANSS | 0 | 0% |
| Mustonen et al. (2019), III | 81 | 78 (48-108) | Clinical and physical evaluation (NeuPSIG) | 66 | 81.5% |
| Pereira et al. (2017), III | 236 | 13 (0-18) | Clinical and physical evaluation | 32 | 13.6% |
| Schou Bredal et al. (2014), III | 563 | 24 (24-72) | S-LANSS | 78 | 13.9% |
| Wilson et al. (2013), III | 316 | 12 (12-60) | Clinical and physical evaluation | 41 | 13.0% |
| Elkaradawy et al. (2012), II | 43 | 9 (0-12) | Clinical and physical evaluation | 31 | 72.1% |
| Carpenter et al. (1998), III | 54 | 35 (3-151) | Clinical and physical evaluation | 32 | 60.0% |
LoE, Level of evidence; BCS, Breast-conserving surgery; DN4, Douleur Neuropathique-4; S-LANSS, Self-report leeds assessment of neuropathic symptoms and sign.
Table 2.
Axillary surgery and chronic pain.
| Author (year), LoE | Patients after BCS | Patients after BCS + SLNB | Neuropathic pain after BCS + SLNB (%) | Patients after BCS + ALND | Neuropathic pain after BCS + ALND (%) |
|---|---|---|---|---|---|
| Fuzier et al. (2022), III | 137 | 43 | 93.0%a | 43 | 7.0%a |
| Abdallah et al. (2021), II | 3 | 3 | 0% | ||
| Mustonen et al. (2019), III | 81 | 81 | 81.5%* | 81 | 81.5%* |
| Pereira et al. (2017), III | 236 | 189 | 9.5% | 47 | 29.8% |
| Elkaradawy et al. (2012), II | 43 | 43 | 72.1% | ||
| Carpenter et al. (1998), III | 54 | 54 | 60% |
LoE, Level of evidence; BCS, Breast-conserving surgery; SLNB, Sentinel lymph node biopsy; ALND, Axillary lymph node dissection.
In the multivariate analyses, a distinction between chronic and neuropathic pain was not established.
Distinctions between SLNB and ALND were not made among patients undergoing breast-conserving surgery.
Neuropathic pain assessment employed validated instruments such as the Douleur Neuropatique Questionnaire (DN4), with neuropathic pain defined at a cut-off of 3 out of 7, and the Self-report Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS), defining neuropathic pain from 12 points on the questionnaire.27,28,31 Other studies adopted a comprehensive approach, combining clinical assessments, questionnaires, and physical or sensory examinations to evaluate neuropathic pain after breast-conserving surgery.
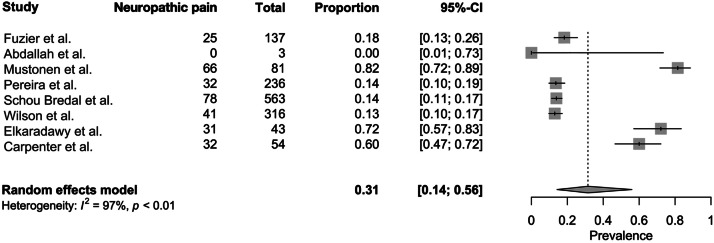
The prevalence of neuropathic pain after breast-conserving surgery ranged from 0% to 82% across the studies included in this review. A meta-analysis was conducted to ascertain the combined prevalence of neuropathic pain following breast-conserving surgery (Figure 2). The synthesis revealed a pooled prevalence estimate of neuropathic pain at 31% (95% CI 0.14-0.56) among patients who underwent breast-conserving surgery.
Figure 2.
Forest plot showing the prevalence of neuropathic pain following breast-conserving surgery.
Six studies investigated axillary procedures, specifically sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND), as a potential risk factor for chronic pain after breast-conserving surgery.27, 28, 29, 30,33,34 Pereira et al. reported a higher risk of neuropathic pain among patients following breast-conserving surgery with ALND, while other studies did not observe a higher risk.30 Two studies, including that of Pereira et al., conducted multivariate analyses revealing no significant associations between axillary procedures and neuropathic pain following breast-conserving surgery.27,30
Discussion
The aim of this systematic review and meta-analysis was to evaluate the prevalence of neuropathic pain following breast-conserving surgery and explore the potential associations between this pain and axillary procedures. The pooled prevalence of neuropathic pain following breast-conserving surgery was found to be 31%, with a CI ranging from 14% to 56%. The review did not provide conclusive evidence indicating the correlations between axillary procedures and neuropathic pain after breast-conserving surgery.
Previous studies mainly focused on the prevalence of chronic pain after breast cancer surgery, reporting rates between 25% and 60%.5, 6, 7, 8, 9 Although the term “chronic pain” is commonly used to cover various pain types, this review specifically focused on neuropathic pain, a subtype arising from damage to the peripheral nerve system. Recent literature estimates the prevalence of neuropathic pain following breast cancer surgery to range between 33% and 58%, highlighting its significant role in post-surgical chronic pain.5,10,11,35,36 Given that targeted interventions hold potential to alleviate neuropathic pain, recognizing the various types of chronic pain and acknowledging the presence of neuropathic pain can improve outcomes in breast cancer survivors.
A notable gap exists in the literature regarding differentiation mastectomy and breast-conserving surgery when reporting chronic pain. Although some studies have explored neuropathic pain prevalence after mastectomy (24% to 36%), comprehensive data on breast-conserving surgery are lacking, despite its predominance in breast cancer surgery.18, 19, 20, 21 This review addresses the gap by specifically studying neuropathic pain following breast-conserving surgery. The findings revealed a prevalence of 31% with a CI ranging from 14% to 56%.
Three of the included studies explored whether the type of surgery (mastectomy or breast-conserving surgery) may contribute to a higher prevalence of neuropathic pain. Bredal et al. (n = 832) and Carpenter et al. (n = 119) reported no significant association between the surgical type and incidence of neuropathic pain.31,34 In contrast, Mustonen et al. (n = 200) observed a higher prevalence of neuropathic pain following breast-conserving surgery compared to mastectomy in their multivariate analysis, even after adjusting for radiotherapy.29 Mustonen et al. suggested that better access and visualization of the axilla during mastectomy procedures might explain the difference in neuropathic pain prevalence.
The axilla is at risk for neuropathic pain during breast cancer surgery owing to the intercostobrachial nerve passing through it.10,21 Axillary surgeries, including SLNB and ALND, are potential risk factors for chronic pain.9,36, 37, 38, 39, 40 The secondary aim of the review was to investigate the correlation between neuropathic pain after breast-conserving surgery and axillary procedures. Among the 6 studies that focused on this aspect (n = 460), only Pereira et al. (n = 47) reported a relatively increased risk of neuropathic pain with ALND, though no statistical significance was found.30 The studies by Bredal et al. and Wilson et al. were excluded from the secondary evaluation as they did not differentiate between mastectomy and breast-conserving surgery in their reported outcomes. However, these studies (n = 412) revealed a significant correlation between ALND and neuropathic pain after breast cancer surgery.31,32 Overall, the current literature findings suggest a trend toward an increased risk but lack statistical support for a direct correlation between axillary procedures and neuropathic pain following breast-conserving surgery.
Broadly, neuropathic pain after breast-conserving surgery may arise from direct surgical injury to the intercostal and intercostobrachial nerves or because of indirect nerve damage caused by excessive stretching or compression due to scar formation.13, 14, 15, 16 This nerve injury triggers dysregulation of miRNAs and lncRNAs, contributing to neuropathic pain development through neuroinflammation and altered ion channel expression.41 Neuropathic pain manifests as burning, shooting pains, or altered skin sensations in the affected nerve's distribution area. The breast and chest wall are innervated by intercostal nerves T2-T6, whereas the intercostobrachial nerve supplies the upper medial arm and anterolateral chest wall.42 Pain sketches, where patients draw painful areas on a human body illustration, can help to identify the pain location and its distribution area, providing insights into which nerves may be affected.29,31 The studies included in this review generally lacked details on nerve pathology or identification of the specific nerve affected by the surgery. Mustonen et al. was an exception, and focused on patients with intercostobrachial nerve dissections and reported the highest neuropathic pain prevalence at 85.5%. This high prevalence was likely due to the selective inclusion of patients with confirmed nerve dissections. Interestingly, among the 81 patients studied, 15 had confirmed nerve damage without concurrent neuropathic pain, raising questions on the connection between nerve damage and the manifestation of neuropathic pain. Studies assessing neuropathic pain should prioritize identification of the affected nerve(s) and confirmation of its nerve-related nature, to enhance preventive or surgical management of neuropathic pain after breast-conserving surgery.
No gold standard exists for diagnosing neuropathic pain following breast-conserving surgery. Studies often use questionnaire-based assessments such as the DN4 and S-LANSS. However, these methods may miss 10%-20% of the clinically diagnosed cases.43,44 The Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain suggests a comprehensive approach, combining medical history, clinical examination, and diagnostic tests.44,45 Ilhan et al. compared various screening tools, revealing that the DN4 tends to show higher prevalence rates, aligning closely with the NeuPSIG criteria.35 Abdallah et al. directly compared the DN4 with NeuPSIG, reporting 90% sensitivity and 60% specificity for the DN4.46 Combining standardized screening tools such as the DN4 with in-depth evaluations guided by NeuPSIG criteria is recommended for a comprehensive understanding of neuropathic pain after breast cancer surgery.35
The systematic review encountered several limitations. Despite an extensive search effort, the final analysis included a limited number of studies (n = 8). This can be attributed to the scarcity of available research, coupled with challenges in distinguishing between chronic pain types (nociceptive or neuropathic) and surgical procedures (mastectomy or breast-conserving surgery). Additionally, the use of diverse assessment tools for diagnosing neuropathic pain across studies hindered inter-study comparisons. Notably, the absence of standardized guidelines for neuropathic pain assessment was evident. Furthermore, the differences in surgical inclusion criteria, such as specific nerve dissections or variations in axillary procedures, complicated direct comparisons of neuropathic pain prevalence between studies, as certain patient groups may have been more predisposed to neuropathic pain than the others. Lastly, the lack of studies with extended follow-up hindered a comprehensive long-term analysis. Longer follow-up, surpassing the conventional 3-month threshold, could provide a more complete understanding of neuropathic pain after breast-conserving surgery.
This systematic review offers valuable insights into the prevalence of neuropathic pain after breast-conserving surgery. Nevertheless, the identified limitations underscore the pressing need for future studies to employ more comprehensive and standardized approaches in investigating post-breast cancer surgery pain. Central to this issue is the need to accurately assess neuropathic pain within the broader scope of chronic pain and to confirm its nerve-related origin. Moreover, healthcare professionals should heighten their awareness of the neuropathic component in breast cancer survivors. A deeper understanding of neuropathic pain following breast-conserving surgery is key to tailoring interventions that can reduce long-lasting neuropathic pain experienced by the survivors of post-breast-conserving surgery.
Conclusion
The systematic review and meta-analysis focused on neuropathic pain post-breast-conserving surgery, revealing a prevalence of 31%, with a CI ranging from 14% to 56%. These results highlight the considerable burden of neuropathic pain within this specific population. The review did not identify any substantial correlations between the axillary procedures and development of neuropathic pain after breast-conserving surgery.
Acknowledgments
Acknowledgments
The authors wish to thank W. Bramer, biomedical information specialist from the Erasmus MC for developing the search strategies.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
None.
Ethical approval
Not required.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2024.07.021.
Contributor Information
Esmee Kwee, Email: e.kwee@erasmusmc.nl.
J. Michiel Zuidam, Email: j.zuidam@erasmusmc.nl.
Appendix. Supplementary materials
References
- 1.Taylor C, McGale P, Probert J, Broggio J, Charman J, Darby SC, et al. Breast cancer mortality in 500 000 women with early invasive breast cancer diagnosed in England, 1993-2015: Population based observational cohort study. BMJ. 2023 Jun 13;381:e074684. doi: 10.1136/bmj-2022-074684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo F, Kuo Y, Shih YCT, Giordano SH, Berenson AB. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer. 2018 Sep 1;124(17):3500–3509. doi: 10.1002/cncr.31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: An investigation of women's experiences. Pain. 1995;61:61–68. doi: 10.1016/0304-3959(94)00162-8. [DOI] [PubMed] [Google Scholar]
- 4.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 5.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725e746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Kudel I, Edwards RR, Kozachik S, et al. Predictors and consequences of multiple persistent postmastectomy pains. J Pain Symptom Manage. 2007;34:619e627. doi: 10.1016/j.jpainsymman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: A prospective study. J Pain. 2006;7:626e634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Gartner R, Jensen MB, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 11.Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: A systematic literature review. Pain. 2013;154:95e102. doi: 10.1016/j.pain.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 12.IASP international association for the study of pain. Available at: https://www.iasp-pain.org/advocacy/global-year/neuropathic-pain/.
- 13.Dini D, Bertelli G, Gozza A, Forno GG. Treatment of the post-mastectomy pain syndrome with topical capsaicin. Pain. 1993;54:223–226. doi: 10.1016/0304-3959(93)90213-9. [DOI] [PubMed] [Google Scholar]
- 14.Elliott K, Foley KM. Neurologic pain syndromes in patients with cancer. Neurol Clin. 1989;7:333–360. [PubMed] [Google Scholar]
- 15.Murtha SD. In: Decision Making in Pain Management. Ramamurthy S, Rogers JN, editors. Mosby; New York: 1993. Postmastectomy pain; pp. 114–115. [Google Scholar]
- 16.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: Proposed classification and research update. Pain. 2003;104:1e13. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 17.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: Understanding the perioperative process. Ann Surg. 2013;257:403–412. doi: 10.1097/SLA.0b013e3182701a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y, Tan Q, Qin Q, Wei C. Prevalence of postmastectomy pain syndrome and associated risk factors: A large single-institution cohort study. Medicine (Baltimore) 2020 May;99(20):e19834. doi: 10.1097/MD.0000000000019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakati B, Nair N, Chatterjee A. Post mastectomy pain syndrome at an Indian tertiary cancer centre and its impact on quality of life. Indian J Cancer. 2023 Apr-Jun;60(2):275–281. doi: 10.4103/ijc.ijc_861_21. PMID: 37530253. [DOI] [PubMed] [Google Scholar]
- 20.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99:604–610. doi: 10.1038/sj.bjc.6604534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson JA, Rubenstein RN, Haglich K, Chu JJ, Yin S, Stern CS, et al. Analysis of a trend reversal in US lumpectomy rates from 2005 through 2017 using 3 nationwide data sets. JAMA Surg. 2022 Aug 1;157(8):702–711. doi: 10.1001/jamasurg.2022.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The preferred reporting items for systematic reviews and meta-analyses (PRISMA). Available at: http://www.prisma-statement.org/. [DOI] [PubMed]
- 23.Jovell AJ, Navarro-Rubio MD. Evaluación de la evidencia científica [Evaluation of scientific evidence] Med. Clin. 1995;105:740–743. (In Spanish) [PubMed] [PubMed] [Google Scholar]
- 24.National Heart, Blood and Lung Institute. Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 25.Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999 Oct;20(5):448–452. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.4. 2023. www.training.cochrane.org/handbook updated August 2023CochraneAvailable from. [Google Scholar]
- 27.Fuzier R, Salvignol G, Gilbert O, Bremaud M, Bataille B, Izard P. Influence of deep serratus anterior plane block on chronic pain at 3 months after breast-conserving surgery: Prospective, cohort study. Clin J Pain. 2022 Jun 1;38(6):418–423. doi: 10.1097/AJP.0000000000001035. [DOI] [PubMed] [Google Scholar]
- 28.Abdallah FW, Patel V, Madjdpour C, Cil T, Brull R. Quality of recovery scores in deep serratus anterior plane block vs. sham block in ambulatory breast cancer surgery: A randomised controlled trial. Anaesthesia. 2021 Sep;76(9):1190–1197. doi: 10.1111/anae.15373. [DOI] [PubMed] [Google Scholar]
- 29.Mustonen L, Aho T, Harno H, Sipilä R, Meretoja T, Kalso E. What makes surgical nerve injury painful? A 4-year to 9-year follow-up of patients with intercostobrachial nerve resection in women treated for breast cancer. Pain. 2019 Jan;160(1):246–256. doi: 10.1097/j.pain.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes J, et al. Neuropathic pain after breast cancer treatment: Characterization and risk factors. J Pain Symptom Manage. 2017 Dec;54(6):877–888. doi: 10.1016/j.jpainsymman.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Bredal IS, Smeby NA, Ottesen S, Warncke T, Schlichting E. Chronic pain in breast cancer survivors: Comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014 Nov;48(5):852–862. doi: 10.1016/j.jpainsymman.2013.12.239. [DOI] [PubMed] [Google Scholar]
- 32.Wilson GC, Quillin RC, Hanseman DJ, Lewis JD, Edwards MJ, Shaughnessy EA. Incidence and predictors of neuropathic pain following breast surgery. Ann Surg Oncol. 2013 Oct;20(10):3330–3334. doi: 10.1245/s10434-013-3156-6. [DOI] [PubMed] [Google Scholar]
- 33.Elkaradawy S, Nasr M, Elkerm Y, Deeb ME, Yassine O. The effect of multimodal balanced anaesthesia and long term gabapentin on neuropathic pain, nitric oxide and interleukin-1β following breast surgery. Egypt J Anaesth. 2012 Jan 1;28(1):67–78. [Google Scholar]
- 34.Carpenter JS, Andrykowski MA, Sloan P, Cunningham L, Cordova MJ, Studts JL, et al. Postmastectomy/postlumpectomy pain in breast cancer survivors. J Clin Epidemiol. 1998 Dec;51(12):1285–1292. doi: 10.1016/s0895-4356(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 35.Ilhan E, Chee E, Hush J, Moloney N. The prevalence of neuropathic pain is high after treatment for breast cancer: A systematic review. Pain. 2017;158:2082–2091. doi: 10.1097/j.pain.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 36.Mejdahl MK, Andersen KG, Gartner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: Six year nationwide follow-up study. BMJ. 2013;346:f1865. doi: 10.1136/bmj.f1865. [DOI] [PubMed] [Google Scholar]
- 37.Smith WC, Bourne D, Squair J, Phillips DO, Chambers WA. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91–95. doi: 10.1016/s0304-3959(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 38.Wallace MS, Wallace AM, Lee J, Dobke MK. Pain after breast surgery: A survey of 282 women. Pain. 1996;66:195–205. doi: 10.1016/0304-3959(96)03064-3. [DOI] [PubMed] [Google Scholar]
- 39.Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156:2413–2422. doi: 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 40.Meretoja TJ, Leidenius MH, Tasmuth T, Sipila R, Kalso E. Pain at 12 months after surgery for breast cancer. JAMA. 2014;311:90–92. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]
- 41.Jiang M, Wang Y, Wang J, Feng S, Wang X. The etiological roles of miRNAs, lncRNAs, and circRNAs in neuropathic pain: A narrative review. J Clin Lab Anal. 2022 Aug;36(8):e24592. doi: 10.1002/jcla.24592. Epub 2022 Jul 9. PMID: 35808924PMCID: PMC9396192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beederman M, Bank J. Post-breast surgery pain syndrome: Shifting a surgical paradigm. Plast Reconstr Surg Glob Open. 2021 Jul 22;9(7):e3720. doi: 10.1097/GOX.0000000000003720. PMID: 34316427PMCID: PMC8301281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010e1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 44.Haanpa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 46.Abdallah FW, Morgan PJ, Cil T, Escallon JM, Semple JL, Chan VW. Comparing the DN4 tool with the IASP grading system for chronic neuropathic pain screening after breast tumor resection with and without paravertebral blocks: A prospective 6-month validation study. Pain. 2015;156:740–749. doi: 10.1097/j.pain.0000000000000108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.