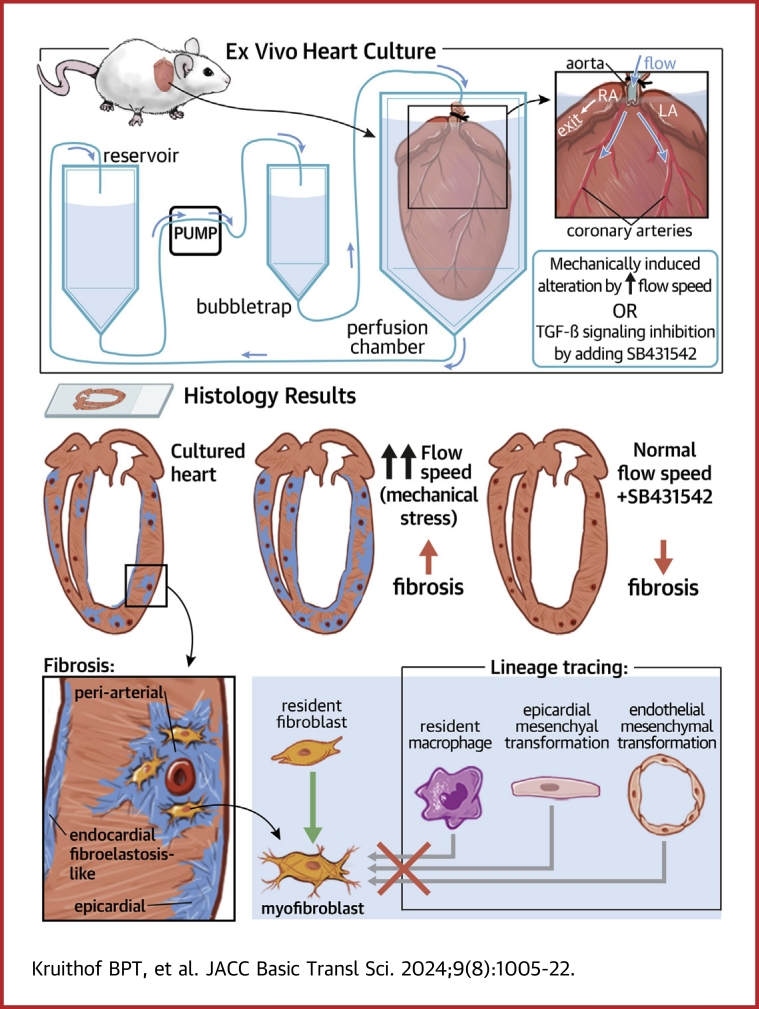
Visual Abstract
Key Words: flow model, mechanical environment, MTCS, preclinical translational, TGFbeta
Highlights
-
•
No effective pharmacological treatment exists for cardiac fibrosis caused by absence of relevant preclinical translational models.
-
•
This study demonstrates the development of an ex vivo flow system, which allows induction of cardiac fibrosis in intact adult mouse hearts.
-
•
The extent of fibrosis is dependent on the level of mechanical stress.
-
•
Inhibition of fibrosis by the SB431542 compound demonstrates that the model can be used as a preclinical test model for new therapeutic agents.
Summary
Fibrosis is a characteristic of many cardiac diseases for which no effective treatment exists. We have developed an ex vivo flow system, which allows induction of cardiac fibrosis in intact adult mouse hearts. Lineage-tracing studies indicated that the collagen-producing myofibroblasts originated from the resident fibroblasts. The extent of fibrosis was flow rate dependent, and pharmacological inhibition of the transforming growth factor beta signaling pathway prevented fibrosis. Therefore, in this powerful system, the cellular and molecular mechanisms underlying cardiac fibrosis can be studied. In addition, new targets can be tested on organ level for their ability to inhibit fibrosis.
Fibrosis is a common pathophysiological process of multiple cardiac diseases including hypertension, myocardial infarction, and nonischemic cardiomyopathy. It leads to myocardial stiffness and loss of contractility, which eventually will lead to life-threatening heart failure. More than 5% of the population older than 65 years harbors heart failure for which heart transplantation is the only treatment. Fibrosis is also a feature of the aging heart, which undergoes structural and functional changes rendering the heart more susceptible to heart diseases. Developing new strategies to control fibrosis is therefore crucial to treat many cardiac diseases.1
Cardiac fibrosis is characterized by the excessive expansion of cardiac fibroblasts and the accumulation of extracellular matrix (ECM) components, in particular collagens I and III, in the myocardium of the heart.2 The activated fibroblast (myofibroblast) of the heart is considered responsible for the production and is triggered by mechanical and biochemical stimuli from the environment. Understanding the regulation of myofibroblast behavior is crucial to interfere with the fibrotic process in the heart.
The transforming growth factor (TGF)-β signaling pathway is considered as the central molecular player steering fibrosis.3,4 The TGF-β pathway is activated by binding of TGF-β to a complex of the TGF-β type II and I receptor (ALK5), which results in SMAD-dependent and -independent regulation of gene expression.5 SMAD-dependent TGF-β signaling is initiated by phosphorylation of the intracellular signal transducer and transcriptional modulators SMAD2 and SMAD3 (pSMAD2/3) by the activated ALK5 kinase. pSMAD2/3, after complex formation with SMAD4, translocates to the nucleus to regulate the activation or repression of target gene expression.4 The TGF-β SMAD-independent signaling pathway includes the extracellular signal-related kinase and p38 mitogen-activated protein kinase (p38MAPK), which have also been implicated in the regulation of cardiac fibrosis.5,6 Although the TGF-β pathway is an attractive therapeutic target for treating cardiac fibrosis, the pleiotropic and context-dependent actions of TGF-β, which can be both protective and detrimental; the complexity of its signaling cascades; and the multistep process of cardiac fibrosis greatly complicate the therapeutic translation.7,8 To better understand the cell-specific actions of TGF-β and other fibrosis-related factors in vivo, effective fibrosis models are required.
In vitro experiments in which fibroblasts are cultured in 2 dimensions provide great experimental control but lack the complexity of the 3-dimensional (3D) architecture of the heart with a delicate interplay between biochemical and mechanical signals steering cell behavior. Currently, more advanced 3D culture models for the study of cardiac fibrosis are generated, where multiple cardiac cell types are present in a 3D configuration.9 Although the level of significance in comparison with 2-dimensional cell cultures is greater, the required maturation state of the cardiac cells and their organization, interaction, and embedding in the surrounding ECM do not equal the native complexity of the myocardium. Further, these 3D setups represent a microenvironment, which provides test settings for applications like toxicity screens and cellular response, but does not facilitate disease modeling on an organ level.
Several in vivo mouse models to study cardiac processes exist where cardiac fibrosis is induced by volume and/or pressure overload generated by genetic alteration,10 by pharmacological stimulation,11 or surgically by constriction of ascending aorta or pulmonary constriction.12 Further, myocardial ischemia-associated cardiac fibrosis is induced by ligation of coronary arteries,13 and dilated cardiomyopathy-related cardiac fibrosis is induced by genetic alteration.14 Whereas the myocardial infarction that is induced by ligation of the coronary arteries results in acute local fibrotic scarring, pressure/volume overload and dilated cardiomyopathy-related fibrosis are more chronic diffuse fibrosis. Although in vivo mouse experiments provide the best native (patho)physiological conditions and are crucial for obtaining insights in the pathophysiology of cardiac diseases and test new therapeutic strategies,15 their utilization still suffers from important shortcomings. The surgical procedures are technically challenging, the follow-up can take months, and especially the lack of controllability of conditions and therefore, establishing consistent reproducible conditions can hamper the analysis. In addition, the testing of new therapeutics in in vivo mouse models is often hampered by the unfamiliarity with the optimal delivery method, administration route, dose, and frequency of administration of a specific therapeutic.16
The aim of this study was therefore to establish a controllable ex vivo culture model for whole mouse heart in which cardiac fibrosis can be induced and manipulated. Therefore, we have adapted the Miniature Tissue Culture System (MTCS), which has been used in previous studies to study valvular heart disease.17, 18, 19, 20 We found that fibrosis takes place in the whole mouse heart after prolonged ex vivo culture with differential characteristics in the ventricular and atrial myocardium and subepicardium. Fibrosis was determined by increased population of myofibroblasts and increased expression of collagen and other fibrosis-associated ECM components. We show that the extent of fibrosis can be modulated by changing mechanical and biochemical signals, and that the fibrotic process is TGF-β-dependent, demonstrating that with this model, the mechanisms underlying cardiac fibrosis can studied and potential new targets can be identified and tested.
Methods
Animals
All animal experiments were performed in 2- to 15-month-old mice with a mixed genetic background (B6;129) according to protocols approved by the animal welfare committee of the Leiden University Medical Center and conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
To trace the fate of endothelium-derived cells, macrophages, and epicardium-derived cells, the VE-cadherin-CreERT2 mouse21 crossed with the B6.129(Cg)-Gt(ROSA)26-Sortm4(ACTB-tdTomato,–EGFP)Luo/J reporter mouse (R26-mT/mG mouse), the LysMCre∗Rosa26Sortm1 enhanced yellow fluorescent (EYFP) mice,22, 23, 24 and the Wilms’ tumor-1 (Wt1)-CreERT2 mouse crossed with the R26-mT/mG mouse were used, respectively. To detect the EYFP and enhanced green fluorescent protein (GFP) expression, immunofluorescent staining was performed for GFP.
Ex vivo culture of whole mouse hearts
Mouse hearts were cultured in the MTCS as previously described.17,19,20 In short and with the following modifications: mice were anesthetized with 4% isoflurane and the hearts were in situ perfused with salt solutions, removed, and transferred to the perfusion chambers. For each heart, the inflow needle of the perfusion chamber was inserted into the aorta and ligated with a suture. Flow (500-1,500 μL/min) was introduced using a pump, and medium (Dulbecco's Modified Eagle Medium, Life Technologies, 31966047; supplemented with 10% fetal bovine serum, antibiotic/antimycotic [100 units of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B/ml; Invitrogen], and insulin-transferrin-selenium [10 μg/mL insulin, 5.5 μg/mL transferrin, 6.7 ng/mL selenium; Fisher Scientific]) was directed from the reservoir through the air bubble trap and into the perfusion chamber where it flowed through the aorta toward the closed aortic valve into the coronary circulation (Supplemental Figure 1). The medium exited the heart via the right atrium and recirculated to the reservoir. The medium was replaced every 3 to 4 days. The ALK5-kinase inhibitor SB431542 (5 μmol/L, Tocris Bioscience) and TGF-β3 (1 ng/mL, R&D) were added to inhibit or stimulate the TGF-β-signaling pathway, respectively. 4OH-tamoxifen (1 μmol/L, Sigma) was added to the medium at the start of the culture to induce recombination when using the VE-cadherin-CreERT2 or Wt1-CreERT2 mouse hearts. The hearts were cultured for up to 2 weeks followed by fixation overnight with 4% PFA/PBS.
Histology
Histology was performed as previously described.17,25 In short, fixed mouse hearts were dehydrated through a graded series of ethanol, cleared in xylene, embedded in paraffin, and sectioned at 6 μm. The sections were deparaffinized and hydrated before subsequent staining. Sections were stained with picro-Sirius Red (Sigma) to visualize collagen fibers using polarized cell microscopy26 and alcian blue (Klinipath) to visualize glycosaminoglycans (GAGs), and counterstained with nuclear-fast red (Sigma). For immunofluorescent staining, the slices were boiled for 8 or 35 minutes in Antigen Retrieval Buffer (10 mmol/L Tris [pH9]/1 mmol/L EDTA/0.05% Tween-20) using a pressure cooker. After blocking with 1% BSA in 0.1% Tween-PBS, sections were incubated overnight with the primary antibodies directed against platelet endothelial cell adhesion molecule (PECAM-1) (R&D, AF3628; 1:1,000), proliferating cell nuclear antigen (PCNA) (Sigma, P8825; 1:1,000), smooth muscle actin-alpha (α-SMA, Sigma; A2547; 1:20,000), collagen I (SouthernBiotech, 1310-01; 1:200), GFP (Abcam, ab13970, 1:1,000), collagen III (SouthernBiotech, 1330-01; 1:200), phospho-p38 mitogen-activated protein kinase (pp38MAPK) (Cell Signaling, #4631; 1:100), periostin (Santa Cruz, SC398631; 1:100), versican B (Chemicon, AB1033; 1:100), fibronectin (Sigma, F3648; 1:200), cardiac troponin-I (cTnI) (HyTest, 4T21/2; 1:1,000), matrix metalloproteinase 2 (MMP2) (Invitrogen, #436000; 1:200), vimentin (Cell Signaling, #5741; 1:200), mannose receptor (CD206, Abcam; ab64693; 1:300), F4/80 (Invitrogen, 14-4801-82; 1:100), MAC3 (CD107b, BD Biosciences, #550292; 1:200), CD68 (Santa Cruz, SC20060; 1:100), and sarcomeric myofilaments (MF20, Developmental Studies Hybridoma Bank, University of Iowa) followed by incubation with Alexa-conjugated secondary antibodies (Molecular Probes). Staining for pSMAD2/3 and Wt1 was performed as previously described.13,27 Slides are mounted using 4',6-diamidino-2-phenylindole (DAPI) containing ProlongGold Antifade reagent (Thermo Fisher). Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) (in situ cell death detection kit; Roche Applied Science) staining was performed according to the manufacturer’s guidelines. All slides were scanned with the Pannoramic 250 slide scanner (version 1.23, 3DHISTECH Ltd) and analyzed using Caseviewer (version 2.4, 3DHISTECH Ltd). Polarized light microscopy was performed to visualize collagen fibers on the Sirus Red-stained sections.
Quantifications
Quantifications were performed using Caseviewer (version 2.4, 3DHISTECH Ltd) and ImageJ.
To determine the cellular composition in noncultured and ex vivo-cultured hearts, the numbers of DAPI-positive endothelial cells and cardiomyocytes cells were quantified in a specific region of the right ventricle using the endothelial cell marker PECAM-1 and myocardial cell marker cTnI. The relative contribution of endothelial cells, cardiomyocytes, and remainder cells to the total number of DAPI-positive cells was calculated.
To determine the extent of the initiation of fibrosis at specific locations in the heart, the periarterial fibrosis, ie, the α-SMA-positive area surrounding the coronary arteries; the epimyocardial fibrosis, ie, the α-SMA-positive area in the myocardium adjacent to the epicardium; and the endocardial fibroelastosis-like fibrosis, ie, the α-SMA-positive area lining the left ventricular lumen were scored. For the periarterial and epimyocardial fibrosis, a score ranging from 0 to 4 was given, with a score of 0 indicating no α-SMA-positive cells and the scores 1 to 4 indicating an increasing α-SMA-positive area. For the endocardial fibroelastosis-like fibrosis, a score ranging from 0 to 2 was given with a score 0 indicating no α-SMA-positive cells, a score 1 indicating α-SMA-positive cells neighboring part of the left ventricular lumen, and a score 2 indicating α-SMA-positive cells surrounding the whole left ventricular lumen. Examples of these scores can be found in Supplemental Figure 2.
For the quantifications, hearts were used in the age range of 2 to 15 months because no significant correlation was found between age and the extent of fibrosis as determined by α-SMA expression (see Supplemental Appendix).
Statistics
Statistical analysis was performed using GraphPad Prism version 9.5.1 for Windows (GraphPad Software). Differences were analyzed as indicated in each legend using analysis of variance (ANOVA) with Tukey’s or Šidák’s correction, or Kruskal-Wallis, if the normality test (Shapiro-Wilk normality test) failed, with Dunn's correction for multiple groups. Data are reported as the mean ± SEM. A P value <0.05 was considered statistically significant.
Results
Cell remodeling in ex vivo cultured hearts
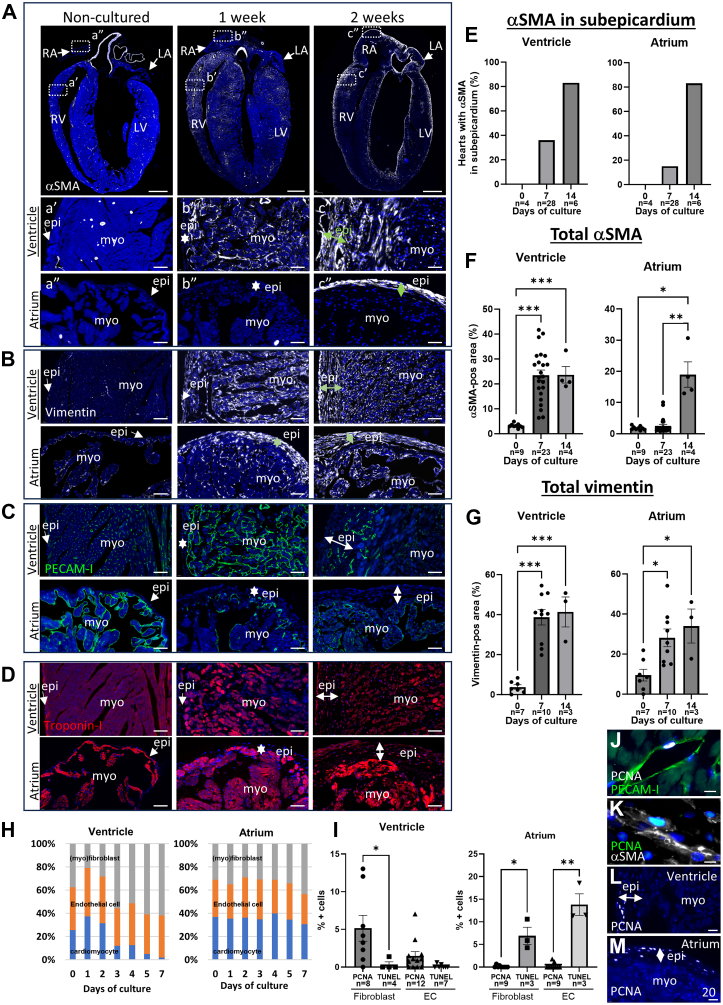
To develop an ex vivo model for cardiac fibrosis, we adapted the MTCS17, 18, 19, 20 to allow perfusion of the coronary circulation (for details see Methods section). Wild type mouse hearts were cultured in the MTCS for increasing times and analyzed by histology for the presence of remodeling in the atrial and ventricular myocardium. Noncultured hearts displayed α-SMA expression predominantly in the smooth muscle cells of the coronary arteries (Figure 1A). Low expression of the intermediate filament marker vimentin was observed in nonmyocardial cells (Figure 1B), and the endothelial cell-marker PECAM-1 visualized the capillaries throughout the myocardium, the inner lining of the coronary arteries, and the endocardium (Figure 1C). The myocardial marker cTnI showed homogeneous expression throughout the myocardium (Figure 1D). After 7 days of culture, an increased number of α-SMA-positive interstitial cells (ie, myofibroblasts) was found in the ventricular myocardium (Figures 1A and 1F), but virtually none were found in the atrial myocardium (Figures 1A and 1F). The subepicardium was thickened in parts of ventricles and atria (Figure 1A), and expression of α-SMA in the ventricular or atrial subepicardium was present in a relative low number of hearts (Figure 1E). Increased vimentin expression was observed in nonmyocardial cells of the myocardium and subepicardium of ventricles and atria (Figures 1B and 1G). Vascular remodeling was observed in the ventricular myocardium after 1 week of culture, whereas decreased PECAM-1 expression was observed in subset of endothelial cells in the atrial myocardium (Figure 1C). The ventricular myocardium displayed loss of its integrity, whereas this was not observed in the atrium (Figure 1D). Different subsets of ventricular cardiomyocytes were observed that displayed an atrophic phenotype, contained vacuoles, expressed the cell death marker TUNEL, or expressed α-SMA (Supplemental Figure 3). After 2 weeks of culture, the subepicardium was overall thicker and displayed α-SMA expression in most cultured hearts overlapping with vimentin expression (Figures 1A and 1E). Detailed comparison of the differential timing of α-SMA and vimentin expression in the subepicardium of ex vivo cultured hearts is shown in Supplemental Figure 4. Further remodeling of the vasculature and loss of myocardial integrity were observed after 2 weeks (Figures 1C and 1D).
Figure 1.
Cell Dynamics in Ex Vivo Cultured Hearts
Representative pictures of noncultured mouse hearts and hearts cultured for 1 or 2 weeks stained for α-smooth muscle actin (SMA) (A), vimentin (B), PECAM-1 (C), and cardiac troponin-I (D). Boxed areas in A are shown at higher magnification in a’, a’’ ,b’, b’’, c’, c’’. (E) Graphs depicting the percentage of hearts with α-SMA-expression in the subepicardium of the ventricle and atrium after culture for 0, 7, or 14 days. (F and G) Graphs depicting the relative α-SMA-positive area (F) and the relative vimentin-positive area (G) in the ventricular or atrial myocardium of hearts cultured for 0, 7, or 14 days. (H) Graphs depicting the relative contribution of the cardiomyocytes (blue), endothelial cells (EC) (orange), and (myo)fibroblast (gray) in the ventricular or atrial myocardium of hearts cultured for 0 (n = 6), 1 (n = 2), 2 (n = 2), 3 (n = 3), 4 (n = 2), 5 (n = 3), and 7 days (n = 11). (I) Graphs depicting the proliferating cell nuclear antigen (PCNA)- and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-positive (myo)fibroblasts and EC in the ventricle and atrium of hearts cultured for 7 days. (J to M) Representative pictures of hearts cultured for 7 days stained for PCNA and PECAM-1 (J), PCNA and α-SMA (K), or PCNA (L,M). 4',6-diamidino-2-phenylindole (DAPI) is used as counterstain in all pictures. Data are presented as means ± SEM. To evaluate significant differences 1-way analysis of variance followed by Tukey’s multiple comparisons test was performed for graphs (F [ventricle] and G). 1-way analysis of variance followed by Šidák’s multiple comparisons test was performed for graphs (F [atrium] and I). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Scale bar A: 1,000 μm; a’ to c’, a’’ to c’’, and B to D: 50 μm; and J to M: 20 μm. Epi = epicardium; LA = left atrium; LV = left ventricle; myo = myocardium; RA = right atrium; RV = right ventricle.
To get insight into the changes in cellular composition of ex vivo-cultured hearts, the relative contributions of cardiomyocytes, endothelial cells, and the remainder cells, presumably fibroblasts, were determined in the ventricular and atrial myocardium. Quantification of the number of nuclei in combination with specific cell markers demonstrated that from day 3 of culture the relative contribution of cardiomyocytes in the ventricular myocardium decreased, with only minimal viable cardiomyocytes being present at day 7 (Figure 1H). The percentage of endothelial cells slightly increased, whereas the contribution of (myo)fibroblasts increased (Figure 1H). In contrast, the relative contributions of the cell types in the atrial myocardium remained constant up until 5 days of culture; after this, a slight decrease was observed for the relative contributions of the cardiomyocytes and endothelial cells after 7 days of culture, whereas the contribution of (myo)fibroblasts slightly increased (Figure 1H). Quantification of PCNA- and TUNEL-positive nuclei observed in the (myo)fibroblast and endothelial cell population indicated that these 2 cell populations proliferated in the ventricular myocardium, whereas they undergo cell death in the atrial myocardium (Figures 1I to 1K). PCNA-positive cells were observed in subepicardium of both the ventricle and atrium (Figures 1L and 1M).
The loss of myocardial cells, vascular reorganization, and the increase and activation of myofibroblasts indicate active cellular remodeling processes similar to those observed during the initiation of cardiac fibrosis.
Matrix remodeling in ex vivo cultured hearts
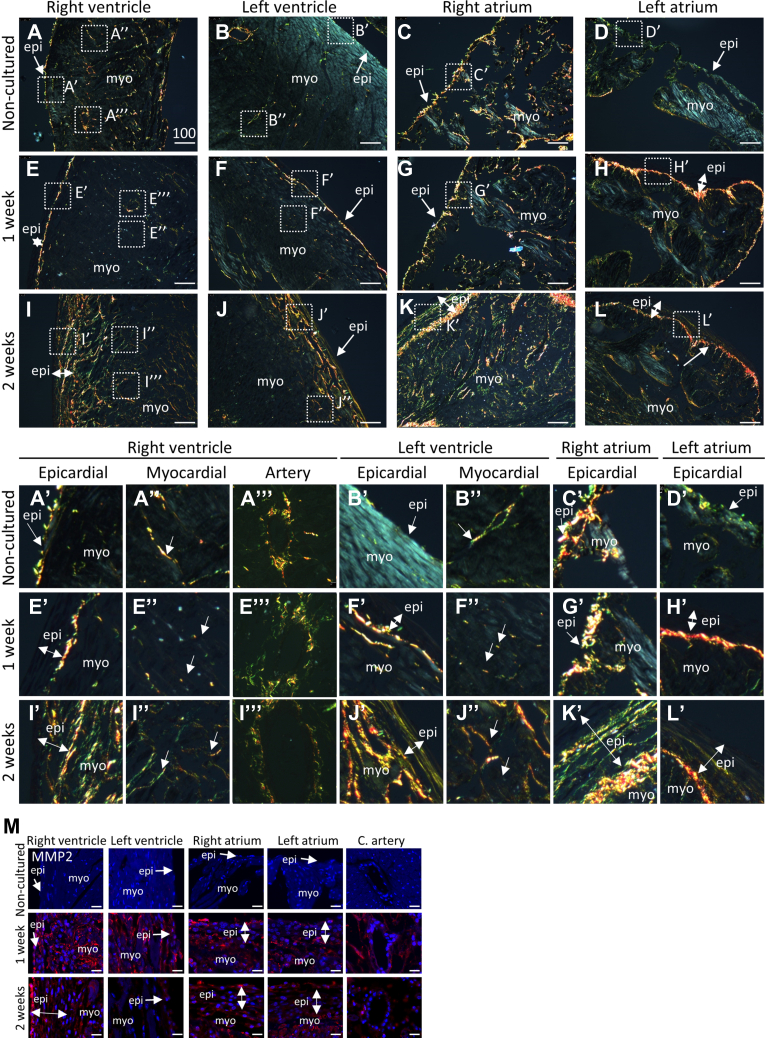
To study the dynamics of collagen fiber distribution in the ex vivo cultured hearts, polarized light microscopy was performed on Sirius Red stained sections to visualize the collagen fibers. Noncultured hearts displayed collagen fibers in the subepicardium and intermyocardial space of the ventricles and atria, surrounding the larger vessels in the ventricles and parts of the subendocardium (Figures 2A to 2D). The fibers in the subepicardium of the right ventricle and atrium were overall more pronounced then in the left ventricle and atrium (Figures 2A to 2D). After 7 days of culture, the collagen fibers in the ventricular myocardium were fragmented (Figures 2E” and 2F”), but not in the atrial myocardium. The fibers were observed in higher intensity in the subepicardium on the border between the subepicardium and myocardium of both ventricles and atria (Figures 2E’ to 2H’). Collagen fibers were disrupted but overall slightly increased in expression surrounding the coronary arteries (Figure 2E”’). After 2 weeks of culture, collagen fibers were observed in the expanded layer of the subepicardium in both ventricles and atria (Figures 2I’ to 2L’), as well as the ventricular wall (Figures 2I’, 2I”, 2J’, and 2J”). These observations suggest the degradation and the subsequent formation of collagen fibers in the ventricular myocardium.
Figure 2.
Collagen Fiber Dynamics in Ex Vivo Cultured Hearts
Representative polarized light microscopy pictures of collagen fibers in the noncultured mouse hearts (A to D) and hearts cultured for 1 week (E and F) or 2 weeks (I to L). Boxed areas in A to L are shown at higher magnification in A’ to L’, A”, B”, E”, F”, I”, J”, A’”, E”’, and I’”. DAPI is used as counterstain. Green: thin collagen fibers; orange thick collagen fibers. (M) Representative pictures of noncultured mouse hearts and hearts cultured for 1 or 2 weeks stained for MMP-2. Scale bar A to L: 100 μm; M: 20 μm. C. artery = coronary artery; other abbreviations as in Figure 1.
Collagen fiber dynamics is regulated by both its production and degradation. Expression of the collagen-degrading MMP-2 was absent at the start of the culture (Figure 2M, noncultured), but was present in the myocardium and (sub)epicardium after 1 week, and to a lesser extent after 2 weeks of culture (Figure 2M). The presence of MMP-2 indicates that the initial degradation of collagen fibers is achieved by protease activity.
Collagen I and III protein expression, as visualized by antibody staining, was present in the intercellular spaces of the myocardium, at the border between the epicardium and myocardium, and surrounding the coronary arteries of the noncultured heart (Figure 3A, Supplemental Figure 5A). After 1 week of culture, collagen I was up-regulated in the ventricular myocardium (Figures 3A, 4A, and 4B, Supplemental Figure 5A), in the thicker subepicardium of the ventricle, and to a lesser extent, in the subepicardium of the atrium (Figures 3A, Supplemental Figure 5A), whereas collagen III protein expression remained at the border between the epicardium and the myocardium (Figure 3A, Supplemental Figure 5A). After 2 weeks of culture, collagen I expression increased further, whereas only a minimal up-regulation of collagen III was observed (Figure 3A). Little change in collagen I and III protein expression was observed surrounding the vessels (Figure 3A). Up-regulation of collagen and the increase of the myofibroblast population are hallmarks for the process of fibrosis. To further validate this observation, we examined the expression of molecules associated with cardiac fibrosis. GAGs, including hyaluronic acid, the matricellular glycoprotein periostin, the proteoglycan versican, and the glycoprotein fibronectin, are up-regulated during cardiac fibrosis.28, 29, 30 In 1 week, cultured mouse hearts, GAGs, visualized by Alcian Blue staining, periostin, versican, and fibronectin, were up-regulated in the ventricular myocardium, surrounding the coronary arteries and in the subepicardium of both ventricles and atria (Figure 3B, Supplemental Figure 5B). Whereas this up-regulation was further increased for GAGs, versican, and fibronectin after 2 weeks of culture, periostin expression was diminished (Figure 3B, Supplemental Figure 5B).
Figure 3.
Extracellular Matrix Dynamics in Ex Vivo Cultured Hearts
Representative pictures of the right ventricle, right atrium, and coronary arteries in noncultured mouse hearts and hearts cultured for 1 or 2 weeks stained for collagen I, collagen III (A), alcian blue, periostin, versican B, and fibronectin (B). DAPI is used as counterstain in all fluorescent pictures. Scalebar: 20 μm. Abbreviations as in Figure 1.
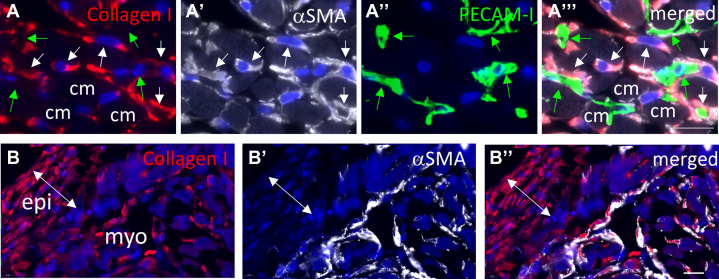
Figure 4.
Collagen-Expressing Cells in the Myocardium and Subepicardium of Ex Vivo Cultured Hearts
(A) Costaining of collagen I, α-SMA, and PECAM-1 in the ventricular myocardium of a mouse heart cultured ex vivo for 1 week. α-SMA-expressing cells (white arrows) display cytoplasmic collagen expression. Endothelial cells (green arrows) and cardiomyocytes (cm) do not show cytoplasmic collagen expression. (B) Costaining of collagen I and α-SMA in subepicardial region of a mouse heart cultured ex vivo for 1 week displaying collagen I-positive/α-SMA-negative cells in the epicardial layer. DAPI is used as counterstain in all pictures. Scale bar: 20 μm. Abbreviations as in Figure 1.
Together these observations suggest active remodeling associated with cardiac fibrosis including degradation and synthesis of ECM components.
Origin of myofibroblasts in the ex vivo cultured hearts
To determine which cell type was responsible for the increased collagen expression, labelling studies were performed using antibodies directed against α-SMA, PECAM-1, and collagen I. In the ventricular wall of ex vivo cultured hearts, cytoplasmic collagen I staining was observed in α-SMA-positive cells and not PECAM-1-positive cells or cardiomyocytes (Figure 4A), suggesting that the α-SMA-positive interstitial cells were the main producers of collagen. In the subepicardium of some hearts, the cytoplasmic collagen was present in α-SMA-negative cells, indicating that α-SMA expression is not required for collagen production (Figure 4A).
Possible origins for the myofibroblasts in the ex vivo cultured are endothelial cells by the process of endothelial-to-mesenchymal transformation (EndoMT), epicardial cells by the process of epithelial-to-mesenchymal transformation, and subsequent migration of subepicardial cells in the myocardium, resident macrophages, and/or resident fibroblasts.31,32 To determine the origin of myofibroblasts in the ex vivo cultured hearts, lineage studies were performed using cell type-specific Cre-mouse models and reporter mouse models (Supplemental Figure 6).
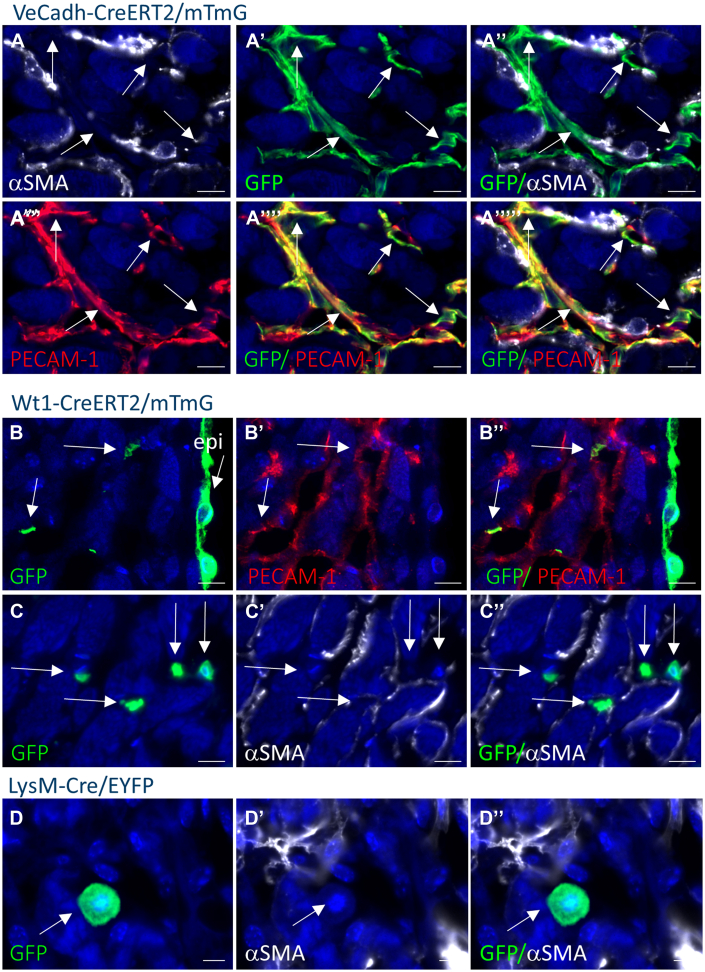
To determine whether EndoMT contributed to myofibroblast formation, inducible Ve-cadherin-CreERT2 mice21 were crossed with the mTmG reporter mice (Supplemental Figure 6). Labeling of the endothelial cells is achieved by exposure of the hearts to tamoxifen. Culturing these hearts with tamoxifen added at the start of the culture resulted in labelling of 90% of the endothelial cells demonstrating high labeling efficiency (Supplemental Figure 7A). All GFP expression was found in PECAM-1 positive cells after 7 days of culture (n = 5) (Figure 5A, Supplemental Figure 8), indicating that EndoMT did not contribute to the myofibroblast population ex vivo.
Figure 5.
Origin of Myofibroblasts in the Myocardium of Ex Vivo Cultured Hearts
Representative pictures of α-SMA and green fluorescent protein (GFP) costaining with or without PECAM-1 costaining. GFP represents the expression of the reporter in the VeCadh-CreERT2/mTmG (A), Wilms’ tumor-1 (Wt1)-CreERT2/mTmG (B and C), and LysM-Cre/enhanced yellow fluorescent protein (EYFP) (D) mice. All GFP-positive cells in the VeCadh-CreERT2/mTmG mouse hearts are positive for PECAM-1 and not for α-SMA (arrows in A). GFP-positive cells in the myocardial layer of Wt1-CreERT2/mTmG mouse hearts are not α-SMA-positive, but mostly PECAM-1 positive (arrows in B and C). GFP staining in LysM-Cre/EYFP mouse hearts does not overlap with α-SMA staining (arrows in D). DAPI is used as counterstain in all pictures. Scale bar: 10 μm. Abbreviations as in Figure 1.
To determine whether epicardial cells contribute to myofibroblasts, first, Wt1 expression studies were performed to determine whether the epicardium becomes activated during the ex vivo culture of the heart. Increased expression of Wt1 was observed in the epicardium, which became thicker at specific parts associated with areas of fibrosis, and in the endothelial cells of the heart (Supplemental Figure 9) as reportered earlier in fibrotic mouse hearts.13 As shown in the previous text, endothelial cells do not contribute to myofibroblasts; therefore, insight into the contribution of epicardium-derived cells to myofibroblasts in the ex vivo cultured hearts can be obtained by performing lineage tracing of Wt1-positive cells using the inducible Wt1-CreERT2 mice crossed with the mTmG reporter mice (Supplemental Figure 6). Culturing these hearts with tamoxifen at the start of the culture to induce labeling of the Wt1-expressing cells resulted in GFP expression in 73% of the epicardial layer (n = 4) (Figure 5B, Supplemental Figure 7) and in a part of the endothelial cells of the heart (Figure 5B). GFP expression was not observed in myofibroblasts (n = 4) (Figure 5C). Sporadic GFP expression was observed in PECAM-1-negative cells residing in the myocardium (not shown). These observations indicate a minimal contribution, if any, of epicardial cells to the myofibroblasts in the ex vivo cultured hearts.
To determine a contribution of the resident macrophages to the myofibroblasts in the ex vivo cultured hearts, the LysM-Cre/EYFP reporter mice were used to label the resident macrophages with YFP (Supplemental Figure 6).22, 23, 24 Cross-reactivity of the GFP antibody with YFP allowed visualization of these cells. In noncultured hearts, GFP expression was observed in numerous interstitial cells that stain positive for the macrophage markers CD206, F4/80, and MAC3 (Supplemental Figures 7D to 7G), indicating efficient labeling of the resident macrophages. Further, some cardiomyocytes in the interventricular septum and left myocardial wall were found positive as reported earlier24 (Supplemental Figure 7C). After 7 days of culture, the number of GFP-positive cells overall increased, and GFP expression was observed in both macrophages, as identified by CD68 expression (Supplemental Figure 7H), and cardiomyocytes (Supplemental Figure 7I). No GFP-/α-SMA-positive cells were found, indicating that the resident macrophages did not contribute to the increase in the myofibroblast population (n = 6) (Figure 5D, Supplemental Figure 8).
Together, our observations suggest that the myofibroblasts present in the ex vivo cultured heart are likely derived from resident proliferating fibroblasts.
Mechanical regulation of cardiac fibrosis
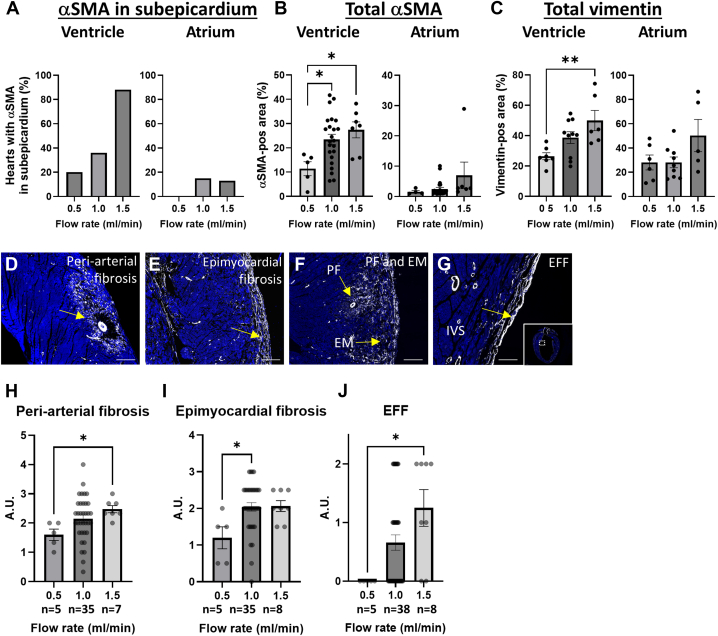
Mechanical stress is considered an important inducer/regulator of fibrosis.33 Shear stress, pressure, and (pulsatile) stretch can induce intracellular pathway modifications leading to altered behavior or phenotype of endothelial cells, fibroblasts, and cardiomyocytes favoring fibrosis. To determine whether mechanical alterations can lead to cellular modification, increasing flow rates were applied and the extent of vimentin, as a measure of cardiac cell activation, and α-SMA, as a measure for myofibroblast formation, were determined after 1 week of culture. Increasing flow rate resulted in relatively more hearts expressing α-SMA in the ventricular subepicardium (Figure 6A), and more α-SMA and vimentin expression in the ventricular myocardium (Figures 6B and 6C), indicating that increased mechanical stress results in cellular modifications associated with cardiac fibrosis. These modifications upon increased mechanical stress were not observed in the atria (Figures 6A to 6C). The distribution of myofibroblast in the ventricular wall was not homogeneous but was concentrated in specific locations. They were observed in the myocardium surrounding the coronary arteries, ie, periarterial fibrosis (Figures 6D and 6F); in the outer myocardial part of the right and left ventricular wall underneath the epicardial layer, ie, epimyocardial fibrosis (Figures 6E and 6F); and surrounding the left ventricular lumen, ie, endocardial fibroelastosis-like fibrosis (Figure 6G). Multiple locations of fibrosis were found in one heart (Figure 6F). Because different parts of the ventricular myocardium might be differently sensitive to mechanical stress, the α-SMA expression, as a measure of myofibroblast activation, was scored in each of these locations in hearts cultured with increasing flow rates, as described in the Methods section, with examples in Supplemental Figure 2. Applying this approach demonstrated that myofibroblast activation in the identified locations was increased when the hearts were cultured with higher flow rates (Figures 6H to 6J). Therefore, the increase in myofibroblast activation in the ventricular myocardium upon increased mechanical stress is not restricted to a specific location.
Figure 6.
Mechanical Regulation of Fibrosis in Ex Vivo Cultured Hearts
(A) Graphs depicting the percentage of hearts with α-SMA expression in the subepicardium of the ventricle and atrium after culture for 7 days with a flow rate of 0.5, 1.0, and 1.5 mL/min. (B and C) Graphs depicting the relative α-SMA-positive area (B) and the relative vimentin-positive area (C) in the ventricular or atrial myocardium of hearts cultured for 7 days with a flow rate of 0.5, 1.0, and 1.5 mL/min. Representative pictures of α-SMA staining on hearts cultured for 7 days indicating the locations of the periarterial fibrosis (PF) (D and F), the epimyocardial fibrosis (EM) (E and F), and endocardial fibroelastosis-like fibrosis (EFF) (G). (H to J) Graphs depicting the quantification of the PF (H), EM (I), and EFF (J) in hearts cultured ex vivo at different flow rates. DAPI is used as counterstain in all pictures. Data are presented as mean ± SEM. To evaluate significant differences, 1-way analysis of variance followed by Tukey’s multiple comparisons test was performed for graphs (B [ventricle] and C). Kruskal-Wallis test followed by Dunn’s multiple comparisons test was performed for graphs (B [atrium] and H to J). ∗P < 0.05, ∗∗P < 0.01. Scale bar D to F: 200 μm; G: 100 μm. Abbreviations as in Figure 1.
Molecular regulation of cardiac fibrosis
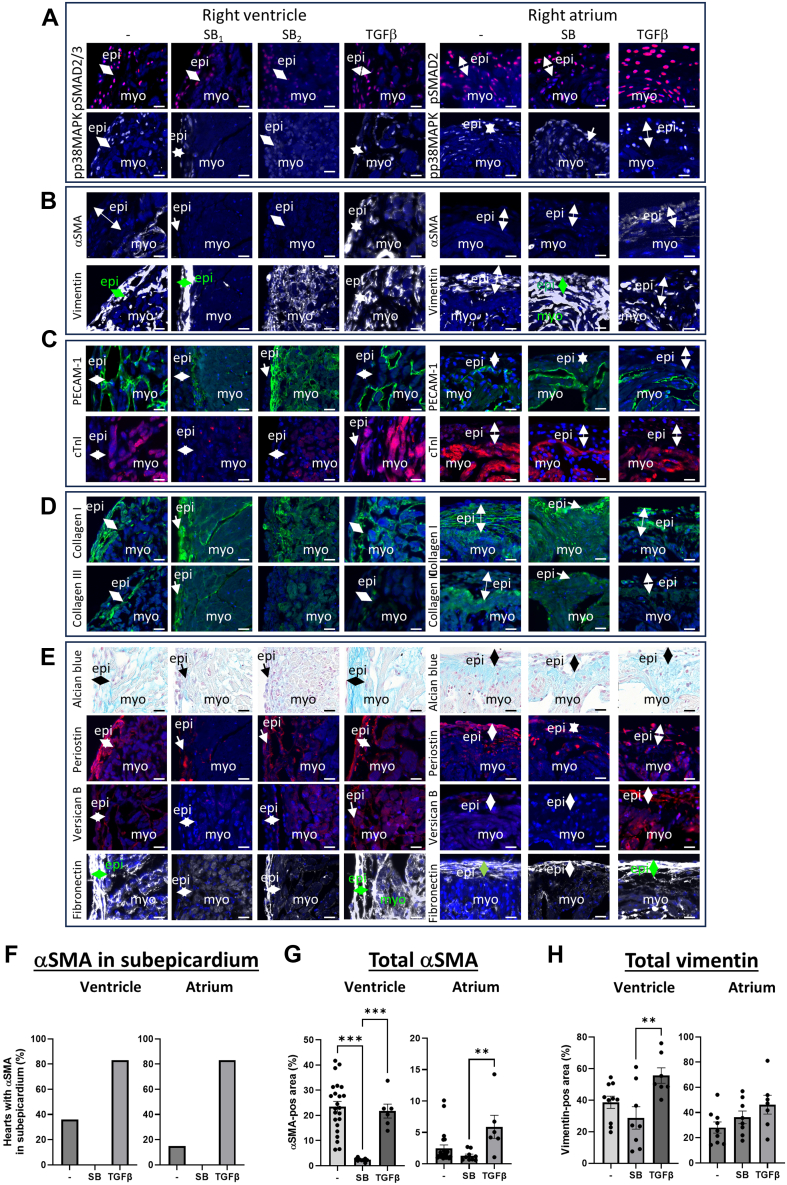
TGF-β is an important player in the cellular alterations and ECM remodeling in cardiac fibrosis and exerts its effect via SMAD-dependent and -independent signaling pathways.5 To determine whether both pathways are activated in ex vivo cultured hearts, the expression of pSMAD2/3 and phospho-p38MAPK (pp38MAPK) was determined, downstream signaling molecules of the SMAD-dependent and -independent TGF-β pathways, respectively. In noncultured hearts, low nuclear pSMAD2/3 expression was observed throughout the heart, whereas pp38MAPK was not expressed (Supplemental Figure 10). In ex vivo cultured hearts, pSMAD2/3 and pp38MAPK were up-regulated, specifically in areas of vimentin expression in the myocardium and subepicardium (Figure 7A). To gain more insight in the role of TGF-β in the remodeling observed in the ex vivo cultured hearts, the ALK5 kinase inhibitor SB431542 was used to inhibit the TGF-β signaling pathway, while TGF-β was added to the culture to activate the TGF-β signaling pathway. α-SMA expression was completely inhibited in the subepicardium and myocardium of SB-treated hearts (Figures 7B, 7F, and 7G, Supplemental Figure 11B), and was only present in vascular smooth muscle cells. On the other hand, the impact of TGF-β signaling inhibition on expression of pSMAD2/3, pp38MAPK, and the other examined fibrotic markers was different between hearts as well as in different regions of the hearts (as indicated by SB1 and SB2 in Figure 7). pSMAD2/3 and pp38MAPK expression was overall inhibited (SB1 in Figure 7A and Supplemental Figure 11A), but clearly present in other parts (SB2 and SB in Figure 7A and Supplemental Figure 11A). Vimentin expression was inhibited in parts of the ventricular myocardium (SB1 in Figures 7B and 7H and Supplemental Figure 11B) and appeared more intense in other parts (SB2 and SB in Figure 7B and Supplemental Figure 11B). Similarly, vascular remodeling was inhibited in parts of ventricular myocardium (SB1 in Figure 7C, Supplemental Figure 11C), although PECAM-1 expression was present between the subepicardium and ventricular myocardium, and more intense in other parts (SB2 in Figure 7C). The endocardial cell layer in the atrium appeared more intact (Figure 7C). The expression of cTnI was low to absent in most parts of the heart (Figure 7C, Supplemental Figure 11C). In some regions of the SB cultured hearts, the expression of ECM components collagen I and III was restored and organized as observed in noncultured hearts (SB1 in Figure 7D and Supplemental Figure 11D), whereas GAGs, periostin, versican, and fibronectin were inhibited in the presence of SB (SB1 in Figure 7E and Supplemental Figure 11E). Addition of TGF-β to the culture did not result in a clear increase of pp38MAPK and pSMAD2/3 (Figure 7A, Supplemental Figure 11A). Whereas the number of hearts with α-SMA in the subepicardium increased upon TGF-β addition (Figure 7F), total α-SMA did not increase in the ventricle or the atrium (Figures 7B and 7G, Supplemental Figure 11B). In addition, a slight increase in the expression of ECM components like GAGs and versican was observed (Figure 7E, Supplemental Figure 11E). These observations show that in the ex vivo cultured hearts, TGF-β is an important player in the formation of fibrosis and that fibrosis in the ex vivo cultured hearts can be molecularly modulated.
Figure 7.
Molecular Regulation of Fibrosis in Ex Vivo Cultured Hearts
Representative pictures of the right ventricle and the right atrium of hearts cultured for 7 days under standard conditions (−), in the presence of SB431542 (SB, SB1, SB2) or transforming growth factor-beta (TGF-β) for (A) phospho-SMAD2/3 (pSMAD2/3) and phospho-p38 mitogen-activated protein kinase (pp38MAPK), (B) α-SMA and vimentin, (C) PECAM-1 and cTnI, (D) collagen I and collagen III, and (E) Alcian blue, periostin, versican B, and fibronectin. (F) Graphs depicting the percentage of hearts with α-SMA expression in the subepicardium of the ventricle and atrium after culture for 7 days under standard conditions (−), in the presence of SB431542 (SB) or TGF-β. (G and H) Graphs depicting the relative α-SMA-positive area (G) and the relative vimentin-positive area (H) in the ventricular or atrial myocardium of hearts cultured for 7 days under standard conditions (−), in the presence of SB431542 (SB) or TGF-β. DAPI was used as counterstain in all fluorescent pictures. Data are presented as means ± SEM. To evaluate significant differences, 1-way analysis of variance followed by Tukey’s multiple comparisons test was performed for graphs (G and H). Kruskal-Wallis test followed by Dunn’s multiple comparisons test was performed for graph (G [atrium]). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Scale bar: 20 μm. Abbreviations as in Figure 1.
Discussion
In this study, we have established a novel ex vivo fibrosis model for intact mouse hearts. Fibrosis could be induced in initially healthy hearts of wild type mice that were perfused through their coronary circulation. Upon extended culture, an increase in myofibroblasts, a decrease in viable cardiomyocytes, and up-regulation of fibrosis-associated ECM components were observed. We found that the myofibroblasts likely originated from resident fibroblasts and demonstrated that the extent of fibrosis is dependent on the level of mechanical stress and the activation of the TGF-β signaling pathway.
Fibrosis is a complex multistep process that involves the degradation of existing ECM and the production, secretion, and maturation of new ECM.34 We observed fragmented collagen fibers and expression of MMP2 after 1 week of culture, indicating active degradation of collagen fibers, but also the production of collagen protein by (myo)fibroblasts in the myocardium and subepicardium. In addition, we observed increased expression of GAGs, fibronectin, periostin, and versican, ie, ECM components associated with fibrosis for local mechanical support. After 2 weeks of culture, new collagen fibers had formed. These fibers resembled more diffuse, interstitial fibrosis rather than scar formation, indicating that reactive fibrosis, stimulated by maladaptive signaling, is the leading fibrotic process. In myocardial fibrosis, the collagen I/III ratio determines the mechanical properties of the myocardium because collagen I fibers are stiffer and collagen III fibers are more compliant. A decreased collagen I/III ratio is associated with ischemic heart disease, whereas a higher ratio is more associated with hypertensive hearts.35,36 We observed a clear increase in collagen I expression compared with collagen III expression, which is in line with the reactive type of fibrosis observed in our ex vivo model.
Cellular modifications were also observed in the ex vivo cultured hearts, including myofibroblast differentiation, vascular remodeling, and loss of myocardial cells. The myofibroblast is considered responsible for the increased production of ECM components leading to fibrosis and is characterized by α-SMA.37 ECM-production in cardiac pathological conditions however is not necessarily dependent of myofibroblast activation,38 as we have also shown in our model where we have observed cytoplasmic collagen expression in subepicardial α-SMA-negative fibroblasts. Myofibroblast activation is suggested to consist of 2 stages, with the initial activation being the differentiation of the fibroblast into so-called proto-myofibroblast induced by mechanical tension or profibrotic factors like TGF-β.37,39,40 Stress fibers are formed but α-SMA is not expressed, whereas markers like periostin and vimentin41,42 might be present. The second stage is the differentiation of the proto-myofibroblast into the α-SMA-expressing myofibroblast by sustained or increased mechanical tension and exposure to profibrotic factors. After 1 week of culture, we observed many α-SMA-positive interstitial cells in the ventricular myocardium with cytoplasmic collagen expression, indicating the differentiation of fibroblasts into myofibroblast with matrix-synthetic phenotype. In addition, we observed vimentin+/periostin+/α-SMA− cells in the subepicardium of ex vivo cultured hearts with cytoplasmic collagen expression suggesting the formation of proto-myofibroblasts. After 2 weeks of culture, an increased presence of α-SMA was observed in the subepicardial cells, indicating that the 2 stages of myofibroblast activation manifested in the ex vivo culture system.
The myofibroblasts in the ventricular myocardium were in tight contact with endothelial cells that up-regulated PECAM-1 expression, showed increased proliferation, and became part of an altered vascular network. We observed myocardial cell death in the ex vivo cultured mouse heart, which resulted in less viable cardiomyocytes after 7 days of culture in the ventricular myocardium. In addition, we observed small detached cardiomyocytes indicative for atrophy. Cardiomyocytes with vacuoles were found, mostly located at the border of fibrotic area, which is reminiscent of the cardiomyocytes in the border zone after myocardial infarction in vivo43 and described as perinuclear sarcomere depletion.44 Furthermore, coexpression of α-SMA and the myocardial marker cTnI was observed in a subset of cells indicating the presence of dedifferentiation.44 Reduced cardiomyocyte viability and changes of myocardial phenotype are features of cardiac fibrosis in vivo.45,46
The origin of myofibroblasts in the fibrotic heart has been shown to be primarily the resident fibroblasts and not the endothelial, epicardial, or immune cells in both the infarction- and pressure overload-induced cardiac fibrosis mouse models.47,48 We have performed lineage tracing using cell type specific cre-mouse models and found that the myofibroblasts in the ventricular myocardium were not derived from endothelial cells, epicardial cells, or macrophages. These observations suggest that the myofibroblasts present in the ex vivo cultured heart are derived from resident proliferating fibroblasts similar to that observed in the in vivo models of cardiac fibrosis. Lineage tracing studies specific for resident fibroblasts should, however, be performed to confirm this.
The stimuli that led to the active fibrotic remodeling in the ex vivo cultured hearts can be multiple and of both a mechanical and biochemical nature. We exposed the ex vivo cultured heart to an altered hemodynamic and molecular environment, which might have affected cellular behavior leading to the initiation of the fibrotic process. Increasing the flow rate in the ex vivo cultured hearts resulted in more myofibroblast activation, whereas blocking the TGF-β signaling pathway resulted in complete inhibition of myofibroblast activation, indicating that both mechanical and molecular factors are involved in the remodeling process. Regional differences were, however, observed in the remodeling of the heart. Myofibroblast differentiation in the ventricles was concentrated around the vessels, in the subepicardium, and in the myocardium neighboring the subepicardium. In the atria, myofibroblast differentiation was mostly observed in the subepicardium after culture for 2 weeks or upon addition of TGF-β. Whereas the fibroblasts and PECAM-1+ cells proliferated in the ventricular myocardium, they underwent cell death in the atria. In the ventricular myocardium, cardiomyocytes mostly lost viability and obtained various phenotypes, whereas in the atria, the cardiomyocytes maintained their viability and integrity. Upon treatment with the compound SB431542, myofibroblast formation was completely inhibited throughout the heart. Parts of the heart even resembled the noncultured hearts, indicating inhibition of overall remodeling. In other parts, however, the remodeling was still present or even intensified, demonstrating that fibrosis is a multifactorial process that is the result of a delicate interplay between biochemical and mechanical signals steering cell behavior. Because the heart is a highly heterogenous organ with unique compositions of different cell types and ECM components exposed to their specific set of biochemical factors and mechanical stresses, it is crucial to consider these context-dependent mechanisms in the study of cardiac fibrosis. Whole heart-culture as the MTCS does allow a more accurate modeling of cardiac fibrosis under various controlled states and conditions.
Currently, no effective pharmacological treatment for cardiac fibrosis exists, and biomarkers for assessment of cardiac fibrosis are mostly inconclusive or do not correlate with extent of cardiac fibrosis.49 Development of new drugs for cardiac fibrosis has been hampered by the mostly unsuccessful validation of the in vivo efficacy of potential treatments.49 A better selection of drug candidates can be achieved by the improvement of preclinical screening methodologies, where potential candidates and targets can be assessed for their therapeutic potential. The ex vivo culture of the whole heart might provide such a modality because fibrosis can be induced in a controllable, reproducible, and consistent manner and fibrotic aspects can easily be monitored. Further, the closed circulation allows the accumulation of fibrosis-related secretions in the perfusion fluid, potentiating the search for specific biomarkers.
Study limitations
The hearts were perfused with medium and not with blood. This likely resulted in an absent or altered inflammatory response, including the removal of dead cardiomyocytes and the promotion of fibrosis. Interestingly, fibrosis still takes place in the absence of blood and this setting allows to investigate the endogenous response of the heart under specific conditions. Furthermore, blood cells can be added back to the system to study their role in the remodeling process of the heart.
The hearts were not beating the largest part of the culture; therefore, the cardiac cells were not exposed to cyclic stretch but rather to a continuous stretch condition. This continuous stretch might have contributed to the activation of fibroblasts into myofibroblast.50 Pulsatile flow can, however, be introduced to create cyclic stretch of the cardiac cells allowing to study its effect on the formation of fibrosis.
The ex vivo culture of the hearts were limited to 2 weeks, in comparison with lifelong follow-up possibility in in vivo models. One week of ex vivo culture, however, already presented clear readouts for aspects of cardiac fibrosis like expansion and activation of fibroblasts and the up-regulation of fibrosis-associated ECM components, and therefore, provides the opportunity for short-term experimental studies assessing the initial stages of cardiac fibrosis.
Conclusions
With the MTCS, we can now control and study cardiac fibrosis in whole mouse hearts. This provides the possibility to elucidate the molecular mechanisms underlying fibrosis in a whole heart setting and contribute in the search for new treatments targeting fibrosis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Fibrosis is a common pathophysiological process of multiple cardiac diseases, which can lead to life-threatening heart failure. No effective treatment exists because of the lack of suitable test systems. Our new ex vivo culture model allows the induction of fibrosis in intact whole mouse hearts and provides a preclinical test system for new targets for the treatment of fibrosis on organ level.
TRANSLATIONAL OUTLOOK: Although the ex vivo model provides a preclinical test system that approaches the in vivo situation in much greater extent than most in vitro models because of its much greater complexity, systemic influences on the formation of fibrosis are not taken into account. In addition, multiple pathways might lead to the formation of fibrosis, which might require different approaches for its modulation. Further studies are needed to incorporate the potential systemic influences and to test the potential different molecular pathways underlying different types of fibrosis. Our new model provides the opportunity to get more insight in these important issues regarding the regulation of fibrosis.
Funding Support and Author Disclosures
This project has received funding form the Ministry of Economic Affairs under TKI-Allowance under the TKI-programme Life Sciences & Health and the Dutch Heart Foundation / Hartstichting. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Karien Wiesmeijer for expert technical assistance and Dr Anke Smits for the Wt1-CreERT2/mTmG mice.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Diez J., de Boer R.A. Management of cardiac fibrosis is the largest unmet medical need in heart failure. Cardiovasc Res. 2022;118:e20–e22. doi: 10.1093/cvr/cvab228. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis N.G. Transforming growth factor-beta in myocardial disease. Nat Rev Cardiol. 2022;19:435–455. doi: 10.1038/s41569-021-00646-w. [DOI] [PubMed] [Google Scholar]
- 4.Goumans M.J., Ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol. 2018;10(2):a022210. doi: 10.1101/cshperspect.a022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bujak M., Frangogiannis N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner N.A., Blythe N.M. Cardiac fibroblast p38 MAPK: a critical regulator of myocardial remodeling. J Cardiovasc Dev Dis. 2019;6(3):27. doi: 10.3390/jcdd6030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna A., Frangogiannis N.G. The Role of the TGF-beta superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6:140. doi: 10.3389/fcvm.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahimi R.A., Leof E.B. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 9.Picchio V., Floris E., Derevyanchuk Y., et al. Multicellular 3D Models for the Study of Cardiac Fibrosis. Int J Mol Sci. 2022;23(19):11642. doi: 10.3390/ijms231911642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teekakirikul P., Eminaga S., Toka O., et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley S.D., Gurley S.B., Herrera M.J., et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Deel E.D., de Boer M., Kuster D.W., et al. Exercise training does not improve cardiac function in compensated or decompensated left ventricular hypertrophy induced by aortic stenosis. J Mol Cell Cardiol. 2011;50:1017–1025. doi: 10.1016/j.yjmcc.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Duim S.N., Kurakula K., Goumans M.J., Kruithof B.P. Cardiac endothelial cells express Wilms' tumor-1: Wt1 expression in the developing, adult and infarcted heart. J Mol Cell Cardiol. 2015;81:127–135. doi: 10.1016/j.yjmcc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J., Burgon P.G., Wakimoto H., et al. Cardiac myosin binding protein C regulates postnatal myocyte cytokinesis. Proc Natl Acad Sci U S A. 2015;112:9046–9051. doi: 10.1073/pnas.1511004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaragoza C., Gomez-Guerrero C., Martin-Ventura J.L., et al. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey M.L., Brunt K.R., Kirk J.A., et al. Guidelines for in vivo mouse models of myocardial infarction. Am J Physiol Heart Circ Physiol. 2021;321:H1056–H1073. doi: 10.1152/ajpheart.00459.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruithof B.P.T., van de Pol V., Los T., et al. New calcification model for intact murine aortic valves. J Mol Cell Cardiol. 2021;156:95–104. doi: 10.1016/j.yjmcc.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Lieber S.C., Kruithof B.P., Aubry N., Vatner S.F., Gaussin V. Design of a miniature tissue culture system to culture mouse heart valves. Ann Biomed Eng. 2010;38:674–682. doi: 10.1007/s10439-010-9922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruithof B.P.T., Paardekooper L., Hiemstra Y.L., et al. Stress-induced remodelling of the mitral valve: a model for leaflet thickening and superimposed tissue formation in mitral valve disease. Cardiovasc Res. 2020;116:931–943. doi: 10.1093/cvr/cvz204. [DOI] [PubMed] [Google Scholar]
- 20.Kruithof B.P., Lieber S.C., Kruithof-de Julio M., Gaussin V., Goumans M.J. Culturing mouse cardiac valves in the miniature tissue culture system. J Vis Exp. 2015:e52750. doi: 10.3791/52750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monvoisin A., Alva J.A., Hofmann J.J., Zovein A.C., Lane T.F., Iruela-Arispe M.L. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 22.Clausen B.E., Burkhardt C., Reith W., Renkawitz R., Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 23.Ye M., Iwasaki H., Laiosa C.V., et al. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 24.Stadtfeld M., Ye M., Graf T. Identification of interventricular septum precursor cells in the mouse embryo. Dev Biol. 2007;302:195–207. doi: 10.1016/j.ydbio.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Kruithof B.P.T., van Wijngaarden A.L., Mousavi Gourabi B., Hjortnaes J., Palmen M., Ajmone Marsan N. Superimposed tissue formation in human aortic valve disease: differences between regurgitant and stenotic valves. J Cardiovasc Dev Dis. 2021;8(7):79. doi: 10.3390/jcdd8070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittaker P., Kloner R.A., Boughner D.R., Pickering J.G. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 27.Alkema M., Goumans M.J., Kruithof B.P.T. Immunofluorescent visualization of BMP signaling activation on paraffin-embedded tissue sections. Methods Mol Biol. 2019;1891:191–200. doi: 10.1007/978-1-4939-8904-1_14. [DOI] [PubMed] [Google Scholar]
- 28.Sasi A., Romaine A., Erusappan P.M., et al. Temporal expression and spatial distribution of the proteoglycan versican during cardiac fibrosis development. Matrix Biol Plus. 2023;19-20:100135. doi: 10.1016/j.mbplus.2023.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobaczewski M., Bujak M., Zymek P., Ren G., Entman M.L., Frangogiannis N.G. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 30.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits A.M., Dronkers E., Goumans M.J. The epicardium as a source of multipotent adult cardiac progenitor cells: their origin, role and fate. Pharmacol Res. 2018;127:129–140. doi: 10.1016/j.phrs.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Tallquist M.D. Cardiac fibroblasts: from origin to injury. Curr Opin Physiol. 2018;1:75–79. doi: 10.1016/j.cophys.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesce M., Duda G.N., Forte G., et al. Cardiac fibroblasts and mechanosensation in heart development, health and disease. Nat Rev Cardiol. 2023;20:309–324. doi: 10.1038/s41569-022-00799-2. [DOI] [PubMed] [Google Scholar]
- 34.de Boer R.A., De Keulenaer G., Bauersachs J., et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail. 2019;21:272–285. doi: 10.1002/ejhf.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee D., Sen S. Alteration of cardiac collagen phenotypes in hypertensive hypertrophy: role of blood pressure. J Mol Cell Cardiol. 1993;25:185–196. doi: 10.1006/jmcc.1993.1021. [DOI] [PubMed] [Google Scholar]
- 36.Cleutjens J.P., Verluyten M.J., Smiths J.F., Daemen M.J. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 37.Frangogiannis N.G. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alex L., Russo I., Holoborodko V., Frangogiannis N.G. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;315:H934–H949. doi: 10.1152/ajpheart.00238.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibb A.A., Lazaropoulos M.P., Elrod J.W. Myofibroblasts and fibrosis: mitochondrial and metabolic control of cellular differentiation. Circ Res. 2020;127:427–447. doi: 10.1161/CIRCRESAHA.120.316958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 41.Farbehi N., Patrick R., Dorison A., et al. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife. 2019;8:e43882. doi: 10.7554/eLife.43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avagliano A., Ruocco M.R., Nasso R., et al. Development of a stromal microenvironment experimental model containing proto-myofibroblast like cells and analysis of its crosstalk with melanoma cells: a new tool to potentiate and stabilize tumor suppressor phenotype of dermal myofibroblasts. Cells. 2019;8(11):1435. doi: 10.3390/cells8111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Driesen R.B., Verheyen F.K., Dijkstra P., et al. Structural remodelling of cardiomyocytes in the border zone of infarcted rabbit heart. Mol Cell Biochem. 2007;302:225–232. doi: 10.1007/s11010-007-9445-2. [DOI] [PubMed] [Google Scholar]
- 44.Dispersyn G.D., Mesotten L., Meuris B., et al. Dissociation of cardiomyocyte apoptosis and dedifferentiation in infarct border zones. Eur Heart J. 2002;23:849–857. doi: 10.1053/euhj.2001.2963. [DOI] [PubMed] [Google Scholar]
- 45.Piek A., de Boer R.A., Sillje H.H. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21:199–211. doi: 10.1007/s10741-016-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lodrini A.M., Goumans M.J. Cardiomyocytes cellular phenotypes after myocardial infarction. Front Cardiovasc Med. 2021;8:750510. doi: 10.3389/fcvm.2021.750510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanisicak O., Khalil H., Ivey M.J., et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Comm. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore-Morris T., Guimaraes-Camboa N., Banerjee I., et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gyongyosi M., Winkler J., Ramos I., et al. Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail. 2017;19:177–191. doi: 10.1002/ejhf.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yong K.W., Li Y., Huang G., et al. Mechanoregulation of cardiac myofibroblast differentiation: implications for cardiac fibrosis and therapy. Am J Physiol Heart Circ Physiol. 2015;309:H532–H542. doi: 10.1152/ajpheart.00299.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.