Abstract
To investigate the pathogenicity of a virus originating in a chimpanzee with AIDS (C499), two chimpanzees were inoculated with a plasma-derived isolate termed human immunodeficiency virus type 1NC (HIV-1NC). A previously uninfected chimpanzee, C534, experienced rapid peripheral CD4+ T-cell loss to fewer than 26 cells/μl by 14 weeks after infection. CD4+ T-cell depletion was associated with high plasma HIV-1 loads but a low virus burden in the peripheral lymph node. The second chimpanzee, C459, infected 13 years previously with HIV-1LAV, experienced a more protracted course of peripheral CD4+ T-cell loss after HIV-1NC inoculation, resulting in fewer than 200 cells/μl by 96 weeks postinoculation. The quantities of viral RNA in the plasma and peripheral lymph node from C459 were below the lower limits of detection prior to inoculation with HIV-1NC but were significantly and persistently increased after superinfection, with HIV-1NC representing the predominant viral genotype. These results show that viruses derived from C499 are more pathogenic for chimpanzees than any other HIV-1 isolates described to date.
The development of AIDS in individuals infected with human immunodeficiency virus type 1 (HIV-1) is most often characterized by a chronic depletion of CD4+ T cells. The destruction of CD4+ T cells results in the development of severe immunodeficiency, which leads to the emergence of opportunistic infections and neoplasia, ultimately culminating in death (9). Despite the development of a number of animal models of pathogenic lentiviral infection, including simian immunodeficiency virus (SIV) (2, 13, 25) and HIV-2 infection of macaques (22, 28), and the development of pathogenic HIV-SIV chimeric viruses (21, 31), an animal model of pathogenic HIV-1 infection has not been developed.
Experimental infection of chimpanzees with HIV-1 was first demonstrated in 1984, soon after the discovery of the virus (1, 11). Subsequently, a large number of chimpanzees have been experimentally inoculated with various isolates of HIV-1 as part of HIV pathogenesis investigations or vaccine technology research. Most HIV-1 isolates have been shown to be nonpathogenic in chimpanzees; thus, the relevance of vaccine protection in this model has been the subject of considerable controversy. The absence of disease development in HIV-1-infected chimpanzees has stimulated investigations aimed at understanding the mechanisms responsible for apathogenic lentivirus infection (4, 7, 8, 10, 17–19, 27, 32, 37). However, no conclusions have been drawn regarding a definitive mechanism of resistance to disease development.
To date, only one chimpanzee infected with HIV-1 has developed AIDS (29). That animal, C499, was euthanized as a result of severe CD4+ T-cell loss, thrombocytopenia, and chronic diarrhea due to Cryptosporidium infection. Transfusion of blood from C499 to an HIV-1-negative chimpanzee, C455, resulted in rapid CD4+ T-cell loss in the latter animal (29). This observation suggested that a chimpanzee-pathogenic strain of HIV had evolved in C499, and subsequent genetic analyses of viruses isolated from C499 and C455 showed that the virulent strain was likely a recombinant of HIV-1LAV1b and HIV-1SF2 (26). To confirm that virus from C499 is pathogenic for chimpanzees, we inoculated two additional chimpanzees with a virus isolated from the plasma of C455. (The Yerkes Regional Primate Research Center is fully accredited by the American Association for the Accreditation of Laboratory Animal Care, and all animals were housed in accordance with Animal Welfare Act guidelines.) The results presented here conclusively demonstrate the in vivo evolution of an HIV-1 variant that is pathogenic for chimpanzees.
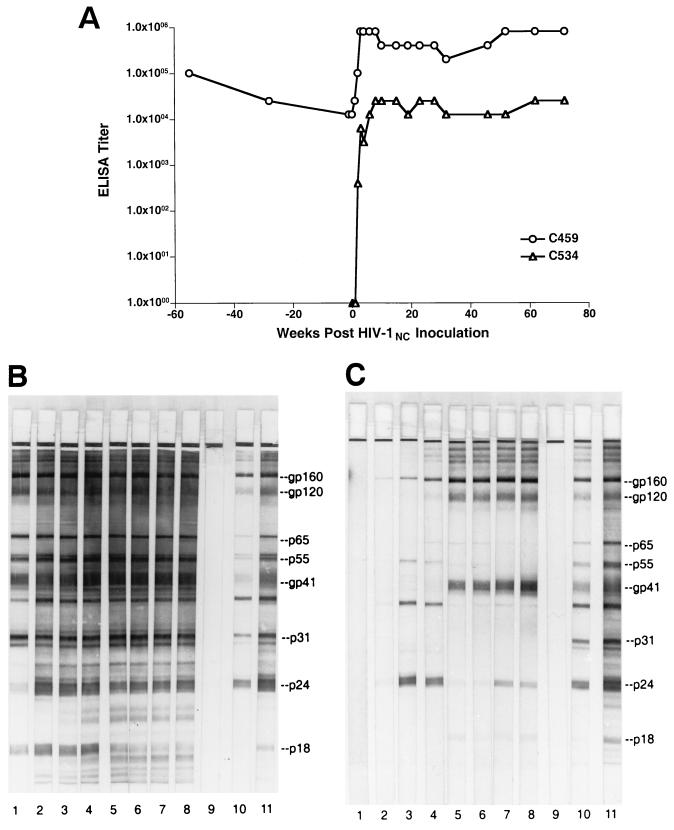
The HIV-1NC isolate, derived from the plasma of chimpanzee C455, has been described previously (26). It was grown in chimpanzee peripheral blood mononuclear cells (PBMC) and, at peak reverse transcriptase activity, cell-free supernatant was harvested, aliquoted, and stored under liquid nitrogen. This virus stock had a titer of 104 50% tissue culture infective doses (TCID50)/ml and had an HIV-1 p24 antigen concentration of 295.8 ng/ml. Two chimpanzees, C459 and C534, were intravenously inoculated with a 104 TCID50 of HIV-1NC. C534 was a naive animal; however, C459 was HIV positive, having been previously infected with the LAV isolate of HIV-1 (HIV-1LAV) in 1984 (14). Prior to infection with HIV-1NC, C459 had a vigorous antibody response to infection with HIV-1LAV as measured in plasma by enzyme-linked immunosorbent assay (ELISA) (Fig. 1A) and Western blotting (Fig. 1B). After superinfection, C459 showed a dramatic increase in HIV-1-specific antibody, from a titer of 1:12,800 on the day of HIV-1NC inoculation to a titer of 1:819,000 at 3 weeks postinfection. Western blot analysis using commercially available HIV-1 strips (Cambridge Biotech, Rockville, Md.) (Fig. 1B) revealed that increases in reactivity to HIV-1-specific proteins were primarily directed against the p24 and p18 capsid antigens following superinfection with HIV-1NC.
FIG. 1.
HIV-specific antibody responses following HIV-1NC infection in chimpanzees. (A) Plasma samples obtained from C459 and C534 at the indicated times postinfection were used to quantify HIV-specific antibody responses using a commercially available whole-virus ELISA. (B and C) Qualitative analyses of viral antibody were performed using commercially available Western blot strips. In panel B, plasma samples were from animal C459; in panel C, plasma samples from animal C534. Lane 1, day of inoculation; lane 2, 2 weeks postinoculation; lane 3, 8 weeks postinoculation; lane 4, 15 weeks postinoculation; lane 5, 32 weeks postinoculation; lane 6, 53 weeks postinoculation; lane 7, 63 weeks postinoculation; lane 8, 80 weeks postinoculation; lane 9, plasma from an uninfected chimpanzee; lane 10, weak positive control serum; lane 11, strong positive control serum.
In the previously uninfected animal, C534, a rapid antibody response to HIV-1 developed following inoculation with the NC isolate. As measured by ELISA, HIV-1-specific antibody titers rose rapidly and stabilized at 1:25,600 by 8 weeks postinoculation (Fig. 1A). While humoral responses of this magnitude are not typically observed during primary infection of chimpanzees with tissue culture-adapted viruses such as HIV-1LAV and HIV-1SF2, responses of this intensity are observed in macaques infected with highly pathogenic isolates of SIV (5, 24, 34). Western blot analysis showed that the humoral response generated by C534 during acute infection was directed primarily against Gag proteins p24 and p55, with lesser reactivity toward Env gp160 (Fig. 1C). At 32 weeks postinfection, a decline in p24 reactivity was observed, with an increase in Env gp41 and gp120 reactivities. As shown, the peak antibody titer observed for C534 did not approach that of C459.
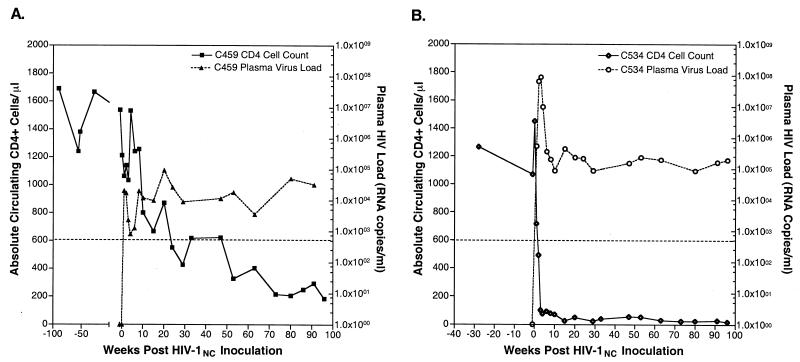
Virus could be isolated from PBMC collected from both C459 and C534 at multiple times following inoculation (data not shown). The quantity of HIV-1 RNA was measured in plasma specimens from both chimpanzees throughout the course of HIV-1NC infection by quantitative competitive PCR as described previously (29). The quantity of viral RNA in previously infected chimpanzee C459 was below the lower limit of detection (800 copies/ml) prior to superinfection (Fig. 2A). At 1 week post-HIV-1NC inoculation, C459 had a plasma virus load of 2 × 104 RNA copies/ml. The plasma virus load in this animal peaked at 19 weeks postinfection (9 × 104 copies/ml). Subsequently, the viral setpoint for this animal has been maintained at between 3 × 103 and 6 × 104 RNA copies/ml. For C534, plasma virus load peaked by 3 weeks postinfection at 8.8 × 107 RNA copies/ml and has subsequently achieved equilibrium between 2 × 104 and 2 × 105 RNA copies/ml (Fig. 2B).
FIG. 2.
Peripheral CD4+ T-cell levels and plasma HIV loads in HIV-1NC-infected chimpanzees. At the indicated times pre- and post-HIV-1NC inoculation, blood was obtained from chimpanzees C459 (A) and C534 (B) for use in determination of circulating levels of CD4+ CD2+ T cells by flow cytometry and for use in the quantitation of plasma viral RNA levels. The dotted line represents the cutoff level for the quantitation of plasma HIV levels (800 copies/ml).
The absolute number of peripheral CD3+/CD4+ T lymphocytes was measured in whole blood specimens from both chimpanzees following HIV-1NC inoculation. CD4+ T cells decreased during the first weeks after HIV-1NC inoculation of C459 but remained within normal limits (Fig. 2A). After rebounding to 1,500 cells/μl by 4 weeks postinoculation, the number of CD4+ T cells steadily decreased to 196 cells/μl by 96 weeks post-HIV-1NC inoculation. In contrast to the slowly progressive depletion observed for C459, immediate and sustained CD4+ T-cell loss was observed after infection of C534. For C534, the number of CD4+ T cells decreased by 50% to 718 cells/μl by 1 week postinoculation and further decreased to fewer than 100 cells/μl by 4 weeks postinoculation, where they remained for the balance of the study (96 weeks postinoculation).
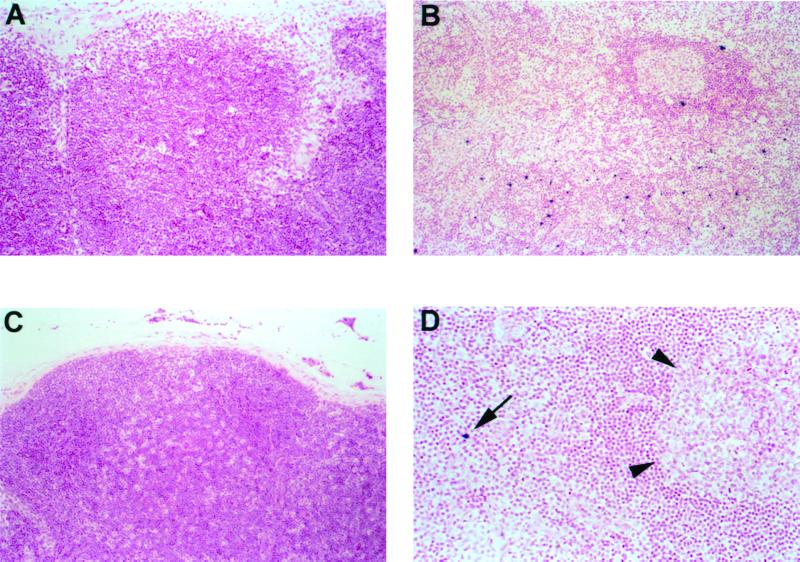
Lymph node biopsies were obtained from C459 and C534 prior to and at 3 weeks after inoculation with HIV-1NC. Lymph nodes from both animals prior to HIV-1NC inoculation showed normal numbers of lymphocytes and little to no secondary follicle formation, similar to other age-matched HIV-negative and HIV-infected nonprogressor chimpanzees (30). The number and distribution of productively infected cells was determined in sections of lymph node by in situ hybridization for viral RNA, using a riboprobe that spans the entire HIV-1 genome, as described elsewhere (29, 30). Virus-infected cells were not observed in sections of lymph node collected from C459 prior to HIV-1NC infection (Fig. 3A). This finding is in agreement with the low virus burden observed in the plasma of C459 on the day of biopsy and is consistent with observations made in other HIV-infected nonprogressor chimpanzees in the Yerkes cohort (30). Moderate numbers of infected cells were localized within germinal centers and paracortical regions of the lymph node specimen collected from C459 3 weeks after superinfection with HIV-1NC (Fig. 3B). Surprisingly, only small numbers of infected cells were observed in sections of lymph node collected from C534 at 3 weeks after infection with HIV-1NC, despite an extremely high plasma virus load measured on the day of biopsy (Fig. 3D). As with C459, infected cells were located primarily within the paracortical regions of the node. In addition, we detected a faint intercellular hybridization signal within the germinal centers, suggesting dendritic cell trapping of immune-complexed virions (data not shown).
FIG. 3.
Distribution of productively infected cells in lymph nodes from HIV-1NC-infected chimpanzees. In situ hybridization for HIV-1 RNA is shown for chimpanzees C459 (A and B) and C534 (C and D), using the chromagen nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) and nuclear fast red counterstain. (A) Productively infected cells are not apparent in sections of lymph ode from HIV-1 infected chimpanzee C459 prior to inoculation with HIV-1NC (×100). (B) Numerous infected cells (dark blue stain) are seen in the paracortex of a lymph node collected from C459 3 weeks after superinfection with HIV-1NC (×100). (C) Lymph node from C543 (uninfected chimpanzee) prior to infection with HIV-1NC (×100). (D) HIV-1-infected cell (arrow) shown in the paracortex of a lymph node from C534 3 weeks after infection with HIV-1NC. Arrowheads delineate the margins of a germinal center (×200). Note the absence of secondary follicles in lymph nodes from HIV-infected, nonprogressor C459 (A) and HIV-negative C534 (C).
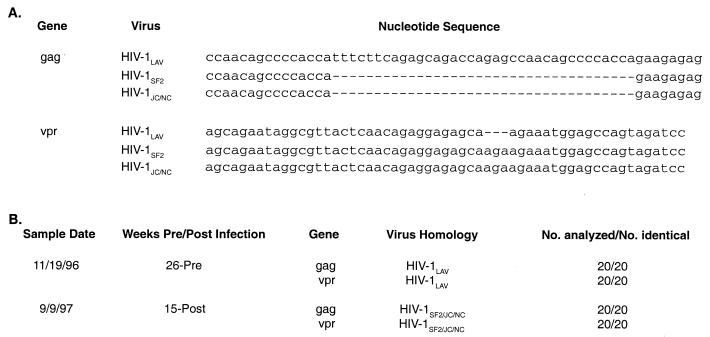
The sudden increase in plasma and lymphoid virus burdens and progressive CD4+ T-cell depletion observed following superinfection of C459 suggested that HIV-1NC had initiated a pathogenic infection in the face of an apathogenic infection with HIV-1LAV. To confirm the identity of the predominant viral genotype present after HIV-1NC inoculation, we analyzed the genotype of virus present in C459 prior to and after inoculation with HIV-1NC. As mentioned above, previous studies indicated that HIV-1NC was a recombinant virus, one derived from both HIV-1LAV1b and HIV-1SF2. Regions of identifiable recombination included both gag and vpr (26). One region in the gag of HIV-1NC specifically resembled HIV-1SF2, containing a 36-bp deletion relative to HIV-1LAV. A second site, located in vpr, also resembled HIV-1SF2, containing a 3-bp insertion relative to HIV-1LAV. These sites were utilized as molecular markers for genotyping virus from C459. Genomic DNA prepared from the PBMC of C459 was utilized to amplify both gag and vpr regions of the HIV-1 genome using typical single-round PCR (High Fidelity Kit; Boehringer Mannheim). The primers used for the amplification of the gag gene were as follows: forward, 5′-TGGCAAAGAAGGGCACATAG-3′; reverse, 5′-TGAGGGAAGTTAAAGGATACAGTT-3′ (these primers amplify a region of gag that is 320 bp long in HIV-1LAV and 284 bp long in HIV-1SF2; the genetic coordinates in HIV-1LAV are 1520 to 1839). The primers used for amplification of the vpr gene were as follows: forward, 5′-ATGGAACAAGCCCCAGAAGACCAAGGG-3′; reverse, 5′-GGATCTACTGGCTCCAGGTCT-3′ (these primers amplify a region of vpr that is 288 bp long in HIV-1LAV and 291 bp long in HIV-1SF2; the genetic coordinates in HIV-1LAV are 5141 to 5428). Twenty clones were analyzed for each time point. Six months prior to inoculation with HIV-1NC, the viral genotype detected in the PBMC of C459 was most like LAV in both gag and vpr (Fig. 4). However, at 15 weeks after superinfection, the predominating virus was SF2-like in gag and vpr, indicating that the replicating virus was the superinfecting HIV-1NC.
FIG. 4.
Nucleotide sequence analysis of HIV-1 genes in chimpanzee C459. (A) Sequence alignments of portions of gag and vpr genes of HIV-1LAV, HIV-1SF2, and HIV-1JC/NC, illustrating major differences. (B) PBMC from chimpanzee C459 obtained pre- and post-HIV-1NC infection (sample dates 11/19/96 and 9/9/97, respectively) were used to prepare genomic DNA for use as template in PCR experiments. Amplified DNA was cloned into the pGEM-T vector. Twenty clones obtained from each sample were analyzed by DNA sequencing. Results show that, prior to HIV-1NC infection, sequences were most similar to HIV-1LAV (the virus used to inoculate animal C459 in 1984) but that, after HIV-1NC inoculation, sequences were most similar to HIV-1SF2 and HIV-1JC/NC (26).
Despite the fact that most HIV-1 isolates have been shown to produce nonpathogenic infections in chimpanzees, continuing efforts have been made to find or derive an isolate of HIV-1 that is more pathogenic for this species (3, 16, 33, 34). Recent findings suggest that chimpanzees are a natural reservoir for SIVcpz and may have served as the source of HIV-1 introduction into the human population (15). Host adaptation to primate lentiviruses most likely explains why experimental infection of chimpanzees with HIV usually results in apathogenic infections. The development of AIDS in C499 confirmed that pathogenic HIV infection can occur in experimentally infected chimpanzees (29). In addition, we recently described progressive HIV infection in other chimpanzees within the Yerkes cohort (30). Transfusion of blood from C499 to an uninfected chimpanzee, C455, resulted in a dramatic decline in CD4+ T cells in the latter animal, which suggested the presence of a novel, chimpanzee-pathogenic HIV-1 isolate. However, because a blood transfusion was utilized, the role of other factors in CD4+ T-cell decline was unknown.
In this study we sought to confirm that the virus that evolved in C499 and was passaged through C455 was consistently pathogenic for chimpanzees. To address this issue, an HIV-1 stock prepared from the plasma of C455 (26) was utilized for the inoculation of two chimpanzees: one uninfected and one previously infected with HIV-1LAV. The kinetics of HIV-1NC infection in naive chimpanzee C534 were remarkably similar to those observed for the transfusion recipient C455. Each animal experienced a rapidly pathogenic course of infection, characterized by high plasma virus loads and rapid and sustained CD4+ T-cell loss, findings reminiscent of SHIV89.6p infection in rhesus macaques (31).
It is uncertain why we observed such great disparity between the plasma virus load (7.4 log10 RNA copies/ml on the day of biopsy) and the cell-associated virus burden in the lymph node biopsy specimen collected from C534 at 3 weeks postinoculation. Variation in the level of virus replication among individual lymphoid organs may account for this discrepancy. Recent studies using the SIV-macaque model have shown that the intestinal tract is a major site of viral replication and CD4+ T-cell depletion at early stages of infection (35, 36). Alternatively, peripheral CD4+ T-cell depletion occurred very rapidly in C534 (101 CD4+ T cells/μl on the day of biopsy). The rate and degree of CD4+ T-cell loss combined with the emerging antiviral immune response at 3 weeks postinoculation may have depleted this node of target cells, resulting in low numbers of surviving HIV+ cells on the day of biopsy.
The slower rate of CD4+ T-cell loss and lower plasma virus burden observed for C459 are most likely the result of preexisting HIV-induced immune responses subsequent to HIV-1LAV infection. Nevertheless, C459 experienced progressive CD4+ T-cell loss over the 96-week course of the study (1,200 cells/μl at inoculation, <200 cells/μl at 96 weeks). Taken together, the persistently elevated virus loads and rapid and sustained CD4+ T-cell loss observed following the inoculation of animals C455, C534, and C459 confirm that virus derived from C499 is more pathogenic for chimpanzees than previous HIV isolates.
The fact that CD4+ T-cell decline was induced in a chimpanzee in the face of an apparently apathogenic HIV-1 infection has implications for vaccine development. In essence, pathogenic HIV-1NC infection was initiated in C459 despite “attenuated” immunization with HIV-1LAV. Preexisting immunity and virus infection were unable to prevent the challenge with a pathogenic virus. Still, these results must be interpreted in light of the route and dose of virus administered to these chimpanzees.
Previous work by others suggested that virus derived from C499 is pathogenic for chimpanzees (6); however, the results presented here indicate that HIV-1JC/NC expresses a more chimpanzee-pathogenic phenotype than does HIV-1JC499. After 3 or more years of infection, however, none of the viruses derived from C499 have induced AIDS in chimpanzees, which may allay concerns over the use of such virulent isolates in chimpanzees (A. M. Prince, Letter, AIDS Res. Hum. Retroviruses 13:1259, 1997; A. M. Prince, J. Allan, L. Andrus, B. Brotman, J. Eichberg, R. Fouts, J. Goodall, P. Marx, K. K. Murthy, S. McGreal, and C. Noon, Letter, Science 283:1117–1118, 1999). The rapid CD4+ T-cell depletion and the high viral loads induced in animals infected with HIV-1NC provide markers for evaluating vaccine efficacy in chimpanzees that were not previously available. Further studies are aimed at defining the mechanisms of HIV-1NC pathogenesis and comparing cellular and humoral immune responses against HIV between these chimpanzees and nonprogressor chimpanzees in the Yerkes cohort.
Acknowledgments
We thank Anne Brodie-Hill for excellent technical assistance. We also thank the clinical veterinarians and animal care technicians at the Yerkes Center, who provided excellent care to all of the animals involved in this study.
This work was supported by NIH grant RO1 AI-40879 to F.J.N. and by grant RR-00165 to the Yerkes Primate Center.
REFERENCES
- 1.Alter H J, Eichberg J W, Masur H, Saxinger W C, Gallo R, Macher A M, Lane H C, Fauci A S. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984;226:549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste R E, Arthur L O, Tsai C C, Sowder R, Copeland T D, Henderson L E, Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986;60:483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogers W M, Koornstra W H, Dubbes R H, ten Haaft P J, Verstrepen B E, Jhagjhoorsingh S S, Haaksma A G, Niphuis H, Laman J D, Norley S, Schuitemaker H, Goudsmit J, Hunsmann G, Heeney J L, Wigzell H. Characteristics of primary infection of a European human immunodeficiency virus type 1 clade B isolate in chimpanzees. J Gen Virol. 1998;79:2895–2903. doi: 10.1099/0022-1317-79-12-2895. [DOI] [PubMed] [Google Scholar]
- 4.Castro B A, Walker C M, Eichberg J W, Levy J A. Suppression of human immunodeficiency virus replication by CD8+ cells from infected and uninfected chimpanzees. Cell Immunol. 1991;132:246–255. doi: 10.1016/0008-8749(91)90023-5. [DOI] [PubMed] [Google Scholar]
- 5.Connor R I, Montefiori D C, Binley J M, Moore J P, Bonhoeffer S, Gettie A, Fenamore E A, Sheridan K E, Ho D D, Dailey P J, Marx P A. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis I C, Girard M, Fultz P N. Loss of CD4+ T cells in human immunodeficiency virus type 1-infected chimpanzees is associated with increased lymphocyte apoptosis. J Virol. 1998;72:4623–4632. doi: 10.1128/jvi.72.6.4623-4632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Rienzo A M, Furlini G, Olivier R, Ferris S, Heeney J, Montagnier L. Different proliferative responses of human and chimpanzee lymphocytes after contact with human immunodeficiency virus type 1 gp120. Eur J Immunol. 1994;24:34–40. doi: 10.1002/eji.1830240106. [DOI] [PubMed] [Google Scholar]
- 8.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin A M, Venet A, Mehtali M, Muchmore E, Michel P, et al. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauci A S, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari G, Place C A, Ahearne P M, Nigida S M, Jr, Arthur L O, Bolognesi D P, Weinhold K J. Comparison of anti-HIV-1 ADCC reactivities in infected humans and chimpanzees. J Acquir Immune Defic Syndr. 1994;7:325–331. [PubMed] [Google Scholar]
- 11.Francis D P, Feorino P M, Broderson J R, McClure H M, Getchell J P, McGrath C R, Swenson B, McDougal J S, Palmer E L, Harrison A K. Infection of chimpanzees with lymphadenopathy-associated virus. Lancet. 1984;ii:1276–1277. doi: 10.1016/s0140-6736(84)92824-1. [DOI] [PubMed] [Google Scholar]
- 12.Fultz P N. Nonhuman primate models for AIDS. Clin Infect Dis. 1993;17(Suppl. 1):S230–S235. doi: 10.1093/clinids/17.supplement_1.s230. [DOI] [PubMed] [Google Scholar]
- 13.Fultz P N, McClure H M, Anderson D C, Swenson R B, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci USA. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fultz P N, McClure H M, Swenson R B, McGrath C R, Brodie A, Getchell J P, Jensen F C, Anderson D C, Broderson J R, Francis D P. Persistent infection of chimpanzees with human T-lymphotropic virus type III/lymphadenopathy-associated virus: a potential model for acquired immunodeficiency syndrome. J Virol. 1986;58:116–124. doi: 10.1128/jvi.58.1.116-124.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao F, Bailes E, Robertson D L, Chen Y, Rodenburg C M, Michael S F, Cummins L B, Arthur L O, Peeters M, Shaw G M, Sharp P M, Hahn B H. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S K, Fultz P N, Keddie E, Saag M S, Sharp P M, Hahn B H, Shaw G M. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology. 1993;194:858–864. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon M L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retrovir. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 18.Gougeon M L, Lecoeur H, Boudet F, Ledru E, Marzabal S, Boullier S, Roue R, Nagata S, Heeney J. Lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to Fas/Apo-1 (CD95)-induced apoptosis and preservation of a T helper 1 phenotype. J Immunol. 1997;158:2964–2976. [PubMed] [Google Scholar]
- 19.Heeney J, Bogers W, Buijs L, Dubbes R, ten Haaft P, Koornstra W, Niphuis H, Nara P, Teeuwsen V. Immune strategies utilized by lentivirus infected chimpanzees to resist progression to AIDS. Immunol Lett. 1996;51:45–52. doi: 10.1016/0165-2478(96)02554-0. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson B D, Aldrovandi G M, Zack J A. The SCID-hu mouse: an in-vivo model for HIV-1 pathogenesis and stem cell gene therapy for AIDS. Semin Immunol. 1996;8:215–221. doi: 10.1006/smim.1996.0027. [DOI] [PubMed] [Google Scholar]
- 21.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looney D J, McClure J, Kent S J, Radaelli A, Kraus G, Schmidt A, Steffy K, Greenberg P, Hu S L, Morton W R, Wong-Staal F. A minimally replicative HIV-2 live-virus vaccine protects M. nemestrina from disease after HIV-2(287) challenge. Virology. 1998;242:150–160. doi: 10.1006/viro.1997.8992. [DOI] [PubMed] [Google Scholar]
- 23.McCune J, Kaneshima H, Krowka J, Namikawa R, Outzen H, Peault B, Rabin L, Shih C C, Yee E, Lieberman M. The SCID-hu mouse: a small animal model for HIV infection and pathogenesis. Annu Rev Immunol. 1991;9:399–429. doi: 10.1146/annurev.iy.09.040191.002151. [DOI] [PubMed] [Google Scholar]
- 24.Meiller T F, Narayan O, Joag S V, Overholser C D., Jr Early appearance of antibodies to simian immunodeficiency virus in saliva and serum of infected macaques. Clin Diagn Lab Immunol. 1995;2:489–491. doi: 10.1128/cdli.2.4.489-491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphey-Corb M, Martin L N, Rangan S R, Baskin G B, Gormus B J, Wolf R H, Andes W A, West M, Montelaro R C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 26.Mwaengo D M, Novembre F J. Molecular cloning and characterization of viruses isolated from chimpanzees with pathogenic human immunodeficiency virus type 1 infections. J Virol. 1998;72:8976–8987. doi: 10.1128/jvi.72.11.8976-8987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nara P, Hatch W, Kessler J, Kelliher J, Carter S. The biology of human immunodeficiency virus-1 IIIB infection in the chimpanzee: in vivo and in vitro correlations. J Med Primatol. 1989;18:343–355. [PubMed] [Google Scholar]
- 28.Nicol I, Flamminio-Zola G, Dubouch P, Bernard J, Snart R, Jouffre R, Reveil B, Fouchard M, Desportes I, Nara P. Persistent HIV-2 infection of rhesus macaque, baboon, and mangabeys. Intervirology. 1989;30:258–267. doi: 10.1159/000150101. [DOI] [PubMed] [Google Scholar]
- 29.Novembre F J, Saucier M, Anderson D C, Klumpp S A, O'Neil S P, Brown II C R, Hart C E, Guenthner P C, Swenson R B, McClure H M. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neil S P, Novembre F J, Hill A B, Suwyn C, Hart C E, Evans-Strickfaden T, Anderson D C, de Rosayro J, Herndon J G, Saucier M, McClure H M. Progressive infection in a subset of HIV-1-positive chimpanzees. J Infect Dis. 2000;182:1051–1062. doi: 10.1086/315823. [DOI] [PubMed] [Google Scholar]
- 31.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuitemaker H, Meyaard L, Kootstra N A, Dubbes R, Otto S A, Tersmette M, Heeney J L, Miedema F. Lack of T cell dysfunction and programmed cell death in human immunodeficiency virus type 1-infected chimpanzees correlates with absence of monocytotropic variants. J Infect Dis. 1993;168:1140–1147. doi: 10.1093/infdis/168.5.1140. [DOI] [PubMed] [Google Scholar]
- 33.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane H C, et al. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sopper S, Sauer U, Hemm S, Demuth M, Muller J, Stahl-Hennig C, Hunsmann G, ter Meulen V, Dorries R. Protective role of the virus-specific immune response for development of severe neurologic signs in simian immunodeficiency virus-infected macaques. J Virol. 1998;72:9940–9947. doi: 10.1128/jvi.72.12.9940-9947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 36.Veazey R S, Lackner A A. The gastrointestinal tract and the pathogenesis of AIDS. AIDS. 1998;12(Suppl. A):S35–S42. [PubMed] [Google Scholar]
- 37.Zarling J M, Ledbetter J A, Sias J, Fultz P, Eichberg J, Gjerset G, Moran P A. HIV-infected humans, but not chimpanzees, have circulating cytotoxic T lymphocytes that lyse uninfected CD4+ cells. J Immunol. 1990;144:2992–2998. [PubMed] [Google Scholar]