Abstract
ZAP-70, a Syk family cytoplasmic protein tyrosine kinase (PTK), is required to couple the activated T-cell antigen receptor (TCR) to downstream signaling pathways. It contains two tandem SH2 domains that bind to phosphorylated TCR subunits and a C-terminal catalytic domain. The region connecting the SH2 domains with the kinase domain, termed interdomain B, has previously been shown to have striking regulatory effects on ZAP-70 function, presumed to be due to the recruitment of key substrates. Paradoxically, deletion of interdomain B preserves ZAP-70 function. Recent structural studies of several receptor tyrosine kinases (RTKs) revealed that their juxtamembrane regions negatively regulate their catalytic activities. In EphB2 and several other RTKs, this autoinhibition depends upon interaction between the kinase domain and tyrosine residues within the juxtamembrane region. Autoinhibition is released when these tyrosines become phosphorylated following receptor stimulation. Sequence homology suggested analogous regulation for ZAP-70. Based on mutagenesis analysis of ZAP-70 interdomain B, we find that this region downregulates ZAP-70 catalytic activity in a similar manner as the juxtamembrane region of EphB2. Similar regulation was also noted for the related Syk kinase. These findings suggest that a general autoinhibitory mechanism employed by RTKs is also used by some cytoplasmic tyrosine kinases.
Stimulation of the T-cell antigen receptor (TCR) leads to a series of signaling events that result in changes in T-cell function and gene expression. Signaling is initiated by two families of protein tyrosine kinases (PTKs) (17). Src-family kinases first phosphorylate specific immunoreceptor tyrosine-based activation motifs (ITAMs) (4) in the cytoplasmic tails of the CD3 and ζ subunits of TCR. Each ITAM is composed of a pair of tyrosines separated by 9 to 11 amino acids which, when phosphorylated, bind the Syk family PTK ZAP-70. In turn, ZAP-70 becomes activated and subsequently phosphorylates a number of key downstream signaling molecules necessary for further signal propagation.
ZAP-70 consists of two N-terminal SH2 domains, responsible for binding to doubly phosphorylated ITAMs, and a C-terminal tyrosine kinase domain (6). The SH2 domains are separated by a linker region, termed interdomain A. The region between the SH2 domains and the kinase domain is known as interdomain B.
Upon binding to phosphorylated ITAMs of the activated TCR, ZAP-70 itself becomes phosphorylated on multiple tyrosine residues, both by Src family PTKs and via autophosphorylation. It is well established that tyrosine phosphorylation plays an important role in ZAP-70 activation, but the precise mechanism responsible is still incompletely understood. Among the best-studied phosphorylation sites are tyrosines 492 and 493 (Y492 and Y493) in the activation loop of the kinase (5, 33, 34). A model has been proposed whereby Y493 becomes phosphorylated first by the Src-family PTK Lck. This phosphorylation is then followed by autophosphorylation at Y492 (5). Phosphorylation at both sites is most likely required for displacement of the activation loop from the catalytic site and, hence, activation of the kinase. However, the recent crystal structure of the isolated nonphosphorylated ZAP-70 kinase domain complexed with staurosporine fails to show blockade of the catalytic site by the activation loop (19). This suggests that some other mechanism may be responsible for regulation of the catalytic domain in the context of the intact ZAP-70 molecule.
Another possible means of regulation could involve other phosphorylation sites, such as Y292, Y315, and Y319, in interdomain B of ZAP-70. Y292 has a negative regulatory function, possibly due to the recruitment of the E3 ubiquitin ligase c-Cbl. The interaction between phosphorylated Y292 and c-Cbl is well documented, and a crystal structure of the Y292-containing phosphopeptide bound to the phosphotyrosine binding (PTB) domain of c-Cbl has been solved (22, 25, 27). However, the importance of this interaction has been challenged by a recent study where knock-in mice expressing a c-Cbl mutant that is unable to bind ZAP-70 did not show major alterations in ZAP-70 expression or function (31).
Interdomain B tyrosines Y315 and Y319 have been proposed to be positive regulatory sites of phosphorylation. It has been demonstrated that both tyrosines become phosphorylated following TCR cross-linking, and ZAP-70 itself (8) and Lck (36) have both been suggested to be responsible for this phosphorylation. Additionally, c-Abl has recently been shown to be able to phosphorylate Y319 (41). In multiple experimental systems, mutations of Y315 or Y319 to phenylalanines substantially impaired ZAP-70-dependent signaling events, thought to be primarily due to the inability of mutated ZAP-70 to recruit downstream effector molecules (8, 12, 13, 23, 36, 37). Probably best studied is the interaction between phosphorylated Y319 and the SH2 domain of Lck, which has been proposed to play an important role in the subsequent Lck-mediated phosphorylation of Y493 of ZAP-70 as a part of a positive feedback mechanism (26, 30). There also is evidence that other molecules, including Vav1 (37), phospholipase C-γ1 (PLC-γ1) (36), and possibly Crk (11) bind interdomain B tyrosines. Therefore, it was rather surprising that an internal deletion which removed most of interdomain B, including Y292, Y315, and Y319, did not have any major effect on ZAP-70-dependent signaling events (40). This suggests that interdomain B has a regulatory function independent of any interacting molecules.
Such regulatory elements have been recently discovered in the juxtamembrane regions of several receptor tyrosine kinases (RTKs) (for review, see reference 15). In RTK EphB2, tyrosines Y604 and Y610 in the juxtamembrane region promote an interaction between the juxtamembrane region and the kinase domain. This leads to substantial alterations in the structure of the kinase domain and inhibition of kinase activity (38). Ligand-induced oligomerization of EphB2 leads to autophosphorylation of Y604 and Y610, which disrupts the autoinhibitory interaction and results in an upregulation of the kinase activity. Based on the sequence similarity between the juxtamembrane region of EphB2 and interdomain B of ZAP-70, we hypothesized that interdomain B plays an analogous role in autoinhibition of ZAP-70 kinase activity. Our data support a novel model of ZAP-70 activation in which Y315 and Y319 of interdomain B play an important structural role in autoinhibition of ZAP-70 and their phosphorylation is an essential prerequisite for putative structural changes leading to the full activation of the kinase.
MATERIALS AND METHODS
Cells, cDNA constructs, and antibodies.
P116, a ZAP-70-deficient Jurkat-derived T-cell line, was obtained from R. Abraham (Burnham Institute, La Jolla, CA). 293 cells, a kidney epithelial cell line, were obtained from the American Type Culture Collection (Manassas, VA). A QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and standard PCR techniques were utilized to prepare the ZAP-70 mutations (Y315/319F, Y315/319A, Y315/319E, K369A, Δ265-331) in the expression plasmid pcDNA3 (Invitrogen, Carlsbad, CA) and the Syk mutations (Y342/346F, Y342/346A) in the expression plasmid pEF6 (Invitrogen). Hemagglutinin (HA)-tagged rat wild-type (WT) Syk was provided by J. B. Bolen (DNAX Research Institute of Molecular and Cellular Biology, Palo Alto, CA). C-terminal, Myc-tagged wild-type, and ΔSH3 c-Abl were constructed by cloning EcoRI fragments of wild-type and ΔSH3 c-Abl from pSRα-MSV-TKneo (obtained from Marie Anne Pendergast, Duke University Medical Center, Durham, NC), into pBS SK. A BglII-XbaI PCR product covering the last 90 coding base pairs of c-Abl with the XbaI site destroying the stop codon was cloned in frame with c-Abl or ΔSH3 c-Abl from pBS (NotI-partial BglII fragment) into NotI-XbaI-digested pEF6Myc/HisA. Lck (24), FynT (7), FLAG-tagged linker for activation in T cells (LAT) (2), non-T-cell activation linker (NTAL) (2), and CD8-TCR-ζ (16) constructs have been described previously. Monoclonal antibody (MAb) 1F6 (anti-Lck) was obtained from J. B. Bolen. Anti-phosphotyrosine MAb 4G10 and mixed MAbs against PLC-γ1 were purchased from Upstate Biotechnology (Charlottesville, VA). Anti-FLAG-tag MAb M2 was from Sigma (St. Louis, MO). Rabbit antisera to PLC-γ1-pY783, LAT-pY132, LAT-pY191, and SLP-76-pY145 were purchased from Biosource (Camarillo, CA); rabbit antiserum against LAT was from Novus Biologicals (Littleton, CO); rabbit antiserum against SLP-76 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); and mouse monoclonal antibody 9B11 against Myc epitope and rabbit antisera against ZAP-70, pY493, and pY319 were from Cell Signaling Technology (Beverly, MA). Rabbit antisera against Fyn were from EMD Biosciences (La Jolla, CA). Monoclonal antibodies C305 (anti-TCR-β) (35), 2F3.2 (anti-ZAP-70) (17), 6B10 (anti-TCR-ζ) (32), and NAP-4 (anti-NTAL) (2) have been described previously.
Transfections and transcriptional assays.
For transient transfections of P116 cells, 20 μg of NFAT luciferase reporter plasmid DNA (29) and 20 μg of ZAP-70 or Syk expression constructs were electroporated at 250 V, 960 μF, using the Bio-Rad Gene Pulser electroporator (Bio-Rad Laboratories, Hercules, CA). Sixteen hours after the transfection, cells were stimulated with anti-TCR (1:1,000 C305 ascites) or phorbol 12-myristate 13-acetate (PMA; 20 ng/ml) and ionomycin (1 μM). Approximately 8 h later, the cells were harvested, lysed, and assayed for luciferase activity. Stably transfected cell lines were generated by transfection of 20 μg WT or mutated ZAP-70 expression constructs into the parental P116 cell line. Clones were isolated by limiting dilution in the presence of G418 (2 mg/ml; Invitrogen). Transient transfections of 293 and Cos cells were carried out in 24-well plates using Lipofectamine and PLUS reagents (Invitrogen) according to the manufacturer's instructions.
Measurement of free intracellular calcium concentration.
Cells were loaded with the fluorescent dye indicator Indo-1 (Molecular Probes, Eugene, OR) and stimulated with anti-TCR MAb C305 (1:1,000 ascites). The fluorescence at 400- and 500-nm wavelengths was measured with a Hitachi F-4500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan), and the concentration of free intracellular calcium was calculated from the ratio of fluorescence at the two wavelengths (14).
Cell stimulation, lysate preparation, immunoprecipitation, and Western blot analyses.
P116 cells (108/ml phosphate-buffered saline) transfected with ZAP-70 constructs were stimulated with anti-TCR MAb C305 (1:100 or 1:1,000 ascites) at 37°C for the time indicated, immediately pelleted, and lysed in ice-cold lysis buffer (20 mM Tris, pH 7.6, 150 mM NaCl, 1% NP-40, 0.01% NaN3, and a cocktail of protease and phosphatase inhibitors). Postnuclear supernatant was either used for immunoprecipitation with antibody prebound to Sepharose-protein A or -protein G beads (Amersham Biosciences, Buckinghamshire, United Kingdom) or diluted with 2× concentrated sodium dodecyl sulfate (SDS) sample buffer containing 2-mercaptoethanol for whole-cell lysates. Where indicated, whole-cell lysates were prepared by resuspending the cells in 2× concentrated SDS sample buffer (200 μl per 5 × 106 P116 cells or 60 μl per well of 293 cells) followed by ultracentrifugation (30 min, 300,000 × g). Samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting with the indicated primary and horseradish peroxidase-conjugated secondary antibody. Proteins were then detected by chemiluminescence (Western Lightning; Perkin-Elmer, Wellesley, MA) on a Kodak Image Station (Kodak, Rochester, NY).
RESULTS
Mutation of tyrosines 315 and 319 of ZAP-70 to alanines does not compromise ZAP-70 function in Jurkat T cells.
In a number of experimental models, mutations of Y315 or Y319 to phenylalanine had adverse effects on ZAP-70-mediated signaling events. Surprisingly, removal of amino acids 265 to 331 from interdomain B, containing these tyrosines, had only mild consequences (40). To reconcile these conflicting observations, we analyzed the amino acid sequence of interdomain B and found that Y315 and Y319 reside within motifs that have striking homology to motifs containing negative regulatory tyrosines located in the juxtamembrane region of EphB2 (Fig. 1A). Similar sequence homology was seen in the Syk PTK. Moreover, the distance of these residues from the kinase domains of ZAP-70 and Syk was similar to that of the tyrosines in EphB2. Therefore, we hypothesized that the segment of interdomain B of ZAP-70 containing Y315 and Y319 autoinhibits its catalytic function via a mechanism similar to that of the EphB2 juxtamembrane region. In EphB2, mutation of juxtamembrane tyrosines Y604 and Y610 to structurally related but unphosphorylatable phenylalanines constrained the kinase into an inactive conformation (38). We reasoned that ZAP-70 could be under similar regulatory constraint. According to this model, replacement of Y315 and Y319 in ZAP-70 with phenylalanines should lock ZAP-70 into an inactive state. We also hypothesized that mutation of these residues to structurally distant alanines could free ZAP-70 from this inhibitory constraint. To test this hypothesis, we made several expression constructs of ZAP-70. In addition to WT ZAP-70, we made a mutant form of ZAP-70 with Y315 and Y319 mutated to phenylalanine residues (YYFF) and other mutants with substitution for Y315 and Y319 with alanine residues (YYAA) or glutamic acids (YYEE). Additional control constructs included a kinase-inactive ZAP-70 (KA) where lysine 369 in the ATP binding site was mutated to alanine and a ZAP-70 construct lacking amino acids 265 to 331 in interdomain B (Δ IB) (Fig. 1B).
FIG. 1.
ZAP-70 sequence analysis and mutant generation. (A) Schematic diagram of ZAP-70 illustrating functional domains and the homology between ZAP-70 interdomain B and EphB2 juxtamembrane region. (B) Diagrammatic representation of ZAP-70 expression constructs. All are in the pcDNA3 expression plasmid backbone.
Stimulation of the TCR on the ZAP-70 deficient Jurkat mutant P116 fails to induce signaling events downstream of the receptor, including the activation of NFAT (nuclear factor of activated T cells) transcriptional reporter constructs, calcium flux, mitogen-activated protein (MAP) kinase activation, and phosphorylation of multiple important downstream signaling molecules. To analyze the role of Y315/Y319 in NFAT activation, we transiently transfected P116 cells with the ZAP-70 constructs described above together with an NFAT-driven luciferase reporter construct and measured TCR-induced luciferase production in these transfectants. We also established clones stably expressing the same ZAP-70 constructs and analyzed them in a similar fashion. The TCR induction of NFAT could be restored by transient or stable transfection of WT ZAP-70 (Fig. 2A and B). Whereas the YYFF mutant and a catalytically inactive (KA) mutant failed to restore NFAT responses, both the interdomain B deletion and the YYAA mutant restored TCR-mediated NFAT responses. These results are consistent with the notion that the Y315/Y319 sites are involved in a negative regulatory function and that recruitment of critical effector molecules is not necessary for downstream signaling. Consistent with this, TCR-induced calcium responses (Fig. 2C) and activation of MAP kinases Erk1 and Erk2 (Fig. 2D) were restored not only by WT ZAP-70 but also by the YYAA mutant in the stable transfectants.
FIG. 2.
Initial functional analysis of ZAP-70 mutants in P116 Jurkat cells. (A) NFAT activation in P116 cells reconstituted with ZAP-70 mutants. P116 cells were transiently transfected with 20 μg NFAT-luciferase and 20 μg vector only or the ZAP-70 expression plasmid. The cells were left unstimulated, stimulated with anti-TCR or with PMA plus ionomycin, and then assayed for luciferase activity. The data are represented as the fraction of the activity obtained with PMA plus ionomycin. Error bars represent the standard deviation. Blots displaying the relative ZAP-70 expression levels are shown below. (B) Representative stable clones expressing ZAP-70 mutants were transfected with 20 μg NFAT-luciferase reporter plasmid and assayed for their TCR-induced activation of NFAT. Data are represented as described for transient assays. (C) Intracellular Ca2+ flux in cells loaded with Indo-1 dye followed by addition of anti-TCR antibody (indicated with arrow). The concentration of Ca2+ was calculated using the ratio of emission at 400 nm to that at 500 nm as described in Materials and Methods. (D) TCR-induced phosphorylation of Erk1 and Erk2 MAP kinases. Cells were left unstimulated or stimulated with anti-TCR antibody for 2, 5, or 20 min, and whole-cell lysates were blotted for phospho-Erk1/2 and total levels of Erk1/2.
YYAA ZAP-70 rescues TCR-dependent tyrosine phosphorylation of signaling proteins in P116 cells.
One of the most proximal measures of ZAP-70-dependent TCR activation is tyrosine phosphorylation of a number of substrates by PTKs. To elucidate the influence of interdomain B tyrosines on TCR-mediated tyrosine phosphorylation of downstream signaling proteins, we analyzed unstimulated and TCR-stimulated P116 cells stably transfected with WT and mutant ZAP-70 constructs by antiphosphotyrosine immunoblotting. Analysis of whole-cell lysates revealed that TCR stimulation of the cells transfected with WT ZAP-70 resulted in phosphorylation of multiple proteins (Fig. 3A), including a 36- to 38-kDa phosphoprotein likely representing an important ZAP-70 substrate transmembrane adaptor protein, LAT (39). Consistent with the data in the previous figure, inducible tyrosine phosphorylation could also be reconstituted by the YYAA mutant, albeit to a modestly reduced extent compared to the wild-type protein. In contrast, the cells expressing the YYFF mutant exhibited reduced responses, while the inactive KA mutant failed to restore inducible tyrosine phosphorylation. An exception to this was the observation of a constitutively hyperphosphorylated 70-kDa phosphoprotein in the lysates of the KA-transfected cells, which most likely represented ZAP-70 itself, as discussed below. Consistent with overall tyrosine phosphorylation, a similar pattern of responsiveness was also observed for several specific substrates as detected by site-directed phospho-specific antibodies against the key downstream signaling molecules, including the ZAP-70 substrates LAT and SLP-76 as well as PLC-γ1 (Fig. 3B).
FIG. 3.
Effects of ZAP-70 mutations on TCR-induced tyrosine phosphorylation. P116 stable transfectants were incubated for 1 min with or without anti-TCR antibody (C305), lysed in 2× concentrated SDS-PAGE sample buffer, and subjected to Western blot analysis with (A) antiphosphotyrosine antibody (4G10) and (B) phospho-specific (as indicated) as well as generic antibodies to LAT, PLC-γ1, and SLP-76.
Recruitment of ZAP-70 to the TCR is altered in ZAP-70 mutant cells.
One of the first events thought to occur following TCR engagement is the phosphorylation of the TCR-ζ chain by the PTK Lck, which leads to the recruitment of ZAP-70 via its SH2 domains to the newly phosphorylated ITAMs (17). Since this is one of the necessary steps in ZAP-70 activation, we next analyzed how mutations in interdomain B affected binding of ZAP-70 to TCR-ζ. We immunoprecipitated TCR-ζ from unstimulated and TCR-stimulated P116 transfectants and analyzed the proteins by immunoblotting (Fig. 4). All cell lines showed an inducible tyrosine phosphorylation of TCR-ζ. However, there was a reproducibly marked increase in basal and inducible TCR-ζ phosphorylation in the YYAA mutant. Inducible TCR-ζ phosphorylation was also reproducibly elevated above wild-type levels in both YYFF and KA mutants.
FIG. 4.
Ability of ZAP-70 mutants to associate with TCR-ζ chain. P116 stable transfectants were incubated with or without anti-TCR antibody, lysates were prepared, and anti-TCR-ζ immunoprecipitations were performed. Immunoprecipitates were subjected to SDS-PAGE and blotted for TCR-ζ, phosphotyrosine, and ZAP-70, as indicated.
In addition to the phosphorylated TCR-ζ, tyrosine-phosphorylated ZAP-70 was also found in these TCR-ζ immunoprecipitates (Fig. 4). Surprisingly, compared to the WT ZAP-70, the KA mutant was hyperphosphorylated in both the basal and activated states, while phosphorylation of YYAA and YYFF was only barely detectable. The blot containing these TCR-ζ immunoprecipitates was stripped and reprobed for the total level of ZAP-70 associated with TCR-ζ. Unexpectedly, in all ZAP-70 mutant-expressing cell lines, there was an increase in the amount of ZAP-70 associated with TCR-ζ. Assuming that some level of tonic signaling is present via the TCR pathway in unstimulated Jurkat cells, as we have previously reported (28), it would appear that the Y315/Y319 sites not only may play a role in negatively regulating ZAP-70 catalytic activity, but also may serve to regulate its association or turnover on TCR-ζ ITAM phosphorylation sites. The intrinsic ability of ZAP-70 mutants to bind more efficiently to TCR-ζ ITAMs may also lead to the protection of TCR-ζ ITAMs from dephosphorylation by cellular phosphatases, thus accounting for the observed increase in TCR-ζ phosphorylation.
Phosphorylation of mutant ZAP-70 proteins.
In an effort to understand the contributions of Y315/Y319 phosphorylation to ZAP-70 activation, we used phospho-specific antibodies to Y319 and Y493 to analyze the phosphorylation status of ZAP-70 proteins in the whole-cell lysates of P116 transfectants. TCR stimulation of cells expressing WT ZAP-70 led to inducible phosphorylation of Y319. Consistent with previous experiments, we observed constitutive hyperphosphorylation of Y319 in the inactive KA mutant that could be further enhanced by TCR stimulation. TCR-inducible phosphorylation of the activation loop Y493 could be detected for all ZAP-70 constructs (Fig. 5A). The phosphorylation of the YYAA mutant at Y493 was reduced compared to WT ZAP-70. The Y493 phosphorylation of YYFF mutant was even more reduced, especially considering the higher expression level of YYFF protein compared to the YYAA. Similar to Y319, Y493 was also hyperphosphorylated in the inactive KA mutant. The constitutive hyperphosphorylation of Y319 and Y493 in the KA-inactive mutant may reflect the failure of negative regulatory pathways to be activated in the absence of an active ZAP-70 kinase.
FIG. 5.
Phosphorylation of ZAP-70 at tyrosines 319 and 493. (A) Lysates of nonstimulated and TCR-stimulated P116 cells stably expressing WT and mutant ZAP-70 proteins were analyzed by immunoblotting with the indicated antibodies. (B) 293 cells were transiently transfected with ZAP-70 WT or mutant constructs and with or without Lck, lysed in 2× concentrated SDS-PAGE sample buffer, and subjected to Western blot analysis. (C) NP-40 lysates of 293 cells, transiently transfected with Lck, CD8-ζ, and different ZAP-70 constructs, were subjected to anti-TCR-ζ immunoprecipitation followed by immunoblotting. In the negative control, the CD8-ζ construct was omitted from the transfection mixture. (D) Cos cells were transiently transfected with ZAP-70 constructs and with Lck, Fyn, c-Abl, or constitutively active cAblΔ SH3 and analyzed as in panel B. The constructs used for transfections are indicated at the top of each panel, with antibodies used for immunoprecipitations (IP) and immunoblotting (BLOT) on the right.
In order to simplify these phosphorylation events and examine the in vivo catalytic function of the ZAP-70 interdomain B double-tyrosine mutants, we overexpressed these mutants in 293 cells alone, or together with Lck, and performed similar analyses using site-directed phospho-specific antibodies. In the absence of Lck, only the YYAA mutant exhibited constitutive phosphorylation of Y493 in the activation loop (Fig. 5B). Phosphorylation of Y493 and Y319 in WT ZAP-70 required the presence of Lck. Interestingly, although Y319 was phosphorylated in the KA mutant, Y493 was not phosphorylated in this mutant or in the YYFF mutant. We also expressed these ZAP-70 constructs together with Lck and a CD8-TCR-ζ chimera (as a simplified TCR surrogate), and immunoprecipitated CD8-TCR-ζ from the lysates of the transfected cells. All constructs appeared to be bound equivalently to phosphorylated TCR-ζ sequences (Fig. 5C). Very similar site-specific tyrosine phosphorylation of ZAP-70 was observed, even in the presence of a CD8-TCR-ζ chain chimera (Fig. 5C). These findings are consistent with the notion that Y319 (and possibly Y315) are Lck phosphorylation sites and regulate accessibility of Y493 in the activation loop to phosphorylation. Results of experiments with single-tyrosine mutants (Y315F, Y319F, Y315A, Y319A) recapitulated those with double mutants; in all cases, the alanine mutants were more active than phenylalanine mutants (data not shown). To study the kinases involved in phosphorylation of Y319 and Y493 in greater detail, we performed a similar experiment using expression constructs of Fyn, c-Abl, and a constitutively active form of c-Abl (c-Abl Δ SH3). Fyn has been shown to interact with and to phosphorylate ZAP-70 (10), although specific phosphorylation sites in ZAP-70 have not been identified. c-Abl has been previously shown to phosphorylate Y319 of ZAP-70 (41). Since we could not observe any kinase activity of FynT in 293 cells, we employed Cos cells in this experiment. As shown in Fig. 5D, all of the kinases were able to induce a similar pattern of ZAP-70 phosphorylation as that induced by Lck. Relative efficiency of the kinases used, however, could not be evaluated since the relative level of their expression could not be estimated in this experiment.
Kinase activity of ZAP-70 and Syk mutants expressed in 293T cells.
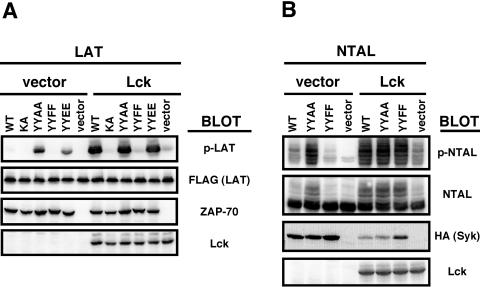
To examine the catalytic activities of these mutants, we immunoprecipitated them from P116 lysates and performed in vitro kinase assays using a glutathione S-transferase (GST) fusion to the cytoplasmic domain of band 3 protein as a substrate, but failed to detect substantial differences in catalytic activity (data not shown). However, when we transfected 293 cells with the lipid raft-anchored ZAP-70 kinase substrate LAT together with the ZAP-70 constructs in the presence or absence of Lck, we observed substantial differences when we examined the in vivo phosphorylation of LAT. Among the constructs used in the previous experiments, only the YYAA mutant could phosphorylate LAT in the absence of Lck (Fig. 6A). Cotransfection of Lck with the WT ZAP-70 was necessary to activate it, as evidenced by LAT phosphorylation. Lck also modestly augmented the ability of the YYAA mutant to phosphorylate LAT. As an additional control, we used a ZAP-70 construct in which Y315 and Y319 were both mutated to glutamic acids (YYEE) to better mimic the negative charge associated with tyrosine phosphorylation at this site. With this mutant, we obtained the same results as with the YYAA construct (Fig. 6A). Similar results were also obtained when another substrate of Syk-family kinases, NTAL (2) (also known as LAB) (18), was used instead of LAT as the ZAP-70 substrate (data not shown). Moreover, the YYEE mutant was able to reconstitute transcriptional activation of NFAT in P116 Jurkat cells to a similar extent as the YYAA mutant (data not shown).
FIG. 6.
Kinase activity of ZAP-70 and Syk mutants in 293 cells. 293 cells transiently transfected with ZAP-70 and LAT-FLAG (A) or Syk and NTAL (B) constructs together with or without Lck as indicated at the top, were lysed in 2× concentrated SDS-PAGE sample buffer and analyzed by immunoblotting with antibody against phosphotyrosine (only the relevant part of the blot corresponding to LAT or NTAL is shown). The expression level of LAT-FLAG, NTAL, ZAP-70, Syk, and Lck was assessed by probing with the respective antibodies. Note the phosphorylation-induced decrease in NTAL electrophoretic mobility.
Our experiments, together with previously published data suggest that inhibition of the kinase activity by tyrosines adjacent to the N terminus of the kinase domain may represent a broadly used regulatory mechanism employed by many receptors as well as cytoplasmic PTKs. Therefore, we next analyzed PTK Syk, the other member of the Syk family of PTKs. It has the same domain structure as ZAP-70, and the interdomain B phosphorylation sites are well conserved between the two members of the family (Fig. 1A). Analogous to previous experiments, we mutated corresponding tyrosines 342 and 346 (orthologs of Y315 and 319 of ZAP-70) to alanines or phenylalanines, expressed these constructs together with the Syk substrate NTAL in the presence or absence of Lck in 293 cells, and analyzed NTAL phosphorylation in the whole-cell lysates (Fig. 6B). Results similar to the previous ZAP-70 experiments were obtained, although this region did not appear to constrain this kinase to the same absolute extent. The YYAA mutant did not require Src family kinases to phosphorylate NTAL in this system, while WT Syk was substantially less active and the YYFF mutant displayed only marginal kinase activity in the absence of Lck. Cotransfection of Lck augmented the in vivo kinase activity of all the Syk constructs, quite surprisingly even that of the YYFF mutant. The previously observed increased activity of Syk (20) may reflect its somewhat reduced dependency on Src kinases to release the inhibitory constraints of interdomain B. The sequence differences in the ZAP-70 and Syk interdomain B (Fig. 1A) might also be partially responsible. On the other hand, it is also possible that the high kinase activity of Syk may be outside the range of sensitivity of the assay.
DISCUSSION
Our findings reveal an autoinhibitory switch in ZAP-70 and Syk and suggest that, in contrast to the previously held view, Y315 and Y319 in ZAP-70 do not play a requisite scaffolding function in order to recruit substrates. Rather, they are primarily responsible for an autoinhibitory switch that regulates kinase function. Our findings suggest that Lck or another kinase activates ZAP-70 by phosphorylating Y319 (and possibly Y315), which, by analogy to the juxtamembrane region of several RTKs (15), relieves a hydrophobic autoinhibitory interaction with the N-terminal lobe and the activation loop of the kinase domain. This likely induces a conformational change that enhances the kinase activity and/or accessibility of the activation loop tyrosines for phosphorylation.
This hypothesis is supported by experiments in 293 and Cos cells as well as in the P116 Jurkat T-cell line. The YYAA mutants of ZAP-70 and Syk appeared dysregulated in 293 cells, as measured by tyrosine phosphorylation of the ZAP-70 activation loop and the ability of both of these YYAA mutants to phosphorylate their substrates. In contrast to the YYFF mutant, YYAA ZAP-70 substantially reconstituted a number of signaling events in P116 cells, including tyrosine phosphorylation, calcium flux, transcriptional activation of NFAT, and activation of the Erk1/2 MAP kinase pathway. Also of note is the fact that we had difficulties generating wild-type expression levels of the interdomain B deletion or YYAA mutants in stable P116 transfectants, suggesting some toxicity of these potentially unregulatable mutants. This may be explained by the fact that activation of the TCR can induce apoptosis in Jurkat cells via Fas-dependent pathways (9).
Although our experiments with the YYAA mutant of ZAP-70 suggest that binding of signal transducing molecules to ZAP-70 tyrosines Y315 and Y319 has only partial amplifying effects on several major TCR-dependent signaling pathways, the scaffolding function of these tyrosines cannot be entirely dismissed. Our observation that some of the signaling events, most notably full tyrosine phosphorylation of LAT, SLP-76, and PLC-γ1, could not be completely rescued by YYAA ZAP-70 in P116 cells suggests that there still may be some, perhaps only quantitative, function for Y315 and Y319 in the recruitment of downstream signaling molecules.
Apart from directly affecting the downstream signaling pathways, the molecules interacting with phosphorylated Y315 and Y319 potentially can be involved in the regulation of ZAP-70 itself. Binding of SH2 domain-containing proteins to phosphorylated interdomain B tyrosines may stabilize the active conformation of ZAP-70 and protect Y315/Y319 from dephosphorylation. In addition, the effector functions of these molecules could directly influence ZAP-70 kinase activity. Indeed, Pelosi et al. suggested that binding of Lck via its SH2 domain to phosphorylated Y319 followed by Lck-mediated phosphorylation of Y493 in the activation loop of ZAP-70 is an important positive feedback mechanism in ZAP-70 activation (26).
Up until now, Lck has been viewed as the major PTK responsible for the phosphorylation of ZAP-70 Y493. This was primarily based on mass spectrometry and two-dimensional analysis of tryptic peptides generated from purified recombinant ZAP-70 protein phosphorylated by Lck (5, 34) as well as the data of Pelosi et al. (26). These studies however, did not investigate the role of other kinases, including Fyn and c-Abl, in ZAP-70 phosphorylation. The first two studies also failed to examine phosphorylation of Y315/Y319 and thus did not take into account the influence of these sites on ZAP-70 activity and the ability to autophosphorylate at Y493. Our data from the 293 cell overexpression system revealed that, unlike WT ZAP-70, mere overexpression of the ZAP-70 YYAA mutant led to its phosphorylation at Y493, suggesting that YYAA was able to autophosphorylate at Y493, perhaps via an intermolecular trans-phosphorylation mechanism (Fig. 7). Moreover, with Lck as well as with Fyn and c-Abl cotransfection, we observed high levels of Y493 phosphorylation in WT ZAP-70 but only marginal phosphorylation of Y493 in the kinase-inactive mutant, despite comparable phosphorylation at Y319. These results suggest that these kinases are not very efficient at phosphorylating Y493, even when Y319 is phosphorylated and potentially available for binding. This seems to suggest that in 293 or Cos cells phosphorylation of interdomain B Y315/Y319 is the only major event dependent on kinase(s) other than ZAP-70 itself, which in turn results in an increase of ZAP-70 kinase activity and its trans-autophosphorylation of the activation loop. These data also seem to rule out the possibility that phosphorylation of Y315/Y319 alone substantially enhances accessibility of Y493 for phosphorylation by Lck, Fyn, or c-Abl. In P116 Jurkat cells, however, the KA mutant was hyperphosphorylated at both Y319 and Y493, and its phosphorylation was further increased upon TCR stimulation. This apparent discrepancy could result from differential regulation of ZAP-70 in the P116 Jurkat T-cell line versus 293 or Cos cells, which are of epithelial origin. T cells likely have multiple regulatory mechanisms to control their key signaling molecules, including ZAP-70. Some of these mechanisms may be missing in 293 or Cos cells because they do not express endogenous ZAP-70 and therefore would have no need to specifically control its activity. The observed hyperphosphorylation of KA ZAP-70 in P116 cells indeed suggests the existence of a ZAP-70-driven negative feedback loop in T cells. By impairing ZAP-70 activity by the KA mutation, this putative regulatory mechanism is turned off and nonphysiological hyperphosphorylation of Y319 and Y493 occurs. A similar regulatory mechanism might also explain the incomplete reconstitution of several tyrosine phosphorylation events in P116 cells expressing the potentially hyperactive YYAA ZAP-70 mutant, which would enhance the activity of the same negative feedback loop. However, considering the difficulties we had in obtaining the cells stably expressing YYAA ZAP-70, it is also possible that only the clones able to compensate for increased ZAP-70 activity by reinforcing some of the negative regulatory pathways were selected.
FIG. 7.
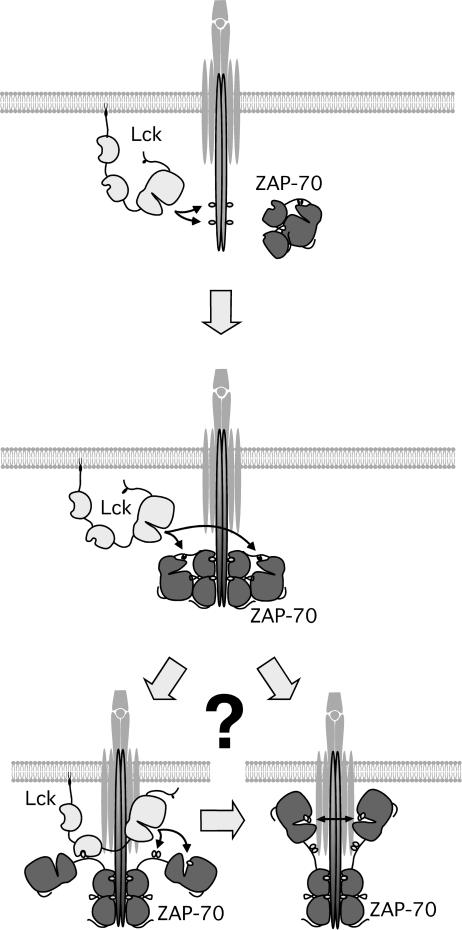
Hypothetical model of the ZAP-70 activation process. Lck phosphorylates ITAMs of activated TCR. ZAP-70 is recruited to phosphorylated ITAMs and subsequently phosphorylated by Lck on interdomain B tyrosines 315 and 319. This leads to a structural rearrangement of ZAP-70 molecule leading to increased accessibility of the activation loop and/or elevated kinase activity. This is followed by autophosphorylation of the activation loop, possibly via the coordinated action of Lck and ZAP-70 itself, and results in fully active ZAP-70 kinase. For simplicity, only Lck is shown in the model, but involvement of other PTKs should also be considered, as discussed in the text.
The observation that KA ZAP-70 could be phosphorylated at Y493 also shows that ZAP-70 is not the only PTK able to phosphorylate Y493 and that other PTKs, most likely Lck (26), participate in phosphorylating this site. Additional studies are needed to dissect the contribution of different kinases to Y493 phosphorylation. Nevertheless, based on currently available data, we favor the possibility that Lck and ZAP-70 are the major kinases phosphorylating Y493 in vivo. It is possible that Lck is responsible only for the initial but rather inefficient phosphorylation of Y493 on several ZAP-70 molecules. These activated ZAP-70 molecules may in turn trans-autophosphorylate other ZAP-70 molecules with which they are dimerized by paired ITAMs in the TCR-ζ and CD3 chains, much like dimerized RTKs. Since there certainly is rapid turnover of ZAP-70 binding to ITAMs (3), it is likely that one active ZAP-70 molecule can activate many other ZAP-70 molecules leading to substantial amplification of the initial signal. Consistent with this model, it has been demonstrated that phosphorylated TCR-ζ chain dimers potentiate ZAP-70 in vitro catalytic activity substantially more than do monomers (21). Also, this mechanism may explain why ITAMs are almost always found as pairs in homo- or heterodimeric non-ligand-binding chains within the receptors of the hematopoietic lineage involved in antigen recognition: i.e., to allow for induced dimers of ZAP-70 or Syk to trans-autophosphorylate.
The importance of the activation loop phosphorylation of Syk family kinases has recently been questioned by the studies describing the structures of the isolated kinase domains of ZAP-70 and Syk (1, 19). In both kinase domains, the unphosphorylated activation loop assumes an extended conformation, typical for an activated kinase. In ZAP-70, this might have been driven by the interactions between the activation loop and the kinase domain of neighboring molecules in the crystal, but it also might suggest that phosphorylation of the activation loop is not as critical for ZAP-70/Syk activation as previously was thought. Interdomain B phosphorylation or YYAA mutation may be sufficient to increase ZAP-70/Syk activity enough to autophosphorylate and/or phosphorylate its downstream substrates. By analogy with EphB2, interdomain B in fact may regulate the activation loop conformation and the observed structure may reflect the lack of interdomain B in the construct used for structure determination. In such a circumstance, phosphorylation of the activation loop probably would have mainly stabilizing effects.
It appears that ZAP-70 activation is a complicated multistep process requiring proper spatial as well as temporal coordination of several key events (Fig. 7). Upon TCR stimulation and phosphorylation of TCR ITAMs by Src family kinases, ZAP-70 is recruited to phosphorylated ITAM sequences. Subsequently, Y315 and Y319 in interdomain B of ZAP-70 become phosphorylated by Src family or Abl/Arg family PTKs. This results in the release of ZAP-70 from an autoinhibited state, the upregulation of kinase activity, and the stabilization of the interaction between ZAP-70 and phosphorylated TCR chains. Subsequent steps are not clear yet, but they likely involve phosphorylation of the ZAP-70 activation loop, possibly via the cooperative action of Lck and ZAP-70 itself resulting in the fully active enzyme.
Collectively, our findings suggest that the cytoplasmic PTKs ZAP-70 and Syk share with RTKs a regulatory mechanism involved in inhibiting catalytic activity that involves two tyrosine residues N terminal to their kinase domains. The paired autoinhibitory tyrosines are localized within the juxtamembrane regions of RTKs, whereas they are within interdomain B of ZAP-70 and Syk. Thus, one could view ZAP-70 and Syk essentially as RTKs in which membrane localization is just another means of regulation. However, once localized to the membrane, these kinases are activated through mechanisms similar to those involved in RTK activation.
Acknowledgments
We thank R. Abraham, J. B. Bolen, C. L. Chan, M. Cooper, V. Horejsi, G. A. Koretzky, M. A. Pendergast, and R. M. Perlmutter for cell lines, constructs, and antibodies. We thank Weiss lab members for advice and discussion.
This work was supported, in part, by A135297 from the National Institutes of Health.
REFERENCES
- 1.Atwell, S., J. M. Adams, J. Badger, M. D. Buchanan, I. K. Feil, K. J. Froning, X. Gao, J. Hendle, K. Keegan, B. C. Leon, H. J. Muller-Dieckmann, V. L. Nienaber, B. W. Noland, K. Post, K. R. Rajashankar, A. Ramos, M. Russell, S. K. Burley, and S. G. Buchanan. 2004. A novel mode of gleevec (STI-571, Imatinib) binding is revealed by the structure of spleen tyrosine kinase (Syk). J. Biol. Chem. 279:55827-55832. [DOI] [PubMed] [Google Scholar]
- 2.Brdicka, T., M. Imrich, P. Angelisova, N. Brdickova, O. Horvath, J. Spicka, I. Hilgert, P. Luskova, P. Draber, P. Novak, N. Engels, J. Wienands, L. Simeoni, J. Osterreicher, E. Aguado, M. Malissen, B. Schraven, and V. Horejsi. 2002. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunnell, S. C., D. I. Hong, J. R. Kardon, T. Yamazaki, C. J. McGlade, V. A. Barr, and L. E. Samelson. 2002. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158:1263-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cambier, J. C. 1995. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL). Immunol. Today 16:110. [DOI] [PubMed] [Google Scholar]
- 5.Chan, A. C., M. Dalton, R. Johnson, G. H. Kong, T. Wang, R. Thoma, and T. Kurosaki. 1995. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14:2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, A. C., M. Iwashima, C. W. Turck, and A. Weiss. 1992. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell 71:649-662. [DOI] [PubMed] [Google Scholar]
- 7.Cooke, M. P., and R. M. Perlmutter. 1989. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1:66-74. [PubMed] [Google Scholar]
- 8.Di Bartolo, V., D. Mege, V. Germain, M. Pelosi, E. Dufour, F. Michel, G. Magistrelli, A. Isacchi, and O. Acuto. 1999. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem. 274:6285-6294. [DOI] [PubMed] [Google Scholar]
- 9.Eischen, C. M., B. L. Williams, W. Zhang, L. E. Samelson, D. H. Lynch, R. T. Abraham, and P. J. Leibson. 1997. ZAP-70 tyrosine kinase is required for the up-regulation of Fas ligand in activation-induced T cell apoptosis. J. Immunol. 159:1135-1139. [PubMed] [Google Scholar]
- 10.Fusaki, N., S. Matsuda, H. Nishizumi, H. Umemori, and T. Yamamoto. 1996. Physical and functional interactions of protein tyrosine kinases, p59fyn and ZAP-70, in T cell signaling. J. Immunol. 156:1369-1377. [PubMed] [Google Scholar]
- 11.Gelkop, S., and N. Isakov. 1999. T cell activation stimulates the association of enzymatically active tyrosine-phosphorylated ZAP-70 with the Crk adapter proteins. J. Biol. Chem. 274:21519-21527. [DOI] [PubMed] [Google Scholar]
- 12.Goda, S., A. C. Quale, M. L. Woods, A. Felthauser, and Y. Shimizu. 2004. Control of TCR-mediated activation of beta 1 integrins by the ZAP-70 tyrosine kinase interdomain B region and the linker for activation of T cells adapter protein. J. Immunol. 172:5379-5387. [DOI] [PubMed] [Google Scholar]
- 13.Gong, Q., X. Jin, A. M. Akk, N. Foger, M. White, G. Gong, J. B. Wardenburg, and A. C. Chan. 2001. Requirement for tyrosine residues 315 and 319 within zeta chain-associated protein 70 for T cell development. J. Exp. Med. 194:507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 15.Hubbard, S. R. 2004. Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 5:464-471. [DOI] [PubMed] [Google Scholar]
- 16.Irving, B. A., and A. Weiss. 1991. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64:891-901. [DOI] [PubMed] [Google Scholar]
- 17.Iwashima, M., B. A. Irving, N. S. van Oers, A. C. Chan, and A. Weiss. 1994. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 263:1136-1139. [DOI] [PubMed] [Google Scholar]
- 18.Janssen, E., M. Zhu, W. Zhang, and S. Koonpaew. 2003. LAB: a new membrane-associated adaptor molecule in B cell activation. Nat. Immunol. 4:117-123. [DOI] [PubMed] [Google Scholar]
- 19.Jin, L., S. Pluskey, E. C. Petrella, S. M. Cantin, J. C. Gorga, M. J. Rynkiewicz, P. Pandey, J. E. Strickler, R. E. Babine, D. T. Weaver, and K. J. Seidl. 2004. The three-dimensional structure of the ZAP-70 kinase domain in complex with staurosporine: implications for the design of selective inhibitors. J. Biol. Chem. 279:42818-42825. [DOI] [PubMed] [Google Scholar]
- 20.Latour, S., L. M. Chow, and A. Veillette. 1996. Differential intrinsic enzymatic activity of Syk and Zap-70 protein-tyrosine kinases. J. Biol. Chem. 271:22782-22790. [DOI] [PubMed] [Google Scholar]
- 21.LoGrasso, P. V., J. Hawkins, L. J. Frank, D. Wisniewski, and A. Marcy. 1996. Mechanism of activation for Zap-70 catalytic activity. Proc. Natl. Acad. Sci. USA 93:12165-12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupher, M. L., Jr., Z. Songyang, S. E. Shoelson, L. C. Cantley, and H. Band. 1997. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J. Biol. Chem. 272:33140-33144. [DOI] [PubMed] [Google Scholar]
- 23.Magnan, A., V. Di Bartolo, A. M. Mura, C. Boyer, M. Richelme, Y. L. Lin, A. Roure, A. Gillet, C. Arrieumerlou, O. Acuto, B. Malissen, and M. Malissen. 2001. T cell development and T cell responses in mice with mutations affecting tyrosines 292 or 315 of the ZAP-70 protein tyrosine kinase. J. Exp. Med. 194:491-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marth, J. D., R. Peet, E. G. Krebs, and R. M. Perlmutter. 1985. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell 43:393-404. [DOI] [PubMed] [Google Scholar]
- 25.Meng, W., S. Sawasdikosol, S. J. Burakoff, and M. J. Eck. 1999. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 398:84-90. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi, M., V. Di Bartolo, V. Mounier, D. Mege, J. M. Pascussi, E. Dufour, A. Blondel, and O. Acuto. 1999. Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J. Biol. Chem. 274:14229-14237. [DOI] [PubMed] [Google Scholar]
- 27.Rao, N., M. L. Lupher, Jr., S. Ota, K. A. Reedquist, B. J. Druker, and H. Band. 2000. The linker phosphorylation site Tyr292 mediates the negative regulatory effect of Cbl on ZAP-70 in T cells. J. Immunol. 164:4616-4626. [DOI] [PubMed] [Google Scholar]
- 28.Roose, J. P., M. Diehn, M. G. Tomlinson, J. Lin, A. A. Alizadeh, D. Botstein, P. O. Brown, and A. Weiss. 17 November 2003, posting date. T cell receptor-independent basal signaling via Erk and Abl kinases suppresses RAG gene expression. PLoS Biol. 1:E53. [Online.] doi: 10.1371/journal.pbio.0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro, V. S., M. N. Mollenauer, W. C. Greene, and A. Weiss. 1996. c-rel regulation of IL-2 gene expression may be mediated through activation of AP-1. J. Exp. Med. 184:1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straus, D. B., A. C. Chan, B. Patai, and A. Weiss. 1996. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J. Biol. Chem. 271:9976-9981. [DOI] [PubMed] [Google Scholar]
- 31.Thien, C. B., R. M. Scaife, J. M. Papadimitriou, M. A. Murphy, D. D. Bowtell, and W. Y. Langdon. 2003. A mouse with a loss-of-function mutation in the c-Cbl TKB domain shows perturbed thymocyte signaling without enhancing the activity of the ZAP-70 tyrosine kinase. J. Exp. Med. 197:503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Oers, N. S., H. von Boehmer, and A. Weiss. 1995. The pre-T cell receptor (TCR) complex is functionally coupled to the TCR-zeta subunit. J. Exp. Med. 182:1585-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wange, R. L., R. Guitian, N. Isakov, J. D. Watts, R. Aebersold, and L. E. Samelson. 1995. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J. Biol. Chem. 270:18730-18733. [DOI] [PubMed] [Google Scholar]
- 34.Watts, J. D., M. Affolter, D. L. Krebs, R. L. Wange, L. E. Samelson, and R. Aebersold. 1994. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J. Biol. Chem. 269:29520-29529. [PubMed] [Google Scholar]
- 35.Weiss, A., and J. D. Stobo. 1984. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 160:1284-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, B. L., B. J. Irvin, S. L. Sutor, C. C. Chini, E. Yacyshyn, J. Bubeck Wardenburg, M. Dalton, A. C. Chan, and R. T. Abraham. 1999. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 18:1832-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, J., Q. Zhao, T. Kurosaki, and A. Weiss. 1997. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J. Exp. Med. 185:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wybenga-Groot, L. E., B. Baskin, S. H. Ong, J. Tong, T. Pawson, and F. Sicheri. 2001. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106:745-757. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, Q., B. L. Williams, R. T. Abraham, and A. Weiss. 1999. Interdomain B in ZAP-70 regulates but is not required for ZAP-70 signaling function in lymphocytes. Mol. Cell. Biol. 19:948-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zipfel, P. A., W. Zhang, M. Quiroz, and A. M. Pendergast. 2004. Requirement for Abl kinases in T cell receptor signaling. Curr. Biol. 14:1222-1231. [DOI] [PubMed] [Google Scholar]