Abstract
The nonstructural NSP4 protein of rotavirus has been described as the first viral enterotoxin. In this study we have examined the effect of NSP4 on polarized epithelial cells (MDCK-1) grown on permeable filters. Apical but not basolateral administration of NSP4 was found to cause a reduction in the transepithelial electrical resistance, redistribution of filamentous actin, and an increase in paracellular passage of fluorescein isothiocyanate-dextran. Significant effects on transepithelial electrical resistance were noted after a 20- to 30-h incubation with 1 nmol of NSP4. Most surprisingly, the epithelium recovered its original integrity and electrical resistance upon removal of NSP4. Preincubation of nonconfluent MDCK-1 cells with NSP4 prevented not only development of a permeability barrier but also lateral targeting of the tight-junction-associated Zonula Occludens-1 (ZO-1) protein. Taken together, these data indicate new and specific effects of NSP4 on tight-junction biogenesis and show a novel effect of NSP4 on polarized epithelia.
Rotavirus is the leading cause of infantile gastroenteritis worldwide and is associated with significant mortality in developing countries. Despite the significant clinical importance, the pathophysiological mechanisms by which rotavirus induces fluid and electrolyte secretion are largely unknown. Rotavirus infects the mature enterocytes in the mid and upper villous epithelium of the small intestine, which ultimately leads to cell death, villous atrophy, and diarrhea. Mechanisms that have been proposed to explain the diarrhea include the following: malabsorption secondary to enterocyte death (9, 16), villus ischemia (30, 34), and a toxin-like effect of the nonstructural protein (NSP4) (2, 11, 38, 40), and the enteric nervous system plays a key role in rotavirus fluid secretion (20).
Calcium has been shown to play an important role during rotavirus replication and cytopathogenicity. Ca2+ homeostasis is thus altered in rotavirus-infected cells (23, 24), and it has been suggested that increased intracellular calcium concentrations may be responsible for cytotoxicity and cell death (23). In addition, it has been shown that the nonstructural NSP4 protein is responsible for increased cytosolic Ca2+ in insect cells and human intestinal cells and can induce diarrhea in young mice (2, 11, 40). Furthermore, an association between increased [Ca2+] and age-dependent Cl− secretion has been reported (26). While the cellular mechanisms by which NSP4 induces fluid and electrolyte changes remain unresolved, it has been observed that NSP4 induces stimulation of inositol 1,3,5-triphosphate (IP3) production, suggesting that NSP4 mobilizes calcium through phospholipase C activation and IP3 release (11).
Changes in intestinal permeability have previously been postulated to contribute to intestinal secretion. Perturbations of the intestinal epithelial barrier by enteric pathogens are not novel but the mechanisms are probably distinct. Clostridium difficile toxins enhance permeability by disrupting actin microfilaments within the perijunctional ring (12, 27). Vibrio cholera (12, 48) produces specific toxins that alter permeability by interfering with tight junctions; other pathogens, including Salmonella (14), Shigella (31), and enteropathogenic Escherichia coli (EPEC) (32), can also disrupt the epithelial barrier. Recent work with EPEC indicates that the decrease in electrical resistance is due to disruption of epithelial tight junctions via EPEC-induced phosphorylation of myosin light chains (49).
Tight junctions maintain the cellular polarity (apical-basolateral) required for vectorial transport across the epithelium and provide a barrier for passive diffusion so that the electrochemical gradient of the epithelium can be maintained. Disruption or interference of intestinal epithelial tight junctions may therefore contribute to microbe-associated diarrhea. Since the distributions of both filamentous actin (F-actin) and Zona Occludens-1 (ZO-1) are altered by certain bacteria and their toxins (17, 25, 36, 48), it is considered that these structural changes are directly or indirectly involved in the pathogenesis of intestinal diseases (17, 47, 48).
We (35) and others (3, 28) have successfully employed the human intestinal Caco-2 cell line to elucidate cytopathological effects of rotavirus. In this study, we deliberately chose a highly polarized epithelial line, MDCK-1 cells, since a putative effect of NSP4 could be applicable to polarized epithelia in general. Madin-Darby canine kidney (MDCK-1) cells rapidly form a highly polarized epithelial-like monolayer on permeable supports and have been intensively used to address cellular and physiological questions and, more recently, also microbe-host cell interactions (4, 15, 33, 41), including rotavirus infections (35).
In this communication, we report the novel observation that the NSP4 enterotoxin of rotavirus causes a decrease in transepithelial resistance across monolayers of MDCK-1 cells and an increase in the paracellular permeability to molecules. The data presented also show that NSP4 prevents lateral targeting of the tight-junction-associated ZO-1 and induces disruption and/or reorganization of F-actin.
MDCK-1 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 10 mM HEPES, and 100 U of penicillin/ml. MDCK-1 cells were grown on glass coverslips or permeable-filter supports as described elsewhere (35, 48). Recombinant NSP4 (SA-11) and VP6 (SA-11) were produced in baculovirus-infected Sf9 cells and were purified as previously described (39). Cells were harvested 4 days postinfection in Hank's medium and lysed with lysis buffer (10 mM Tris-HCl [pH 8.1]–0.1 mM EDTA–1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}). NSP4 was first semipurified by fast protein liquid chromatography using a quaternary methylamine anion-exchange column and immunoaffinity column onto which purified rabbit immunoglobulin G against NSP4 had been immobilized. Vp6 was purified from the medium of Sf9 cells infected with baculovirus recombinant pAC461/Sa-11 by pelleting oligomers through a 35% sucrose cushion followed by equilibrium gradient centrifugation in CsCl (39). The baculovirus glycoprotein gp67 was purified from lysated cells infected with wild-type baculovirus and used as control. Protein was purified from cells solubilized with CHAPS followed by fast protein liquid chromatography and affinity chromatography on a concanavalin A column.
Polarized MDCK-1 cells grown on 6-mm-diameter Transwell Clear 0.4-μm-pore-size filters (0.33 cm2) (Costar) were measured for transepithelial electrical resistance (TER) as previously described (35, 48) by use of a Millicell-ERS resistance apparatus (Millipore). Electrical resistance values obtained in the absence of cells were considered background. The net resistance was calculated by subtracting the background. The permeability of the epithelial monolayer was assessed by measuring the rate at which fluorescein isothiocyanate (FITC)-dextran (molecular mass, 20,000 Daltons) was transported across the epithelium. FITC-dextran was dissolved in Krebs-Ringer glucose phosphate buffer (KRG), pH 7.3, to 20 mg/ml. Subsequently, 200 μl of this marker were added to the apical surface of MDCK-1 monolayers and 100-μl aliquots were removed from the basolateral compartment for a period of 46 h and placed in 2 ml of KRG for measurements. Measurements were performed using a fluorescence spectrometer (Perkin-Elmer Ltd., Beaconsfield, Buckinghamshire, England) with an excitation wavelength of 483 nm and an emission wavelength of 517 nm. Monolayers were grown on permeable filters or glass coverslips. After incubation for the desired time with NSP4, cells were washed twice in PBS and fixed with 2.5% paraformaldehyde for 45 min on ice, washed once in PBS, and then incubated two times for 10 min each in 0.5-mg/ml NaBH4 to quench free aldehyde groups. After another rinse with PBS, the cells were permeabilized with 0.3% Triton X-100 for 7 min at room temperature and stained with tetramethyl rhodamine isocyanate (TRITC)-labeled phalloidin (Sigma) diluted 1:200 in PBS for 45 min at 37°C in the dark. The ZO-1 protein was detected with the primary rat anti-ZO-1 monoclonal antibody (1520; Chemicon Int., Inc., Temecula, Calif.) diluted 1:200 in PBS for 1 h at 37°C, then washed three times in PBS, and then incubated for 1 h at 37°C with Alexa 488-conjugated-labeled goat anti-rat antibody (Molecular Probes, Eugene, Oreg.). After staining and a final wash in PBS, the cells were mounted in 4 ml of Citifluor-glycerol (Citifluor L.T.D., London, United Kingdom), 2 g of Airvol-203 (Air Products, Utrecht, Netherlands), and 8 ml of 0.2 M Tris-HCl (pH 8.5). Cells were examined with a 60× immersion objective (numerical, 1.4) in a confocal laser scanning microscope (Sarastro 2000; Molecular Dynamics, Sunnyvale, Calif.). The 514/488 nm line of the argon laser was used to excite TRITC.
Apically administered NSP4 cause a decrease in TER in MDCK-1 cells.
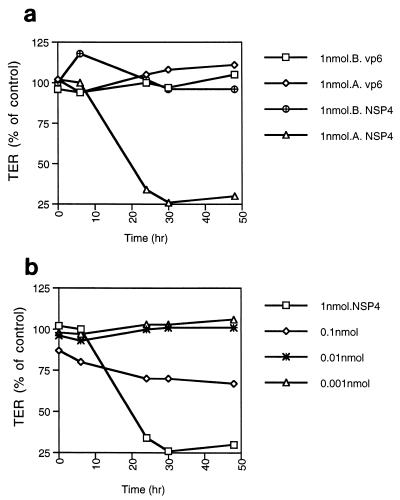
The permeability of polarized epithelial monolayers can be assessed by measurement of electrical resistance across the monolayer, which measures the integrity of the tight junction and paracellular permeability. To determine whether NSP4 has any effect on the permeability on polarized epithelial cells, MDCK-1 cells were grown on permeable supports. MDCK-1 monolayers had a TER of ≥1000 to 2000 ohm/cm2 at the time of the experiments. To determine any effect of NSP4 on the permeability, MDCK cells were treated from the apical or basolateral compartment with a 50-μl volume containing 1 nmol (20 μM) of NSP4, VP6, or buffer followed by measuring the electrical resistance at different times (Fig. 1A). The amount (1 nmol) was chosen because it is capable of inducing diarrhea in mice (2). As illustrated in Fig. 1A, apical but not basolateral administration of NSP4 caused a time-dependent decrease in TER. A separate set of experiments was performed to investigate whether any effect of NSP4 would occur within the first 4 h. No effect was seen (not shown), nor were any effects with VP6 observed at any time point. The viability of polarized MDCK-1 monolayers treated with NSP4 was examined by light and confocal microscopy during the experimental period. By this criterion, the cells remained viable during the experimental period. This is in agreement with our and others' previous observations that rotavirus induces a transepithelial leak on filter-grown polarized epithelia before development of cythopathic effect and cell death (28, 35).
FIG. 1.
(a) Asymmetric effect of NSP4 on the integrity of polarized MDCK-1 cells. NSP4 and VP6 were administered (50 μl) to the apical (A) or the basolateral (B) surfaces of filter-grown polarized MDCK-1 monolayers, followed by determination of TER at different time points. TER is presented as a percent of the TER of mock-treated cells. Mean values are presented (n = 4). (b) NSP4 demonstrates a dose-dependent effect on MDCK-1 cells. NSP4 and VP6 were administered (50 μl) in different concentrations to the apical surface of filter-grown MDCK-1 cells, and resistance was determined after different time points. Mean values are presented (n = 4).
To investigate if NSP4 causes a dose-dependent effect, MDCK-1 cells were incubated with various amounts of NSP4, from 1 to 0.001 nmol. Figure 1B shows that the effect of NSP4 is dose dependent, with no effects at amounts of 0.01 nmol or lower and a limited effect at 0.1 nmol.
NSP4 induces paracellular passage of FITC-dextran in MDCK cells.
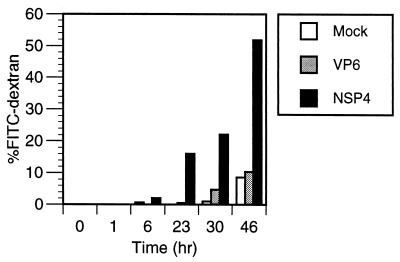
To further examine the effect of NSP4 on epithelial integrity, MDCK-1 monolayers grown on permeable filters were incubated with 1 nmol of NSP4. FITC-dextran and NSP4 were added to the apical surface at day 2 when the cells had reached an integrity of 2,000 ohm. FITC-dextran was chosen as a paracellular marker, and flux was measured by collecting the media from the basolateral domain at the indicated times. As shown in Fig. 2, FITC-dextran was transported from the apical domain to the basolateral chamber more significantly in NSP4-treated cells than in mock-treated cells, suggesting that NSP4 alters the tight-junction complex. Vertical confocal sectioning revealed no sign of transcellular passage of dextran (not shown). The kinetics of the transport corresponds well with the kinetics of reduction in TER shown in Fig. 1 and suggests that NSP4 alters tight-junction structure and formation.
FIG. 2.
NSP4 (1 nmol) but not VP6 (1 nmol) increases the paracellular flux of FITC-dextran (molecular weight, 20,000) across polarized filter-grown MDCK-1 monolayers. Shown is the total amount of FITC-dextran (in percent) transported from the apical to the basolateral compartment after various time points.
Epithelial integrity is restored by removing NSP4.
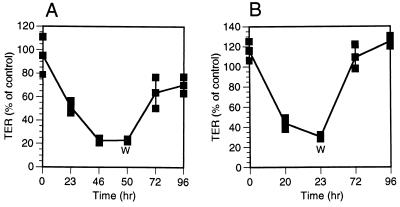
The fact that NSP4 decreased the TER in a dose-dependent and time-dependent manner raised a question of whether the effect would continue even after removal of NSP4. To address this question, 1 nmol of NSP4 was added to MDCK-1 monolayers followed by monitoring the electrical resistance. At 23 or 50 h postadministration, when the electrical resistance was 40 and 20% of the resistance of mock-treated cells, respectively, NSP4 was removed, and the apical surface of the cells was washed twice with DMEM and then incubated in DMEM for up to 96 h. As illustrated in Fig. 3A, the TER recovered and reached about 70% of the control level. Removal of NSP4 at 23 h (Fig. 3B) had a similar effect, except that the regeneration of electrical resistance was slightly more efficient, most probably due to the longer recovery time (73 h versus 46 h).
FIG. 3.
TER is recovered after removing NSP4. (A) NSP4 (1 nmol) was added to the apical surface of polarized MDCK-1 monolayers and then washed out (W) with DMEM after 50 h, followed by incubation of the cells in DMEM up to 96 h. Values are means ± 1 standard deviation) and are presented as a percent of the resistance measured in mock-treated cells (n = 3). (B) Recovery of TER after removing NSP4 (1 nmol) by washing out NSP4 after 23 h (n = 3).
NSP4 prevents development of electrical resistance and lateral targeting of the tight-junction-associated ZO-1 protein.
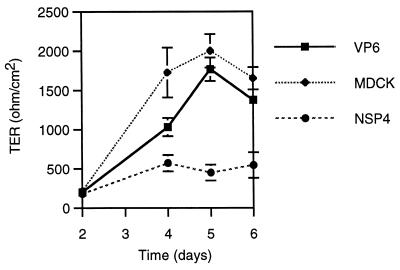
Given the effect of NSP4 on permeability, we next investigated whether NSP4 could prevent development of a tight polarized epithelium. MDCK-1 cells were seeded on permeable supports for 2 days, followed by incubation with 1 nmol of NSP4, 1 nmol of VP6, or mock treatment. Figure 4 shows that coculturing of MDCK-1 cells with 1 nmol of NSP4 significantly (day 5) prevented the development of electrical resistance. Note that NSP4-treated cells had a slight increase in electrical resistance from the day of administration (day 2) to day 4. This most likely reflects the incubation time required for NSP4 to show any measurable effect on MDCK-1 cells (Fig. 1 and 2).
FIG. 4.
NSP4 prevents development of a tight polarized epithelium. MDCK-1 cells were cocultured with 50 μl containing 1 nmol of NSP4 (20 μM) or VP6 from the first day of seeding the cells onto filters. Electrical resistance measurements started on day 2 postseeding of cells on filters. Values of TER presented are means ± 1 standard deviation (n = 3). MDCK, cultivation without protein in the medium.
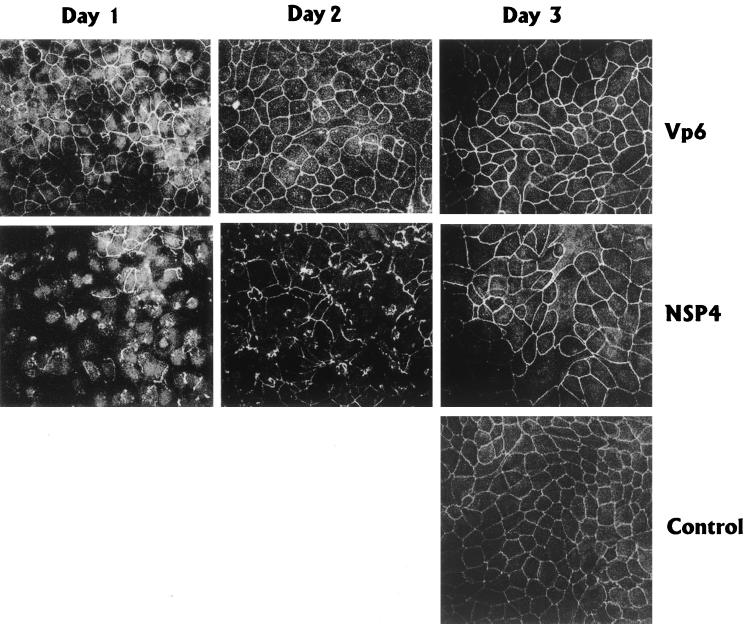
The fact that NSP4 prevented development of electrical resistance raised the question of whether this was due to interference with ZO-1, a ubiquitous tight-junction-associated protein in MDCK-1 cells (1). To investigate if the targeting of ZO-1 to tight junctions was affected by NSP4, MDCK-1 cells grown on a coverglass were incubated at days 1, 2, and 3 postseeding with 1 nmol of NSP4 or VP6, and the distribution of ZO-1 was analyzed by confocal microscopy. In contrast to mock- and VP6-treated cells, NSP4 prevented lateral targeting of ZO-1 and its association with tight junctions led to a weaker and interrupted distribution of ZO-1 (Fig. 5). The effect was most pronounced in cells treated for 48 h, with NSP4 added at the time of seeding (day 1), and was absent or weak when NSP4 was incubated for 24 h to already-confluent MDCK-1 monolayers (day 3). This suggests that NSP4 interfered with the targeting of ZO-1 to tight junctions during biogenesis and formation of an epithelial barrier.
FIG. 5.
Ability of NSP4 to redistribute ZO-1 in polarized MDCK-1 cells. Day 1, MDCK cells mixed with 1 nmol of NSP4 or VP6 and seeded on filters for 48 h; day 2, MDCK-1 cells incubated 24 h from day 2 with NSP4 or VP6; day 3, incubation of MDCK-1 cells with NSP4 or VP6 for 24 h from day 3 postseeding on filters.
NSP4 alters the distribution of F-actin.
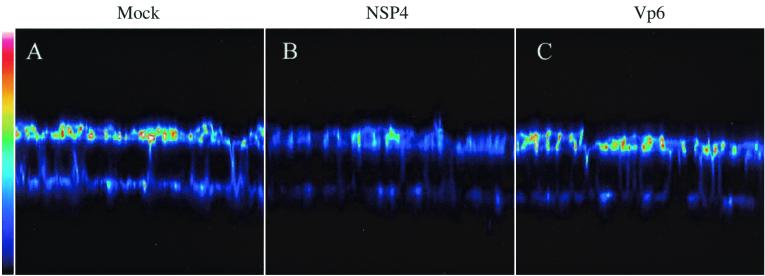
Actin filaments are a key in maintaining cell shape and regulating tight junction permeability, and several bacterial enterotoxins previously have been found to alter actin microfilaments (27, 42, 47). We therefore examined if the viral NSP4 toxin had any effect on the distribution of F-actin in MDCK-1 cells. After a 24-h incubation of confluent MDCK-1 monolayers with 1 nmol of NSP4, the cells were stained with phalloidin, which binds specifically to F-actin, and examined by confocal microscopy. Control or VP6-treated monolayers revealed a punctate surface staining consistent with the F-actin bundles present in the apical microvilli. As illustrated with a vertical section (Fig. 6), the apical concentration of F-actin was much lower in MDCK-1 cells after incubation with NSP4 than in mock- or VP6-treated cells. Colors reflect different relative concentrations of F-actin (linear 8-bit scale = 256 levels). Figure 6 shows that the predominant amount of F-actin is localized mainly to the apical surface.
FIG. 6.
NSP4 perturbs F-actin distribution in polarized MDCK-1 cells. Shown is a vertical section (x-z) of mock-treated MDCK-1 cells (A) or MDCK-1 cells treated for 24 h with 1 nmol of NSP4 (B) or 1 nmol of VP6 (C). F-actin was identified by TRITC-labeled phalloidin and analyzed by confocal microscopy. The vertical sections were scanned with a 0.35 pixel size and a pinhole setting of 50 μm. The pseudocolors in the images represent the relative concentration (linear 8-bit scale) of F-actin. White shows the maximum intensity (level 256) and dark blue shows the minimum intensity (level 0).
The mechanism of rotavirus diarrhea is not yet well understood. In several animal species, profuse diarrhea occurs prior to detection of histologic changes, including villus blunting (8, 37, 43), and in vitro studies show that polarized intestinal cells can withstand infection without lysis for a long time (19, 35). It is therefore tempting to speculate that specific signals elicited by infection participate in pathophysiology. In support of this hypothesis, Ball et al. (2) have shown that young mice respond with diarrhea soon after inoculation with purified NSP4 without significant changes of the mucosal morphology. This and similar observations therefore indicate that rotavirus diarrhea somehow involves NSP4 and that fluid secretion is not the only direct result of virus replication and gross morphological changes in the mucosa.
Polarized epithelial cells grown on permeable supports were used to assess whether NSP4 of rotavirus had any effect on membrane permeability. The role of increased permeability in rotavirus-induced diarrhea remains speculative, but an association with diarrhea has been suggested for other diseases (21, 22). We (35) and others (3, 28) have previously shown that rotavirus infection of polarized epithelial (MDCK and Caco-2) cells leads to a paracellular leak and F-actin alterations, and this study extends these observations and associates the NSP4 enterotoxin with this effect. The time and kinetics by which NSP4 induces a paracellular leak are very similar to observations of infectious viruses in polarized epithelia (10, 35). FITC-dextran was used to demonstrate NSP4-induced flux across the epithelium, and the kinetics paralleled the reduction in electrical resistance. As FITC-dextran is not cell permeable, our present results and previous proposal (35) indicate that the reduction in TER is an effect on the paracellular pathway.
The observation that the electrical resistance could be recovered following removal of NSP4 indicates that apical administration of NSP4 does not kill the cell. Instead, the kinetics suggest that NSP4 interacts with a specific receptor on the apical surface of MDCK-1 cells that triggers a signal transduction cascade (11). The decrease in TER followed the kinetics observed for native virus (35) and was slower than that for certain bacteria (4, 7, 14, 48) but similar to that for C. difficile toxin B (17).
Dong et al. (11) have shown that exogenous treatment of cells with NSP4 mobilizes intracellular Ca2+ in human intestinal cells through receptor-mediated phospholipase C activation and inositol-1,4,5-triphosphate production. In cells with phospholipase C activation, diacylglycerol is produced, which can activate protein kinase C. Activation of protein kinase C has previously been reported to participate in the regulation of the paracellular barrier (6), and decreases of the electrical resistance in MDCK-1 cells (29) and may thus offer an attractive explanation for the observed decrease in TER in NSP4-treated cells. Furthermore, it is interesting to note that several bacterial toxins also have specific effects on F-actin, with a concomitant effect on epithelial permeability (5, 13, 17, 18, 48).
Rotavirus has recently been shown to decrease the apical expression of sucrase-isomaltase (19), a brush border disaccharidase expressed in the small intestine. A possible explanation for this reduction could be virus-induced reorganization of the cytoskeleton of microvilli. In fact, rotavirus has recently been shown to perturb the organization of apical F-actin (19), and this observation is in accordance with our earlier observation that microvilli are affected in rotavirus-infected cells (35). The present study, for the first time, associates the perturbing effect on F-actin with the specific protein NSP4. F-actin staining was diminished at the apical pole of NSP4-treated cells, most likely due to depolymerization of actin with further changes on the Zona Occludens and tight junctions. It is interesting to note that Obert et al. (28) and Dickman et al. (10) have recently shown that infection with rhesus rotavirus redistributes occludin and ZO-1. It therefore appears that rotavirus interferes with at least two tight-junction-associated proteins (ZO-1, occludin), and in this study we report that NSP4 specifically interferes with ZO-1.
Jourdan et al. (19) have found that rotavirus perturbs the targeting of sucrase-isomaltase to the apical surface of polarized epithelia and proposed that rotavirus infection interferes with targeting of this molecule to the plasma membrane. Several endogenous proteins and rotavirus VP7 are also mistargeted in neurons infected with rotavirus (44–46), which further supports the proposal that rotavirus interferes with the secretory pathway. In this work, we found that rotavirus not only prevented lateral targeting of the ZO-1 protein to tight junctions during biogenesis but showed, more importantly, that this novel effect could be associated with NSP4.
In summary, our results indicate that NSP4 specifically perturbs the paracellular pathway, reorganizes F-actin, and prevents transport of the ZO-1 protein to tight junctions during biogenesis and thereby impairs normal formation of tight junctions and a tight polarized epithelium.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (projects 10392 [L.S.] and 6251 [K.-E.M.]), Karolinska Institute Research Fund (L.S.), the Lions Fund (K.-E.M.), Swedish Research Council for Engineering Sciences (K.-E.M.), King Gustaf Vth 80-Year Fund (K.-E.M.), the National Institute of Health (grant DK 30144 [M.K.E.]), and the Baylor-Karolinska Institute Scientific Collaboration Program.
Special thanks to Mats Wolving for image analysis and presentations.
REFERENCES
- 1.Anderson J, Stevenson B, Jesaitis L, Goodenough D, Mooseker M. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 3.Brunet J, Cotte-Laffitte J, Linxe C, Quero A-M, Geniteau-Legendre M, Servin A. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J Virol. 2000;74:2323–2332. doi: 10.1128/jvi.74.5.2323-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canil C, Rosenshine I, Ruschkowski S, Donnenberg M, Kaper J, Finlay B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers F G, Koshy S S, Saidi R, Clark D, Moore R, Sears C. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84) in vitro. Infect Immun. 1997;65:3561–3570. doi: 10.1128/iai.65.9.3561-3570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Citi S. The molecular organization of tight junctions. J Cell Biol. 1993;121:485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collington G, Booth I, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins J, Benfield D, Duimstra J. Comparative virulence of two porcine group-A rotavirus isolates in gnotobiotic pigs. Am J Vet Res. 1989;50:827–835. [PubMed] [Google Scholar]
- 9.Davidson G P, Gall D G, Petric M, Butler D G, Hamilton J R. Human rotavirus enteritis induced in conventional piglets. Intestinal structure and transport. J Clin Investig. 1977;60:1402–1409. doi: 10.1172/JCI108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickman K, Scott J, Anderson J, Lippe S, Zhao L, Burakoff R, Shaw R. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am J Physiol Gastrointestin Liver Physiol. 2000;279:G757–G766. doi: 10.1152/ajpgi.2000.279.4.G757. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Zeng C Q, Ball J M, Estes M K, Morris A P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci USA. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper J B, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum S E. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Investig. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finlay B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 15.Fuller S D, von Bonsdorff C-H, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- 16.Graham D Y, Sackman J W, Estes M K. Pathogenesis of rotavirus-induced diarrhea. Preliminary studies in miniature swine piglet. Dig Dis Sci. 1984;29:1028–1035. doi: 10.1007/BF01311255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht G, Koutsouris A, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 18.Hecht G, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Investig. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jourdan N, Brunet J-P, Sapin C, Blais A, Cotte-Laffitte J, Forestier F, Quero A-M, Trugnan G, Servin A. Rotavirus infection reduces sucrase-isomaltase expression in human intestinal cells by perturbing protein targeting and organization of microvillar cytoskeleton. J Virol. 1998;72:7228–7236. doi: 10.1128/jvi.72.9.7228-7236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundgren O, Peregrin A, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:409–411. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 21.Madara J. Contributions of the paracellular pathway to secretion, absoption, and barrier function in the epithelium of the small intestine. In: Lebenthal F, Duffy M, editors. Textbook of secretory diarrhea. New York, N.Y: Raven Press; 1990. pp. 125–138. [Google Scholar]
- 22.Madara J L. Tight junction dynamics: is paracellular transport regulated? Cell. 1988;53:497–498. doi: 10.1016/0092-8674(88)90562-4. [DOI] [PubMed] [Google Scholar]
- 23.Michelangeli F, Liprandi F, Chemello M E, Ciarlet M, Ruiz M C. Selective depletion of stored calcium by thapsigargin blocks rotavirus maturation but not the cytopathic effect. J Virol. 1995;69:3838–3847. doi: 10.1128/jvi.69.6.3838-3847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michelangeli F, Ruiz M-C, Castillo J, Ludert J, Liprandi F. Effect of rotavirus on intracellular calcium homeostasis in cultures cells. Virology. 1991;181:520–527. doi: 10.1016/0042-6822(91)90884-e. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell M, Laughon B, Lin S. Biochemical studies on the effect of Clostridium difficile toxin B on actin in vivo and in vitro. Infect Immun. 1987;55:1610–1615. doi: 10.1128/iai.55.7.1610-1615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris A P, Scott J K, Ball J M, Zeng C Q, O'Neal W K, Estes M K. NSP4 elicits age-dependent diarrhea and Ca(2+) mediated I(−) influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 27.Nybom P, Magnusson K E. Studies with wortmannin and cytochalasins suggest a pivotal role of phosphatidylinositols in the regulation of tight junction integrity. Biosci Rep. 1996;16:265–272. doi: 10.1007/BF01207340. [DOI] [PubMed] [Google Scholar]
- 28.Obert G, Peiffer I, Servin A. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 monolayers. J Virol. 2000;74:4645–4651. doi: 10.1128/jvi.74.10.4645-4651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojakian G. Tumor promoter-induced changes in the permeability of epithelial tight junctions. Cell. 1981;23:95–103. doi: 10.1016/0092-8674(81)90274-9. [DOI] [PubMed] [Google Scholar]
- 30.Osborne M P, Haddon S J, Spencer A J, Collins J, Starkey W G, Wallis T S, Clarke G J, Worton K J, Candy D C, Stephen J. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J Pediatr Gastroenterol Nutr. 1988;7:236–248. doi: 10.1097/00005176-198803000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Perdomo J, Gounon P, Sansonetti P. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayers by Shigella flexneri. J Clin Investig. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philpott P, Mckay D, Sherman P, Perdue M. Infection of T84 intestinal epithelial cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 33.Simons K, Fuller S D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- 34.Starkey W G, Collins J, Wallis T S, Clarke G J, Spencer A J, Haddon S J, Osborne M P, Candy D C, Stephen J. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986;67:2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- 35.Svensson L, Finlay B B, Bass D, von Bonsdorff C H, Greenberg H B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 1991;65:4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thelestam M, Bronnegard Interaction of cytophatogenic toxin from C. difficile with cells in tissue culture. Scand J Infect Dis. 1980;22:16–29. [PubMed] [Google Scholar]
- 37.Thiel K, Bohl E, Cross R, Kohler E, Agnes A. Pathogenesis of porcine rotaviral infection in experimentally inoculated gnotobiotic pigs. Am J Vet Res. 1978;39:213–220. [PubMed] [Google Scholar]
- 38.Tian P, Ball J M, Zeng C Q, Estes M K. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J Virol. 1996;70:6973–6981. doi: 10.1128/jvi.70.10.6973-6981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian P, Estes M K, Hu Y, Ball J M, Zeng C Q, Schilling W P. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian P, Hu Y, Schilling W P, Lindsay D A, Eiden J, Estes M K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Bonsdorff C-H, Fuller S D, Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985;4:2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vouret-Craviari V, Grall D, Flatau G, Pouysségur J, Boquet P, van Obberghen-Schilling E. Effects of cytotoxic necrotizing factor 1 and lethal toxin on human cytoskeleton and V-cadherin localization in human endothelial cell monolayers. Infect Immun. 1999;67:3002–3008. doi: 10.1128/iai.67.6.3002-3008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward L A, Rosen B I, Yuan L, Saif L J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 44.Weclewicz K, Kristensson K, Greenberg H B, Svensson L. The endoplasmic reticulum-associated VP7 of rotavirus is targeted to axons and dendrites in polarized neurons. J Neurocytol. 1993;22:616–626. doi: 10.1007/BF01181488. [DOI] [PubMed] [Google Scholar]
- 45.Weclewicz K, Svensson L, Billger M, Holmberg K, Wallin M, Kristensson K. Microtubule-associated protein 2 appears in axons of cultured dorsal root ganglia and spinal cord neurons after rotavirus infection. J Neurosci Res. 1993;36:173–182. doi: 10.1002/jnr.490360207. [DOI] [PubMed] [Google Scholar]
- 46.Weclewicz K, Svensson L, Kristensson K. Targeting of endoplasmic reticulum-associated proteins to axons and dendrites in rotavirus-infected neurons. Brain Res Bull. 1998;46:353–360. doi: 10.1016/S0361-9230(98)00013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells C L, van de Westerlo E M, Jechorek R P, Feltis B A, Wilkins T D, Erlandsen S L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996;110:1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- 48.Wu Z, Milton D, Nybom P, Sjo A, Magnusson K E. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- 49.Yuhan R, Koutsouris A, Savkovik S, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]