Abstract
Parkinson’s disease (PD) and multiple system atrophy (MSA) can be preceded by isolated REM sleep behavior disorder (iRBD). As excessive sighing during wakefulness is a red flag for MSA in individuals with parkinsonism, we measured sighing during slow wave sleep (N3) and REM sleep as potential biomarkers in 73 participants with MSA, 111 with iRBD, 257 with PD, and 115 controls. The number of sighs/hour of N3 (index) was higher in the MSA group than in the other groups. Sighs were rarer in REM sleep than in N3 sleep. A sigh index greater than 3.4/h of N3 was 95% sensitive in discriminating participants with MSA from controls, and a sigh index greater than 0.8 sigh/h of REM sleep was 87% specific in discriminating participants with MSA from controls. MSA participants with (vs. without) sigh were younger, had a lower apnea-hypopnea index (but no more stridor), and had no other difference in motor, autonomic, cognitive, and sensory symptoms. The sigh index could be used for screening for MSA in the millions of middle-aged persons who receive polysomnography for other purposes. Whether sighing in iRBD predicts preferential conversion towards MSA should be measured in a longitudinal study.
Subject terms: Biomarkers, Neurology, Parkinson's disease
Introduction
Alpha-synuclein-related neurodegenerative diseases are a significant socio-economic burden in aging populations. Identifying alpha-synucleinopathies at their prodromal stage may offer the best opportunity to test neuroprotective and disease-modifying therapies. In this context, it is essential to develop simple, population-based diagnostic biomarkers for identifying prodromal or early alpha-synucleinopathies1. Recently, the nighttime breathing signal, commonly monitored for sleep apnea in middle-aged individuals, was analyzed by artificial intelligence and was demonstrated to accurately recognize Parkinson’s disease (PD) and its severity2. Among the nonmotor signs that occur a decade or more before the development of PD, dementia with Lewy bodies and multisystem atrophy (MSA), isolated REM sleep behavior disorder (iRBD) constitutes the most specific disorder of early-stage alpha-synuclein neurodegeneration1. It is characterized by dream enactment and abnormal muscular activity during REM sleep. Because of this specificity and high conversion rate to overt synucleinopathies, individuals with iRBD are now intensively studied to identify concomitant biomarkers of neurodegeneration.
Of these potential biomarkers, we chose to study sighing during sleep because it is one of the diagnostic red flags for MSA in individuals with parkinsonism3, and sighing during sleep can be efficiently and accurately identified during NREM sleep. A sigh is an abrupt, wide inspiration followed by a prolonged exhalation, isolated in the middle of regular breathing4. Sighs expand the lung, preventing progressive alveolar collapse4–6. They occur when respiratory variability is low in order to relax the lower airway smooth muscles, open the airway (and reduce atelectasis), relax the chest muscles, and restore normal, high respiratory variability7. Sighs are typical, normal events during wakefulness that occasionally occur during sleep in healthy young adults, primarily during the transition from wakefulness to NREM sleep8. The generation of sighs is likely controlled by brainstem central pattern generators responsible for respiratory rhythmogenesis9. Neurologists have observed excessive sighing during the daytime in MSA patients but not in PD patients10. However, the sigh frequency and characteristics are unclear. Only one recent study in 9 patients with MSA showed a higher frequency of sighs than in 9 patients with PD during the awake and light sleep stages (N1 and N2 sleep)11. However, sighs have not yet been studied in people with iRBD, nor during slow wave sleep (N3 sleep), although this is the ideal stage to identify them, as breathing is more stable and regular than during any other stages of sleep or wakefulness. In addition, one may imagine that abnormally frequent sighing in people with iRBD suggests a preferential conversion to MSA.
We hypothesized that excessive sighing during sleep differentiates MSA from PD patients and healthy controls and that it could be observed early in the iRBD stage. For this purpose, we aimed to measure the sighing phenomenon during sleep in individuals with MSA, with PD, and with iRBD, compared to healthy participants, in order to determine sighs frequency and index during N3 sleep across synucleinopathies and to determine whether sighing could be used as a biomarker at the iRBD stage. In addition, we studied the demographical, clinical, and sleep characteristics associated with the presence of sighing within the MSA group, hypothesizing that sighs may be present at the early stage of MSA.
Results
Demographic and polysomnographic characteristics of participants
Among 962 potential participants identified, 253 were excluded because low-quality signal, no N3 or REM stages, total sleep time below 4 h, apnea-hypopnea index greater than 15/h in the control group (Supplementary Fig. A). No patients (including MSA) were excluded because of unclear/differentiated sleep staging. In addition, the information from 153 PD patients or controls was not considered necessary (and would be an additional time-consuming task) as the present sample was large enough. Finally, 556 participants remained to be analyzed in the present study, including 73 participants with MSA, 257 with PD, 111 with iRBD, and 115 controls.
The demographic and polysomnographic characteristics of the groups are summarized in Supplementary Table A. There were more women in the MSA group and control group than in other groups. Age was lower in the MSA group than in the iRBD group, and higher in the PD group and iRBD group than in the control group. Total sleep time was longer in the iRBD group than in the MSA, PD, and control groups. Sleep efficiency was lower in the MSA group than in the control group. Sleep onset latency was longer in the MSA group than in the PD group. REM sleep latency was longer in the MSA and PD groups than in the iRBD and control groups. The distribution of sleep stages was globally within normal ranges in each group. However, the N1 percentage was greater in the iRBD group than in the control group, the N2 percentage was similar across groups, the N3 percentage was greater in the MSA and PD groups than in the control group, and the REM sleep percentage was shorter in the PD group than in the iRBD group. The apnea-hypopnea index was higher in the MSA group than in the PD and control groups, and higher in the PD and iRBD groups than in the control group. The index of obstructive apneas was higher in the PD and iRBD groups than in the control group. Central apneas were more frequent in the MSA group than in the iRBD and control groups. Minimal oxygen saturation was similar across groups. Periodic leg movements index was greater in the MSA group than in the other groups. The arousal index was greater in the iRBD group than in the PD and control groups. None of these sleep measures were affected by the use of antidepressants or benzodiazepines.
Presence of sighing and index of sighs among sleep stages and groups
Isolated sighs (N = 1439, 89.3% of all sighs) were more frequent than sighs followed by post-sigh apnea (N = 150, 9.3% of all sighs, P < 0.001 vs. isolated sighs without apnea). Double sighs were rare (N = 11 double sighs, 0.7%, P < 0.001 vs. isolated sighs). Sighing was silent, except for two MSA patients with stridor at the time of sighing who had increased stridor noise during the inspiratory part of the sigh and a brief moan (but no catathrenia) during the expiratory part of the sigh (Supplementary video clip). More participants had sighs in N3 sleep than in REM sleep (Table 1). There were fewer participants with sighs in N3 sleep in the PD group than in the other groups. The sigh index in N3 sleep was greater in the MSA group than in other groups, whether participants without sighing were included (Table 1) or not (Fig. 1). The results were verified using frequentist probabilities with a Bayesian approach. In terms of sigh index in N3 sleep, the MSA group differed from the other groups with significant evidence BF10 > 100 in general and group by group (Supplementary Table C). These results confirmed the frequentist probabilities. There were more participants with sighing in REM sleep in the MSA group and iRBD group than in the PD group and control group. The sigh index in REM sleep was higher in the MSA group and PD group than in iRBD group and control group, whether participants without sighing were included (Table 1) or not (Fig. 1). Using Bayesian probability, there was substantial evidence for the sigh index in REM sleep to be higher in the MSA group than in the control group and anecdotal evidence for the sigh index in REM sleep to be higher in the MSA group than in the iRBD group (data not shown). The presence and index of sighs during N3 sleep and during REM sleep were not influenced by antidepressant use, age, gender, and apnea-hypopnea index when analyzed using a mixed model (data not shown).
Table 1.
Prevalence and index of sighs in N3 sleep and REM sleep in participants with multiple system atrophy (MSA), Parkinson’s disease (PD), isolated REM sleep behavior disorder (iRBD), and controls
| MSA | PD | iRBD | Controls | P value | Post-hoc test | |
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| Number | 73 | 257 | 111 | 115 | – | – |
| Stage N3 | ||||||
| Participants with sighs, N (%) | 57 (78) | 130 (51) | 73 (65.7) | 79 (68) | <0.001 | A = C = D > B |
| Sigh index/h of N3 | 2.75 (2.40) | 0.93 (1.37) | 1.18 (1.46) | 1.06 (1.14) | <0.001 | A > B = C = D |
| Range | 0–15.9 | 0–6.5 | 0–6.4 | 0–4.7 | ||
| Stage REM | ||||||
| Participants with sighs, N (%) | 24 (32.9) | 38 (14.8) | 28 (25.2) | 9 (7.83) | <0.001 | A = C > B = D |
| Sigh index/h of REM | 0.8 (1.6) | 0.33 (1.2) | 0.26 (0.55) | 0.08 (0.34) | <0.001 | A = B > C = D |
| Range | 0–6.8 | 0–12.9 | 0–3.2 | 0–2.55 | ||
Fig. 1. Index of sighs in N3 and REM sleep.
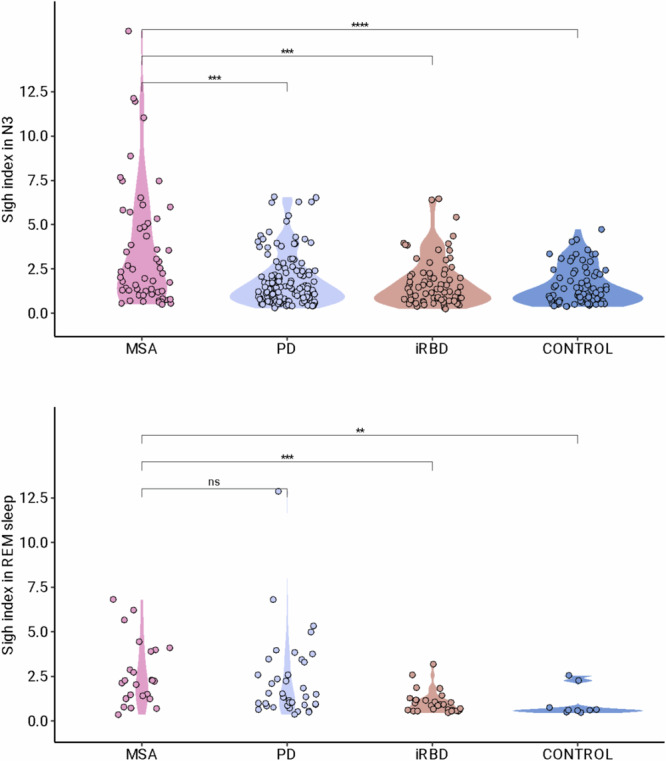
Index of sigh in N3 (upper panel) and in REM sleep (lower panel), violin chart. Participants without any sigh have been excluded. Each circle corresponds to the measure in a single participant. In this violin plot, the shape represents the density estimate of distribution.
Identification of thresholds for sigh index
The receiver operating characteristic analyses leading to identifying sigh index cutoff with the best accuracy is shown in Table 2. A sigh index greater than 3.4/h of N3 yielded a good (95%) sensitivity to distinguish participants with MSA from healthy controls, and a threshold of 4.32/h had a good (93%) sensitivity to distinguish participants with MSA from those with PD, although specificities were low (32–38%). In contrast, a cutoff of 0.8 sighs/h of REM sleep was 87% specific and 78% sensitive to distinguish participants with MSA from controls. The combination of a sigh index greater than 3.4/h in N3 sleep and greater than 0.8 in REM sleep yielded a sensitivity of 90% for discriminating MSA participants from controls. In the iRBD group, 11 (9.9%) participants had a sigh index greater than 3.4/h in N3 sleep. None had stridor. Their smell performances, and their autonomic measures (number of stools/week, orthostatic hypotension) did not differ from the 100 iRBD participants having a sigh index above 3.4/h of N3 sleep (Supplementary Table D). An N3 cut-off of 3.4 discriminated the non-MSA population from the MSA population with excellent sensitivity (91%) and lower specificity (63%).
Table 2.
Optimal cut-off points of sigh index (in N3 sleep and in REM sleep) to distinguish participants with multiple system atrophy (MSA) from those with Parkinson’s disease (PD), isolated REM sleep behavior disorder (iRBD) and controls, evaluated using Receiver Operating Characteristics Curves
| Cutoff (sighs/h) | Specificity (%) | Sensitivity (%) | PPV | NPV | Accuracy | Threshold | Bacc | Auc | |
|---|---|---|---|---|---|---|---|---|---|
| MSA vs PD | |||||||||
| N3 | 4.32 | 32 | 93 | 0.76 | 0.67 | 0.75 | 0.56 | 0.56 | 0.65 |
| REM | 1.19 | 83 | 45 | 0.81 | 0.49 | 0.60 | 0.64 | 0.49 | 0.60 |
| N3 & REM | NA | 57 | 70 | 0.79 | 0.44 | 0.66 | 0.72 | 0.63 | 0.65 |
| MSA vs iRBD | |||||||||
| N3 | 4.35 | 32 | 96 | 0.63 | 0.85 | 0.67 | 0.391 | 0.61 | 0.66 |
| REM | 1.20 | 83 | 79 | 0.84 | 0.77 | 0.80 | 0.65 | 0.74 | 0.83 |
| N3 & REM | NA | 53 | 82 | 0.7 | 0.7 | 0.7 | 0.55 | 0.65 | 0.69 |
| MSA vs Control | |||||||||
| N3 | 3.4 | 38 | 95 | 0.67 | 0.84 | 0.71 | 0.44 | 0.62 | 0.69 |
| REM | 0.8 | 87 | 78 | 0.7 | 0.91 | 0.84 | 0.50 | 0.82 | 0.82 |
| N3 & REM | NA | 57 | 90 | 0.74 | 0.81 | 0.75 | 0.54 | 0.71 | 0.76 |
| MSA vs without MSA | |||||||||
| N3 | 4.35 | 31 | 95 | 0.87 | 0.58 | 0.84 | 0.7 | 0.56 | 0.66 |
| REM | 1.20 | 83 | 61 | 0.92 | 0.40 | 0.66 | 0.80 | 0.50 | 0.72 |
| N3 & REM | 3.4 | 63 | 91 | 0,8 | 0.82 | 0.8 | 0.51 | 0.75 | 0.77 |
Auc area under the curve, Bacc best accuracy, NPV negative predictive value, PPV positive predictive value.
Demographical, clinical, and sleep characteristics associated with sighs in multiple system atrophy
We further compared the demographic, clinical, and sleep characteristics between participants with MSA with (N = 57) or without (N = 16) sighing (at least one sigh) in N3 sleep (Table 3). Compared to participants without sighing, those with sighing were younger at disease onset and at the time of the sleep study but had a non-different disease duration. There were no differences with respect to sex, body mass index, as well as clinical features of MSA, including the proportion of Parkinsonian vs. cerebellar forms of MSA, motor, autonomic, cognitive, and sensory symptoms, as well as scores of the Unified Multiple System Atrophy Rating Scale, the Global Disability Scale score, and the levodopa equivalent doses in the group with vs. without sighing. However, a higher percentage of participants with sighing took antidepressants, and a lower percentage of them took antihypertensive drugs, compared to MSA participants without sighing. There was no difference between groups when focusing on selective serotonin reuptake inhibitors. However, only one patient had no sigh and took this kind of drug, which lacks power for a valid comparison. On sleep measures, MSA participants with sighs had a lower apnea-hypopnea index than those without sighs. Other sleep measures (including the presence of stridor) were not different between groups.
Table 3.
Demographic, clinical, and drug characteristics in multiple system atrophy (MSA) participants with vs. without sighs in N3 sleep
| MSA (N = 73) | p value | ||
|---|---|---|---|
| With sighs | Without sighs | ||
| Number | 57 | 16 | – |
| Demographic characteristics | |||
| Age at disease onset, y | 56.4 (9) | 62.8 (9.2) | 0.015 |
| Age at sleep study, y | 61.2 (8.6) | 69.5 (7.7) | 0.001 |
| Sex, female N (%) | 35 (61.4) | 7 (43.8) | 0.33 |
| BMI, kg/m2 | 24.7 (4.4) | 25.9 (4.1) | 0.31 |
| Clinical characteristics | |||
| Phenotype | |||
| MSA-P, % | 31 (54.4) | 7 (43.8) | 0.76 |
| MSA-C, % | 25 (43.9) | 8 (50) | |
| Disease duration, years | 4.8 (2.3) | 6.7 (4.2) | 0.09 |
| Symptoms | |||
| Axial dystonia, % | 16 (28.1) | 3 (18.8) | 0.78 |
| Oromandibular dystonia, % | 7 (12.3) | 0 | 0.35 |
| Blepharospasm, % | 2 (3.5) | 0 | 1 |
| Limb dystonia, % | 10 (17.5) | 1 (6.2) | 0.53 |
| Dysphonia | 1.9 (0.7) | 1.9 (0.9) | 0.91 |
| Dysphagia | 1.1 (1) | 1.4 (1.2) | 0.31 |
| Abnormal posture | 1.7 (1) | 1.4 (0.8) | 0.41 |
| Body sway | 2.2 (0.8) | 2.2 (1) | 0.94 |
| Altered gait | 2.6 (0.7) | 2.4 (1) | 0.28 |
| Falls | 1.6 (1.3) | 1.8 (1.4) | 0.63 |
| Hyposmia, % | 4 (7) | 1 (6.2) | 1 |
| Constipation, % | 43 (75.4) | 12 (75) | 0.90 |
| Nocturia, % | 38 (66.7) | 10 (62.5) | 1 |
| Urge incontinence, % | 52 (91.2) | 13 (81.2) | 1 |
| Urinary retention, % | 39 (68.4) | 8 (50) | 0.45 |
| Orthostatic hypotension, % | 46 (80.7) | 11 (68.8) | 0.85 |
| Supine hypertension, % | 22 (38.6) | 7 (43.8) | 0.75 |
| Cognitive dysfunction, % | 13 (22.8) | 7 (43.8) | 0.13 |
| Unified Multiple System Atrophy Rating Scale (UMSARS) | |||
| UMSARS I (historical review) | 19.8 (7.2) | 14.3 (8.4) | 0.24 |
| UMSARS II (motor examination) | 23.4 (9.5) | 12.3 (13.1) | 0.09 |
| Global Disability Scale (GDS) | 2.6 (1.1) | 2.6 (1.2) | 0.84 |
| Drugs | |||
| Benzodiazepines, % | 10 (17.5) | 5 (31.2) | 0.40 |
| Melatonin, % | 15 (26.3) | 5 (31.2) | 0.94 |
| Antidepressants, % | 30 (52.6) | 3 (18.8) | 0.03 |
| SSRI antidepressants, % | 22 (38.6) | 1 (6.2) | 0.4 |
| Dopamine agonist, % | 10 (17.5) | 3 (18.8) | 1 |
| Dopaminergic, % | 36 (63.2) | 10 (62.5) | 1 |
| Anti-hypertension, % | 7 (12.3) | 6 (37.5) | 0.03 |
| Anti-hypotension, % | 19 (33.3) | 3 (18.8) | 0.51 |
| L - dopa equivalent daily dose (LEDD) | 598.2 (463.4) | 534 (312.1) | 0.7 |
| Polysomnographic measures | |||
| Cycles | 3.4 (2) | 2.4 (1.4) | 0.06 |
| Total sleep time, min | 389.1 (76) | 372.5 (72.4) | 0.43 |
| Sleep efficiency, % | 77.6 (9.3) | 75.2 (13.7) | 0.52 |
| Latency to, min | |||
| Sleep onset | 28.6 (25.9) | 28.4 (32.7) | 0.98 |
| REM sleep | 160.5 (106.1) | 197.8 (147) | 0.26 |
| Sleep stages (% of total sleep time) | |||
| N1 | 4.5 (3.3) | 5.3 (5.2) | 0.54 |
| N2 | 51 (14.6) | 57.6 (10.5) | 0.09 |
| N3 | 26.6 (9.2) | 23.2 (10.9) | 0.21 |
| REM | 18.4 (8.9) | 13.8 (9.7) | 0.07 |
| Apnea-hypopnea index | 10.7 (12.7) | 25.5 (25.7) | 0.04 |
| Apnea-hypopnea index > 15, N (%) | 16 (28,1) | 7 (43.8) | 0.04 |
| Oxygen saturation in sleep, % | |||
| Minimal oxygen saturation | 86.6 (4.5) | 82.7 (8.7) | 0.097 |
| Mean oxygen saturation | 94.1 (1.8) | 92 (4.3) | 0.07 |
| Arousal index | 9.6 (7) | 13.3 (10.7) | 0.21 |
| Periodic leg movement index | 41.6 (50.8) | 59.2 (74.5) | 0.27 |
| Stridor in the night, N % | 19 (33.3) | 6 (37.5) | 0.76 |
Discussion
Among 556 participants (with PD, N = 257, with MSA, N = 73; with iRBD, N = 111, and controls, N = 115), more participants with MSA (78%), iRBD (65.7%) and control (68%) had sighs during N3 than participants with PD (51%). The sigh index in N3 sleep was higher in the MSA group than in the PD, iRBD, and control groups. Sighs were rarer in REM sleep than in N3 sleep, and their index was higher in the MSA group and PD group than in the iRBD group and control group. A sigh index greater than 3.4/h of N3 was 95% sensitive to identify participants with MSA from healthy controls and non-MSA participants, and 0.77 sighs/h of REM sleep was 87% specific and 78% sensitive to distinguish participants with MSA from controls. Participants with MSA and sighs were younger, had a lower apnea-hypopnea index (but no more frequent presence of stridor), and no other difference in motor, autonomic, cognitive, and sensory symptoms.
How does our study compare with previous works? Although sighs are recurrently signaled among red flags for recognizing MSA in front of parkinsonism12, studies on sighs are scarce in synucleinopathies. They are even scarcer when focusing on sleep rather than wake-associated sighs. A single multicentric study focused on potential red flags of MSA in 57 participants with probable MSA showed that 43% of participants had observed sighs during wakefulness (as reported by clinicians) vs. 3.4% of 116 participants with PD10. In 9 patients with MSA studied with polysomnography, 100% had sighs during wake and N1 and N2 sleep stages vs. 2/9 (22%) patients with PD11. Furthermore, the sigh index during wake and sleep was higher in the MSA group than in the PD group. Here, we confirm these results in a larger series in N3 sleep, as 78% of the MSA group had sighs in N3 sleep. The median sigh index was 1.62 in N1-N2 sleep in the participants with MSA in the single previous study, and the mean sigh index was 2.75 in N3 sleep in our participants with MSA, suggesting that the rate of sighs increases when sleep deepens from N1-N2 to N3 sleep, and then decreases in REM sleep. Half of our 257 participants with PD had sighs in N3 sleep, a lower proportion than in the MSA group, but still a higher proportion than the 3% of participants with PD with observed sighs during daytime10 and the 22% as measured during wake and N1-N2 sleep11. These discrepancies may be due to the strength of a formal, continuous measure during 100 min of N3 sleep (vs. simple visual observations during a brief outpatient clinic10), a different sleep stage (N1-N2 vs. N3 here11), and a larger sample of participants with PD here. We extended this finding to participants with iRBD and controls and found that 65.7% and 68% of them had sighs during N3 sleep. To our knowledge, this is the largest study of sighs in healthy participants during sleep, as previous measures had been performed in 12 normal adults8.
Now, could sighs be used as markers to improve diagnosis categorization? Here, an isolated sigh during N3 does not adequately differentiate participants with MSA from controls and participants with iRBD or PD. In contrast, a sigh index >1.2 during REM sleep was 83% specific for a diagnosis of MSA rather than PD. This cut-off could be used to identify participants with MSA (which is a rare disorder) among all people undergoing polysomnography for atypical parkinsonism, in addition to stridor (which is specific for MSA) and RBD (which is specific for a synucleinopathy). Furthermore, using a sigh index threshold of 3.4 /h of N3 sleep and of 0.8/h of REM sleep was 90% sensitive to recognize MSA patients from other participants. Future studies may help determine whether this biomarker is applicable to polysomnograms performed in middle-aged individuals, primarily in the context of screening for sleep apnea. Nearly 850,000 polysomnograms were performed in the U.S. in 2021, leading to several million polysomnograms performed worldwide each year. There are now large databases of polysomnograms with 10-year follow-up information on disease outcomes. These could be used to test this marker.
After this first step using human eyes to detect sighs, it will be possible to characterize and develop new automated tools to identify sighs in the future. Meanwhile, identifying and scoring sighs (and generating sigh index in N3 sleep) is easy and less complex for a sleep scorer (physician or technologist) than learning how to score apnea or hypopnea. It can be an easy addition to implement in any sleep report in the case of atypical parkinsonism or iRBD. If an algorithm can recognize sighs in N3 sleep and in REM sleep, it could be used in these tests to detect possible MSA. Additionally, it could be interesting to screen patients with iRBD to identify those at a higher risk of later converting to MSA (rather than to PD and dementia with Lewy bodies). To answer this question, one must follow up over 5 to 10 years the participants with iRBD and determine which sigh index thresholds on polysomnography would predict more frequent conversion to MSA than to other neurological disorders. This sign could be added to other non-motor signs more frequently associated with MSA conversion than with PD conversion. It could be another sensitive and robust marker besides normal olfaction which has an excellent negative predictive value13, and stridor, which is exceptional in iRBD but has an 100% specificity for MSA diagnosis14. MSA stands out as a particularly severe and disabling disorder, exhibiting a higher impact on younger patients in comparison to PD. The alpha-synuclein strands are different in MSA and PD, with possible different therapeutic or disease-modifying therapeutic approaches. Therefore, any red flag indicating that a patient with prodromal or early parkinsonism is at higher risk of developing MSA instead of PD would be useful when such therapy is available. Neuroprotective or disease-modifying approaches may be more intensive in suspected MSA which progresses more rapidly and severely than PD and is more lethal.
The exact mechanisms of sighs in MSA is unknown. Here, we found that MSA participants with sighs were younger than those without sighs. The presence of nocturnal sighs was not influenced by disease duration, cerebellar or parkinsonism-subtype of MSA, the severity of motor symptoms, disease severity, levodopa dose-equivalent, and presence of autonomic symptoms (including bowel, urinary, and blood pressure problems). Among other breathing abnormalities, sleep-associated stridor (which corresponds to a larynx obstruction) affected one-third of MSA patients, with no further difference between those with and without sighs. However, the apnea-hypopnea index (which corresponds to a pharynx obstruction) was lower in MSA participants with than without sighs, suggesting different respiratory systems are at play behind these three respiratory markers, and that sighs may paradoxically be a sign of relatively “better health” in MSA. The persistence of sighs in patients on continuous positive airway pressure further suggests that sighs in MSA are not related to pharyngeal or laryngeal obstruction. In addition, since sighs are more frequent at the onset of MSA than later in the disease, they may represent a compensatory mechanism (for chest rigidity and atelectasis due to bronchial congestion in recumbent patients with coughing difficulties, a common problem in Parkinsonism15, or other unknown problems) that later disappears.
What are the neural mechanisms of sighs? Because breathing is a critical function, at least three groups of neurons (containing neurokinin1, serotonin, and epinephrine) interplay in the preBötzinger complex in the medulla oblongata to trigger inspiration, to the point that one group can replace another group when deficient16. The ablation of neurokinin1 neurons in rats decreases the index of typical sighs17. In fact, the neurokinin1 neurons in the ventrolateral medulla (including the preBötzinger complex) are depleted by ~60% in individuals with PD and ~89% in individuals with MSA18. This neuronal loss does not necessarily imply a decrease in sighs as in animal studies because the three groups of neurons (neurokinin1, serotonin, and epinephrine) can reconfigure their interplay and trigger new breathing patterns.
This study has some limitations, such as the lack of identification of the sigh index during wakefulness or N1 and N2 sleep stages. However, we assessed the sigh index in N3 sleep in which the identification of sighs is more reliable than in other stages, because hypopnea and apnea are rare in N3 sleep (hence sighs cannot be confounded with post hypopnea inspirations), and because sighs are neat, ample, isolated figures more visible on the background of regular breathing (regular respiratory rate and amplitude) typical of N3 stage19,20, resulting in a high signal to noise ratio. The raters were not blinded to the group of participants, but they had excellent interrater concordance, illustrating how easy it is to recognize sighs. This is a cross-sectional study conducted over a single night and not a longitudinal study, which does not allow conclusions on the predictive value of sighs. We need to follow up the participants with iRBD who have abnormal sighs index (here 7 of 111 iRBD participants) and compare their rate of conversion to MSA with participants with iRBD and no abnormal sighs. Despite these drawbacks, this study is one of the first to explore sighs during sleep, has a sizable sample, and is controlled. Furthermore, Bayesian statistics supported the usual frequentist findings.
To summarize, the N3 sigh index could be used as a biomarker for screening for MSA (a rare, severe, and rapidly evolutive neurological disorder) in the millions of middle-aged persons who receive polysomnography for another purpose. Whether sighs in iRBD predict a preferential conversion towards MSA should now be measured in a longitudinal study.
Methods
Participants
The selected samples were extracted from two different samples, including a research sample and a clinical sample. The research sample (extracted from the ICEBERG study, a longitudinal, controlled cohort in early PD and iRBD aimed at finding clinical, neurophysiology, and MRI markers of individual disease trajectories) included participants with early PD (aged 18-75 years, with less than four years of evolution from first motor manifestation as reported by the patient to inclusion time21), participants with iRBD, and age and sex-matched healthy controls22. Each had a sleep study at the time of inclusion into the cohort, regardless of their sleep status. The clinical sample included patients diagnosed with MSA, PD or iRBD (who had been referred for a sleep study for various purposes, including screening for sleep apnea, stridor, insomnia, excessive daytime sleepiness, or RBD). Clinical controls were retrospectively selected from 2015 to 2022 amongst the patients referred to the sleep clinic for snoring and suspected sleep apnea, later not confirmed by polysomnography (apnea-hypopnea index lower than 15). These patients did not have any features of neurodegenerative disease, based on clinical examination by neurologists. Patients with PD met the criteria of the Movement Disorders Society for PD23. Patients diagnosed with multiple system atrophy (MSA) fulfilled the criteria of the Movement Disorder Society diagnostic for probable MSA24. They were classified as cerebellar (MSA-C) or parkinsonian-predominant (MSA-P) phenotype, depending on the presence of parkinsonism (bradykinesia, rest tremor, rigidity) or cerebellar syndrome (gait ataxia, ataxic dysarthria, limb ataxia or sustained gaze-evoked nystagmus) at time of the sleep study. Patients with defined iRBD met the criteria of the International Classification of Sleep Disorders-3, including: (i) repeated episodes of sleep-related vocalization and/or complex motor behaviors; (ii) these behaviors were documented by polysomnography to occur during REM sleep or, based on the clinical history of dream enactment, were presumed to occur during REM sleep; (iii) polysomnography recording demonstrated REM sleep without atonia (enhanced tonic chin muscle tone, greater than twice the lowest muscle tone of the night, during a time equal to or greater than 18% of REM sleep); and iv) the disorder was not better explained by another sleep disorder, mental disorder, medication, or substance use. RBD was diagnosed in PD and MSA participants with the same criteria. To be characterized as suffering from isolated RBD, patients should not meet the criteria of another neurological disorder and should not take any drug susceptible to enhancing muscle tone during REM sleep (mostly antidepressants) at the time of iRBD diagnosis. Patients could be diagnosed with iRBD even though videopolysomnography was performed during antidepressant therapy if RBD symptoms had appeared (through spouse history) several years prior to antidepressant initiation. Healthy controls were selected to be unaffected by any neurological disorder, as defined after a medical interview and neurological examination, and matched with other groups for age and sex. From these samples, we selected patients following the flow chart shown in Supplementary Fig. A. After a preliminary analysis, we decided not to exclude participants treated with positive airway pressure (8 participants with PD and 7 participants with iRBD) from the analysis because there was no difference in the production of sighs with or without positive airway pressure. Participants with insufficient (<4 h) total sleep time, absent N3 sleep, absent REM sleep during the video-polysomnography, insufficient clinical details, or unclear/artifact signals were excluded.
All participants agreed to have their clinical and video-polysomnography measures used for further research. The participants in the ICEBERG research cohort signed a consent, and the ethics committee approved the full trial (IRB Paris VI; RCB: 2014-A00725-42 clinicaltrials.gov: NCT02305147). In the clinical cohort, patients did not oppose the reuse of their measures after reading an information form, and their non-opposition was consigned in their file, a procedure which waives to obtain the approval of an ethical committee, following the French regulation on retrospective studies. We hereby certify that we have obtained signed consent from the patients authorizing the publication of their video.
Questionnaires
The neurologists in charge of the participants with MSA completed the Unified Multiple System Atrophy Rating Scale (UMSARS) as a surrogate measure of disease progression, including sections of Parts I (items 2, 8) and II (items 2, 12-14) and the part IV (Global Disability Scale, GDS). In MSA participants with no UMSARS, symptoms were rated as present or absent, including dystonia, urinary dysfunction, constipation, hyposmia, orthostatic hypotension (10 min lying, then after two minutes standing up), supine hypertension, and impaired cognitive function. Some patients were rated from 0 (absent disability) to 4 (most severe disability), including speech, dysphagia, posture, body sway, gait, and falls. Drugs taken by participants with MSA were collected in the medical files. Age, sex, and body mass index were collected from the participants’ files.
Sleep monitoring
Video and sleep monitoring were performed during a single standard, full, attended overnight video-polysomnography using the GraelTM system (Compumedics Ltd, Aus). Electrodes measured electroencephalography (EEG; F1-A2, C3-A2, O1-A2), right and left electrooculography (EOG), electromyography (EMG) of the chin and anterior tibial muscles. The cardiorespiratory activity was measured using nasal pressure, oronasal thermistor, transcutaneous oxyhemoglobin, and pulse measurement via a finger pulse oximeter, thoracic and abdominal plethysmography belts (measuring respiratory efforts), continuous electrocardiography in DII, microphone as a snore sensor. A thoracic belt sensor was used to assess the body posture. Each measurement was calibrated at wakefulness in the evening before recording. Infrared light videography coupled with an ambient microphone was synchronized with physiological signals. Participants slept ad libitum from bedtime (no later than 23:00) to morning time (no later than 7:00) under the supervision of a nursing team. The sleep and video/audio signals recorded by polysomnography were analyzed and visually scored according to the international criteria25.
The measure of sighs during sleep stage N3 and REM sleep
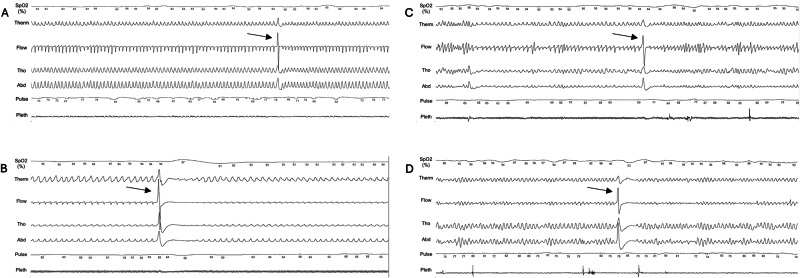
Sighing was defined as an abrupt increase in peak signal excursion in the respiratory sensors, including the nasal pressure transducer and effort belts, of at least double the pre-event baseline breaths, after a previous 10-second period of stable respiration and no movement11. It begins with ample, sudden inspiration, followed by a prolonged expiration, and then back to normal respiratory flow or other respiratory events. Segments containing flow limitations, apneas, hypopneas, movements, and arousals (including post-sigh arousal) were excluded from the analysis, as these events can be associated with pseudo-sighs, which are easy to differentiate with human eyes. In fact, a sigh is characterized by a specific pattern that is easy to recognize visually. The sigh is identified in the midst of stable and regular breathing, which helps distinguish it from large inspirations caused by obstructive events. It is markedly different from the shapes associated with sleep talking and sleep sobbing. Sleepers speak during expiration, which extends the expiratory time and results in a pseudo-flat brief “central-like” appearance on respiratory belts, occurring in clusters. This characteristic is unrelated to sighs. Examples of sighs in N3 sleep and REM sleep, followed or not by a post-sigh central apnea are shown in Fig. 2 and in the Video-Clip. Sighs in N3 and REM sleep were quantified by visual analysis of individual 5-minute segments25, including all N3 and all REM sleep epochs. All sighs were scored twice by two researchers (ZY and EVG), who were not blinded to the participants’ group. The Pearson correlation coefficient agreement between the raters was 81% for the first 15 sleep studies (the rare discrepancies between the raters concerned low-amplitude sighs) and 100% after an agreement meeting and complete review of all studies (1450 sighs) by the raters. A sigh counting training was carried out with the video and audio before performing the total sigh count. Only two MSA participants (0.36%) exhibited stridor at the time of sighing. Otherwise, the sighs were silent, with a total absence of catathrenia. In addition, we quantified isolated and double sighs (immediately consecutive sighs) and sighs followed or not by a central apnea (absence of signal on the nasal flow and naso-oral thermistor and absence of respiratory efforts) lasting at least 10 s. Thus, double sighs were characterized by a minimal duration of 2 seconds and a maximal duration of 3 seconds. The sigh index was defined as the total number of sighs in a given sleep stage divided by the time spent in the stage (in hours). It was calculated during N3 and REM sleep, not during N2 sleep or wakefulness because breathing is more irregular during these last stages.
Fig. 2. Examples of sighs during NREM and REM sleep.
Sighs in NREM N3 stage without (A, participant with PD) and with (B, participant with MSA) post-sigh central apnea. Sighs in REM sleep without (C, participant with MSA) and with (D, participant with MSA) post sigh central apnea, in a 5-min long epoch. Y axis: From the top to the bottom, SpO2: Oxyhemoglobin saturation; Therm: naso-oral thermistor; Flow: nasal pressure; Tho: thoracic movements; Abd: abdomen movements; Pulse: heart rate; Pleth: pulse wave.
Statistical analysis
The normality of the distribution of continuous measures was assessed using the Shapiro-Wilk test. Mixed models were run adjusting for the use of antidepressants or benzodiazepines in all groups. No interaction was observed between antidepressant or benzodiazepine use and sighing, so sighing was examined on a group basis only. The comparisons were performed using the ANOVA test between 4 groups, Student t-test (normal distribution), Wilcoxon rank sum test (non-normal distribution) between 2 groups for continuous variables, and chi-square test for frequencies. If the comparison test was significant, post-hoc tests were performed using the Tukey adjustment test using a p-value < 0.05 as significant. The presence vs. absence of sighs between the groups was analyzed using a generalized linear model followed by a post hoc pairwise test with a Tukey adjustment. The index of sighs was compared between the groups (restricted to participants with sighs) using a linear model and followed by a post hoc pairwise test with a Tukey adjustment. A logarithmic transformation of the sigh index was used to obtain a normal distribution of residues. If the mean sigh index differed in each group from other groups, we determined a sigh index threshold discriminating this group from other groups using a logistic regression model and the Youden index (which balances sensitivity and specificity). The computations were performed using R Studio software program (R [2022]; R Foundation for Statistical Computing, Vienna, Austria). A confirmation of these frequentist statistics was done using the Bayesian model (JASP Team (2023). JASP (Version 0.17.1) [Computer software]).
Supplementary information
Acknowledgements
The PhD in charge of this work received a PhD grant “CIFRE”, an Industrial Convention for Training through Research between the French Ministry of Higher Education, Research and Innovation, and Bioserenity Ltd, France. The ICEBERG study, from which part of the sample is extracted, was financed by Agence Nationale de la Recherche through “Investissements d’avenir” program (ANR-10- IAIHU-06 and ANR-11-INBS-0006); Fondation EDF; Fondation Planiol; Société Française de Médecine Esthétique and Energipole.
Author contributions
E.V.G.: execution, statistical analysis, writing of the first draft of the manuscript; Z.Y.: execution; P.D., S.L.S., C.L., and D.G.: patient recruitment and data collection; MP: patient recruitment, data collection, critique of the manuscript; TS and CS: critique of the manuscript; M.V.: review, and critique of the manuscript; I.A.: conception, organization of research project, review, and critique of the manuscript. All authors reviewed and approved the final manuscript.
Data availability
The raw data containing demographic, clinical, and sleep measures are stored in the Sleep Department (isabelle.arnulf@aphp.fr). They are not publicly available to preserve individuals’ privacy under the European General Data Protection Regulation. The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Competing interests
EVG: PhD with ICM & Bioserenity; ZY: has received an educational grant from the Chinese government; PD, SLS, CL: have nothing to report; MP: Financial activities outside the submitted work; CS: Financial activities outside the submitted work 1.Centre Hospitalier Universitaire de Lille, France grants 2. CARMAT other support; TS, DG, MV: have nothing to report; IA: has no financial disclosure, whether for this work or outside this work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Estefania Vargas Gonzalez, Zhongmei Yang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-024-00765-4.
References
- 1.Miglis, M. G. et al. Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol.20, 671–684 (2021). 10.1016/S1474-4422(21)00176-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang, Y. et al. Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat. Med.28, 2207–2215 (2022). 10.1038/s41591-022-01932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanciulli, A. & Wenning, G. K. Multiple-System Atrophy. N. Engl. J. Med.372, 249–263 (2015). 10.1056/NEJMra1311488 [DOI] [PubMed] [Google Scholar]
- 4.Bendixen, H. H., Smith, G. M. & Mead, J. Pattern Vent. Young Adults J. Appl. Physiol.19, 195–198 (1964). [DOI] [PubMed] [Google Scholar]
- 5.Koch, H. et al. Stable respiratory activity requires both P/Q-type and N-type voltage-gated calcium channels. J. Neurosci.33, 3633–3645 (2013). 10.1523/JNEUROSCI.6390-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cammarota, G. et al. Influence of lung collapse distribution on the physiologic response to recruitment maneuvers during noninvasive continuous positive airway pressure. Intensive Care Med37, 1095–1102 (2011). 10.1007/s00134-011-2239-8 [DOI] [PubMed] [Google Scholar]
- 7.Baldwin, D. N. et al. Effect of sighs on breathing memory and dynamics in healthy infants. J. Appl. Physiol. Bethesda Md 198597, 1830–1839 (2004). [DOI] [PubMed]
- 8.Perez-Padilla, R., West, P. & Kryger, M. H. Sighs during sleep in adult humans. Sleep6, 234–243 (1983). 10.1093/sleep/6.3.234 [DOI] [PubMed] [Google Scholar]
- 9.Lieske, S. P., Thoby-Brisson, M., Telgkamp, P. & Ramirez, J. M. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat. Neurosci.3, 600–607 (2000). 10.1038/75776 [DOI] [PubMed] [Google Scholar]
- 10.Köllensperger, M. et al. Red flags for multiple system atrophy. Mov. Disord.23, 1093–1099 (2008). 10.1002/mds.21992 [DOI] [PubMed] [Google Scholar]
- 11.Parreira, S., Antunes, F., Coelho, M., Bentes, C. & Peralta, R. Sighs during sleep in multiple system atrophy. Sleep. Med78, 75–80 (2021). 10.1016/j.sleep.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 12.Stefanova, N., Bücke, P., Duerr, S. & Wenning, G. K. Multiple system atrophy: an update. Lancet Neurol.8, 1172–1178 (2009). 10.1016/S1474-4422(09)70288-1 [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann, H. et al. Natural history of pure autonomic failure: A United States prospective cohort: Pure autonomic failure. Ann. Neurol.81, 287–297 (2017). 10.1002/ana.24877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silber, M. H. & Levine, S. Stridor and death in multiple system atrophy. Mov. Disord.15, 699–704 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Herer, B., Arnulf, I. & Housset, B. Effects of levodopa on pulmonary function in Parkinson’s disease. Chest119, 387–393 (2001). 10.1378/chest.119.2.387 [DOI] [PubMed] [Google Scholar]
- 16.Doi, A. & Ramirez, J.-M. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J. Neurosci.30, 8251–8262 (2010). 10.1523/JNEUROSCI.5361-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay, L. C., Janczewski, W. A. & Feldman, J. L. Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat. Neurosci.8, 1142–1144 (2005). 10.1038/nn1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay, L. C. & Feldman, J. L. Unilateral ablation of Pre-Bötzinger complex disrupts breathing during sleep but not wakefulness. Am. J. Respir. Crit. Care Med.178, 89–95 (2008). 10.1164/rccm.200712-1901OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burioka, N. et al. Approximate entropy of human respiratory movement during eye-closed waking and different sleep stages. Chest123, 80–86 (2003). 10.1378/chest.123.1.80 [DOI] [PubMed] [Google Scholar]
- 20.Phillipson, E. A. Control of breathing during sleep. Am. Rev. Respir. Dis.118, 909–939 (1978). [DOI] [PubMed] [Google Scholar]
- 21.Dodet, P. et al. Sleep disorders in Parkinson’s disease, an early and multiple problem. NPJ Park. Dis.10, 46 (2024). 10.1038/s41531-024-00642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biondetti, E. et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain J. Neurol.143, 2757–2770 (2020). 10.1093/brain/awaa216 [DOI] [PubMed] [Google Scholar]
- 23.Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results: MDS-UPDRS: Clinimetric Assessment. Mov. Disord.23, 2129–2170 (2008). 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 24.Gilman, S. et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology71, 670–676 (2008). 10.1212/01.wnl.0000324625.00404.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry, R. B. et al. AASM Scoring Manual Version 2.2 Updates: New chapters for scoring infant sleep staging and home sleep apnea testing. J. Clin. Sleep. Med.11, 1253–1254 (2015). 10.5664/jcsm.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data containing demographic, clinical, and sleep measures are stored in the Sleep Department (isabelle.arnulf@aphp.fr). They are not publicly available to preserve individuals’ privacy under the European General Data Protection Regulation. The data supporting this study’s findings are available from the corresponding author upon reasonable request.