Abstract
Mammalian cells have the ability to sense low oxygen levels (hypoxia). An adaptive response to hypoxia involves the induction of the transcription factor hypoxia-inducible factor 1 (HIF-1). The intracellular signaling pathways that regulate HIF-1 activation during hypoxia remain unknown. Here, we demonstrate that p38α−/− cells fail to activate HIF-1 under hypoxic conditions. Cells deficient in Mkk3 and Mkk6, the upstream regulators of p38α, also fail to activate HIF-1 under hypoxic conditions. The p38α−/− cells are able to activate HIF-1 in response to anoxia or iron chelators during normoxia. Furthermore, the hypoxic activation of p38α and HIF-1 was abolished by myxothiazol, a mitochondrial complex III inhibitor, and glutathione peroxidase 1 (GPX1), a scavenger of hydrogen peroxide. Thus, the activation of p38α and HIF-1 is dependent on the generation of mitochondrial reactive oxygen species. These results provide genetic evidence that p38 mitogen-activated protein kinase signaling is essential for HIF-1 activation.
Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that regulates physiological responses to hypoxia, including placental development, and pathophysiological processes such as cancer (41). HIF-1 is composed of two subunits, an oxygen-sensitive HIF-1α subunit and a constitutively expressed HIF-1β subunit. Under normal oxygen conditions, HIF-1α is polyubiquitinated and targeted for degradation by an E3 ubiquitin ligase complex that contains the von Hippel-Lindau tumor suppressor protein (pVHL), elongin B, elongin C, Cul2, and Rbx (32). This process is dependent on the hydroxylation of two proline residues by a family of prolyl hydroxylase (PHD) enzymes, which mediates the binding of pVHL (20, 22, 31). PHDs utilize oxygen as a substrate and iron as a cofactor to hydroxylate proline residues of HIF-1α (6, 12). Oxygen tension also regulates the interaction of HIF-1α with the transcriptional coactivators p300 and CBP. Asparagine hydroxylation of residue 803 of HIF-1α by the enzyme FIH-1 (factor inhibiting HIF-1) blocks the binding of p300 and CBP to HIF-1α, thus inhibiting HIF-1-mediated gene transcription (26, 27, 29). Under hypoxic conditions or in the presence of iron chelators, the rate of proline and asparagine hydroxylation is decreased. Consequently, pVHL cannot target HIF-1α for degradation, thereby allowing HIF-1α to accumulate and dimerize with HIF-1β in the cell. Moreover, p300 and CBP can then be recruited to the HIF-1 complex, allowing transcriptional activation of HIF-1 target genes.
The signaling mechanisms that regulate the hypoxic activation of HIF-1 are not fully understood. Current models suggest that signaling pathways are not involved in the hypoxic activation of HIF-1 (42). This is based on the observation that during hypoxia the decline in oxygen levels directly decreases the activity of the PHDs, thereby preventing hydroxylation of the HIF-1α protein (18). The lack of hydroxylation inhibits pVHL from binding to HIF-1α, allowing HIF-1α to be stabilized. In contrast, there have been reports to indicate that intracellular signaling pathways are required for HIF-1 activation during hypoxia. These include but are not limited to the requirement of diacylglycerol kinase, small GTPases, mitochondrial reactive oxygen species (ROS), and phosphatidylinositol 3-kinase (PI3-K)/AKT (4, 9, 14, 17, 19, 47, 48, 49). The requirement of signaling pathways in the activation of HIF-1 suggest that the hydroxylases are not the sole regulators of HIF-1 during hypoxia.
The p38α mitogen-activated protein kinase (MAPK) is a stress kinase that is activated by dual phosphorylation on Thr and Tyr in a Thr-X-Tyr motif located within the activation loop proximal to the ATP- and substrate-binding sites (36). This phosphorylation is meditated by the upstream MAPK kinases (MAPKKs) MKK3 and MKK6 (10, 37). Mice deficient in p38α MAPK demonstrate embryonic lethality at day 10.5 and display abnormal vascularization associated with the placenta, resembling the phenotype seen in the Hif-1β− embryos (1, 2, 3, 30, 34, 46). The phenotype of the Mkk3/6−/− mice also resembles the p38α−/− embryos (5). Pharmacologic inhibition of p38α MAPK has been shown to decrease hypoxic activation of HIF-1 (17, 44). Nonhypoxic stimuli such as exposure to hepatocyte growth factor (HGF), chromium, or arsenite activates HIF-1 through oxidant induction of p38 MAPK (11, 13, 45). Furthermore, p38α MAPK is activated by mitochondrial ROS during hypoxia (25). Based on these previous reports, in the present study we genetically tested whether mitochondrial oxidant activation of the p38 MAPK signaling pathway is required for the hypoxic activation of HIF-1.
MATERIALS AND METHODS
Cell culture.
p38α−/− and Mkk3/6−/− mouse embryonic fibroblasts were generated as previously described (5, 35). p38α−/− cells were retrovirally infected with a p38α cDNA or vector control. These cells were selected in Dulbecco's modified Eagle media (DMEM) containing hygromycin. Cells were infected with an adenovirus encoding a myc-tagged GPX-1 (28). Hypoxic conditions (1.5% O2, 93.5% N2, and 5% CO2) were achieved in a humidified variable aerobic workstation (INVIVO O2; BioTrace). Anoxic conditions (0% O2, 95% N2, and 5% CO2) were achieved using the Bugbox (BioTrace).
Immunoblot analysis.
HIF-1α protein was analyzed in nuclear extracts prepared from cells. Nuclear extracts (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting with an anti-HIF-1α antibody (1:500; Novus Biological Sciences). To control for loading, membranes were stripped and reprobed with an anti-Pol II antibody (1:200; Santa Cruz Biotechnology, Inc.). For total cell lysates, equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with the appropriate antibodies. To control for loading, an anti-α-tubulin antibody (1:2,000; Sigma-Aldrich, Inc.) was used. The following antibodies were used: p38α MAPK (1:1,000; Cell Signaling Technology, Inc.), phospho-p38 MAPK (1:1,000; Cell Signaling Technology, Inc.), and myc (1:5,000; Invitrogen).
Reporter and ROS assays.
Transfections were done using the Mirus TransIT Transfection reagent (Mirus Bio Corporation) according to the manufacturer's protocol. The HRE-Luciferase reporter gene construct was transfected into cells. After 24 h, the media was replaced and cells were exposed to various conditions. For GAL4-reporter assays, cells were transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites. Data were normalized by using total protein concentration as determined by the Bio-Rad protein assay (Bio-Rad Laboratories). ROS were measured as previously described (9). The data presented for reporter assays and ROS measurement are the mean (± standard errors of the means [SEM]) of four independent experiments.
Real-time reverse transcription-PCR (RT-PCR) analysis.
Total RNA was isolated from cells exposed to various conditions using the RNAqueous-4PCR kit (Ambion, Inc.). First-strand cDNA was synthesized from 1 μg of total RNA using the RETROscript cDNA synthesis kit (Ambion, Inc.) with the random decamer primers. The diluted cDNAs were amplified using the Bio-Rad iCycler iQ system (Bio-Rad Laboratories). Quantitative real-time RT-PCR was carried out using gene-specific dual fluorescently labeled probes. The following primer and probe sequences were used: for Pgk1, the primer sequences were TCTGTTCTTGAAGGATTGTGTGG and CTCTACATGAAAGCGGAGGTTT and the probe sequence was 6-carboxyfluorescein (FAM)-CGAGAATGCCTGTGCCAACCCAGCGG-black hole quencher 1 (BHQ-1); for Glut-1, the primer sequences were AACATGGAACCACCGCATCG and CCGACAGAGAAGGAACCAATCAT and the probe sequence was 6-FAM-AGCCCATCCCATCCACCACACTCACCACGC-BHQ-1; for L19, the primer sequences were CATCAAGCGATCAGGGAATG and GAGGATTATACAGTTCAAAGCAAAT and the probe sequence was Texas Red-CACCTTGTCCTTCAATCGTGTTCCTGAGGG-black hole quencher 2 (BHQ-2). The specificity of primers and probes were first tested under normal PCR conditions before quantitation. Cycle threshold values were normalized for amplification of the mitochondrial ribosomal protein L19. The data presented are the result of triplicate analyses and the error bars indicate SEM.
RESULTS
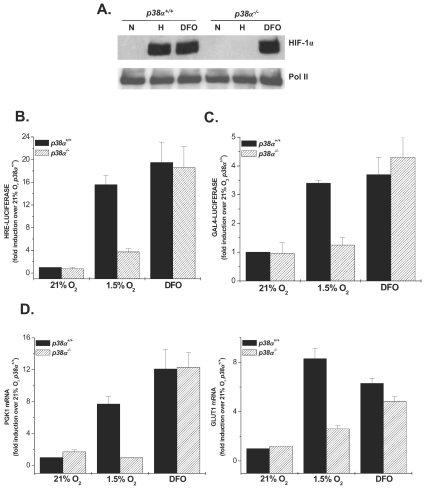
To elucidate the upstream signaling mechanisms that regulate the hypoxic activation of HIF-1, we used fibroblasts isolated from wild-type (WT) and p38α null embryos (35). HIF-1 activity is dependent on the stability of the oxygen sensitive subunit, HIF-1α. WT or p38α−/− cells were exposed to hypoxia (1.5% O2) or normoxia (21% O2) in the presence or absence of the iron chelator desferrioxamine (DFO; 100 μM) to examine whether p38α is required for the stabilization of the HIF-1α protein. Loss of p38α completely suppressed the stabilization of the HIF-1α protein under hypoxia, whereas HIF-1α protein stability was unaffected with treatment of DFO in either the WT or p38α-deficient cells (Fig. 1A). Since PHDs require iron as a cofactor to execute hydroxylation, it is likely that DFO inhibits PHD activity directly by chelating intracellular iron. These results were further confirmed by examining HIF-1 activity using a luciferase reporter assay under the control of a promoter containing three hypoxic response element sites (HRE-Luciferase). Hypoxia and DFO increased HRE-dependent luciferase induction in WT cells (Fig. 1B). In contrast, p38α−/− cells displayed a marked attenuation of luciferase induction under hypoxia but not in the presence of DFO during normoxia (Fig. 1B). These results indicate that the hypoxic activation of HIF-1 is dependent on p38α MAPK and hypoxia and iron chelators have distinct mechanisms for activating HIF-1.
FIG. 1.
p38α MAPK is required for hypoxic activation of HIF-1. (A) HIF-1α protein levels in p38α+/+ and p38α−/− cells exposed to 21% O2 (N) ±100 μM DFO or to 1.5% O2 (H) for 2 h. (B) p38α+/+ and p38α−/− cells transfected with the HRE-Luciferase reporter gene construct and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 16 h. (C) p38α+/+ and p38α−/− cells transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 36 h. (D) p38α+/+ and p38α−/− cells cultured for 16 h under 21% O2 ± 100 μM DFO or to 1.5% O2, and transcription levels of pgk1 and glut1 were determined by quantitative real-time RT-PCR analysis.
HIF-1α contains two oxygen regulated transactivation domains (TADs), which are termed N-TAD (amino acids 531 to 575) and C-TAD (amino acids 786 to 826) (24). The C-TAD is regulated by the hydroxylation of an asparagine residue by FIH to prevent the interaction of the HIF-1α protein with transcriptional coactivators such as p300. To examine the activity of the transactivation domains a GAL4 DNA-binding domain (amino acids 1 to 147) fused to HIF-1α (531 to 826) protein can be utilized. Previous work shows that steady-state levels of this fusion protein do not change between normoxic and hypoxic conditions (24). Therefore, we used the GAL4-HIF-1α (531 to 826) fusion construct to investigate the transcriptional activity of HIF-1α independent of its protein expression level. Transactivation by GAL4-HIF-1α (531 to 826) was induced by hypoxia and DFO in WT cells (Fig. 1C). In contrast, cells deficient in p38α failed to induce transactivation under hypoxia mediated by GAL4-HIF-1α (531 to 826). Furthermore, transactivation by GAL4-HIF-1α (531 to 826) was induced by DFO in p38α null cells, similar to the induction seen in the WT cells. HIF-1 transcriptional activity was further assessed by examining the induction of HIF-1 target genes, phosphoglycerate kinase 1 (pgk1) and glucose transporter 1 (glut1), using real-time quantitative RT-PCR. WT cells exposed to hypoxia displayed an eightfold increase in both pgk1 and glut1 levels, whereas p38α−/− cells had diminished the transcription of pgk1 and glut1 under hypoxic conditions (Fig. 1D). Moreover, pgk1 and glut1 gene induction in WT and p38α−/− cells exposed to DFO were significantly increased to similar levels. These results suggest that p38α MAPK is specifically required for HIF-1α transcriptional activity that is induced by hypoxia, but not by DFO.
Anoxia activation of HIF-1 does not require p38α MAPK.
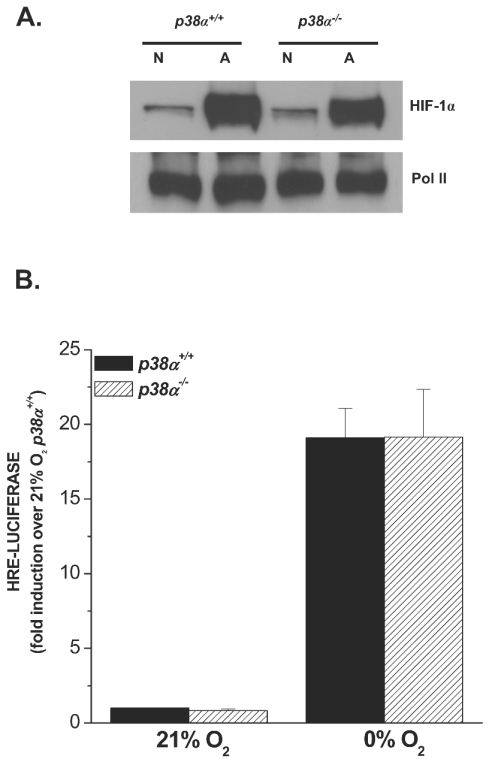
We have previously reported that cells exposed to anoxia (∼0% O2) differ in their mechanism of HIF-1 activation compared to cells exposed to hypoxia (40). Cells exposed to hypoxia fail to activate HIF-1 in the presence of mitochondrial inhibitors or in cells that lack mitochondrial DNA [rho0] cells). In contrast, cells exposed to anoxia retain their ability to activate HIF-1 even in the presence of mitochondrial inhibitors or in [rho0] cells. These results suggest that hypoxia but not anoxia utilizes a functional electron transport chain to activate HIF-1. Anoxia directly limits oxygen availability to the hydroxylases to stabilize HIF-1α protein and thus would not require activation of any signaling mechanisms. To further test whether anoxia requires p38 MAPK signaling, WT or p38α−/− cells were exposed to anoxia or normoxia. Loss of p38α did not affect the stabilization of the HIF-1α protein under anoxia (Fig. 2A). Anoxia also increased HRE-dependent luciferase to similar levels in both WT and p38α−/− cells (Fig. 2B). These results indicate that the anoxic activation of HIF-1 is independent of p38α MAPK and that hypoxia and anoxia have distinct mechanisms for activating HIF-1.
FIG. 2.
p38α MAPK is not required for anoxic activation of HIF-1. (A) HIF-1α protein levels in p38α+/+ and p38α−/− cells exposed to 21% O2 (N) or to 0.0% O2 (A) for 2 h. (B) p38α+/+ and p38α−/− cells transfected with the HRE-Luciferase reporter gene construct and exposed to 21% O2 or to 0.0% O2 for 16 h.
Reintroduction of p38α MAPK rescues hypoxic activation of HIF-1.
To confirm that the hypoxic activation of HIF-1 was directly due to the absence of p38α, p38α−/− cells were stably reconstituted with a p38α cDNA or with vector alone. Immunoblot analysis demonstrates that the reconstituted cells stably express p38α (Fig. 3A). The reintroduction of p38α rescued the hypoxic stabilization of the HIF-1α protein in p38α−/− cells (Fig. 3B). The transcriptional activity of HIF-1, as assessed by HRE-Luciferase and the GAL4-HIF-1α (531 to 826) fusion construct, during hypoxia in p38α−/− cells was also rescued by the expression of p38α (Fig. 3C and D). Furthermore, hypoxia induced gene expression of pgk1 and glut1 in the p38α reconstituted cells (Fig. 3E). Cells treated with DFO during normoxia activated HIF-1 irrespective of the presence or absence of p38α. These results indicate that the suppression of HIF-1 activation under hypoxia in the p38α−/− cells was due to loss of p38α MAPK.
FIG. 3.
Reintroduction of p38α rescues hypoxic activation of HIF-1 in p38α null cells. (A) p38α−/− cells stably reconstituted with a p38α cDNA or with vector alone. (B) HIF-1α protein levels in reconstituted cells exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 2 h. (C) Cells transfected with HRE-Luciferase and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 16 h. (D) Cells transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 36 h. (E) Reconstituted cells cultured for 16 h under 21% O2 ± 100 μM DFO or to 1.5% O2, and transcription levels of pgk1 and glut1 were determined by quantitative real-time RT-PCR analysis.
Upstream MAPKKs MKK3 and MKK6 are required for HIF-1 activation during hypoxia.
MKK3 and MKK6 are the upstream MAPKK isoforms that specifically activate p38α MAPK (5, 10, 37). To investigate if MKK3 and MKK6 are required for the hypoxic activation of p38 MAPK, we used the Mkk3/6+/+ and Mkk3/6−/− cells, exposed them to hypoxia, and performed immunoblot analysis using a phosphospecific antibody for p38 MAPK. During hypoxia, the WT cells demonstrated activation of p38 MAPK (Fig. 4A). In contrast, cells deficient in both Mkk3 and Mkk6 failed to activate p38 MAPK during hypoxia. Next, we examined whether MKK3 and MKK6 were required for the stabilization of HIF-1α. Similar to the p38α null cells, the Mkk3/6−/− cells did not stabilize HIF-1α during hypoxia, whereas cells treated with DFO stabilized HIF-1α irrespective of the status of MKK3/6 (Fig. 4B). To test the requirement of MKK3 and MKK6 for the hypoxic activation of HIF-1, WT and Mkk3/6−/− cells were transfected with HRE-Luciferase under normoxia, hypoxia, and DFO (Fig. 4C). Hypoxia and DFO induced HRE-Luciferase expression in WT cells. In contrast, Mkk3/6−/− cells displayed a severe decline of luciferase induction under hypoxia but not in the presence of DFO during normoxia. Furthermore, cells deficient in Mkk3/6 failed to induce transactivation of HIF-1 under hypoxia (Fig. 4D). Consistent with these data are the observation that MKK3 and MKK6 are essential for the hypoxic induction of the HIF-1 target genes but not for DFO (Fig. 4E). Together these results indicate that the hypoxic activation of p38 MAPK and HIF-1 is dependent on MKK3 and MKK6.
FIG. 4.
MKK3 and MKK6 are essential for hypoxic activation of HIF-1. (A) p38 MAPK activation in WT and Mkk3/6−/− cells exposed to 21% O2 (N) or to 1.5% O2 (H) for 30 min. (B) HIF-1α protein levels in WT and Mkk3/6−/− cells exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 2 h. (C) WT and Mkk3/6−/− cells transfected with HRE-Luciferase and exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 16 h. (D) WT and Mkk3/6−/− cells transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites and exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 36 h (E) WT and Mkk3/6−/− cultured for 16 h under 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H), and transcription levels of Pgk1 and Glut1 were determined by quantitative real-time RT-PCR analysis.
Mitochondrial ROS are required for both the hypoxic activation of HIF-1 and p38 MAPK.
The upstream regulators of the MKK3/6-p38α MAPK signaling pathway remain unknown during hypoxia. Mitochondrial ROS generated within complex III have been implicated as potential regulators of p38 MAPK activation during hypoxia (25). Hypoxia increases the generation of oxidants during the ubiquinione cycle within complex III. Indeed, hypoxia generated ROS, as detected by the oxidation of the 2′,7′-dichlorofluorescin (DCFH) dye, in WT and p38α−/− cells (Fig. 5A). To demonstrate the source of the ROS generation during hypoxia, cells were treated with myxothiazol, a complex III inhibitor. Myxothiazol significantly decreased the oxidation of the DCFH dye during hypoxia (Fig. 5A). To demonstrate the requirement of the mitochondrial complex III for the activation of p38 MAPK, WT cells were treated with myxothiazol under hypoxic conditions. The phosphorylation of p38 MAPK under hypoxic conditions was also suppressed by myxothiazol (Fig. 5B). To demonstrate whether ROS are required for the hypoxic stabilization of HIF-1α and the activation of p38 MAPK, cells were infected with an adenovirus encoding glutathione peroxidase 1 (GPX1), an antioxidant enzyme that converts hydrogen peroxide into water (Fig. 5C). Cells overexpressing GPX1 failed to activate p38 MAPK during hypoxia (Fig. 5D). The activation of HIF-1 during hypoxia has also been shown to be regulated by mitochondrial ROS (8, 9). The increase in oxidant production is required for the hypoxic stabilization of HIF-1α protein. To demonstrate the requirement of the mitochondrial complex III for the stabilization of HIF-1α, WT cells were treated with myxothiazol under hypoxic conditions. Myxothiazol prevented the hypoxic stabilization of the HIF-1α protein but not in response to DFO (Fig. 6A). Cells overexpressing GPX1 failed to stabilize HIF-1α protein during hypoxia (Fig. 6B). These results demonstrate that activation of p38 MAPK signaling links mitochondrial generated ROS to the activation of HIF-1 during hypoxia.
FIG. 5.
Hypoxic activation of p38 MAPK and requires mitochondrial generated oxidants. (A) ROS were determined by incubating p38α+/+ and p38α−/− cells with DCFH-DA (10 μM) exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 ± myxothiazol, a complex III inhibitor, for 4 h. (B) p38 MAPK activation in WT cells exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) ± myxothiazol (1 μM) for 30 min. (C and D) GPX1 and p38 MAPK activation in WT cells infected with null adenovirus (control) or adenovirus expressing myc-tagged GPX1 and subsequently exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) for 2 h.
FIG. 6.
Hypoxic activation of HIF-1α protein levels requires mitochondrial generated oxidants. (A) HIF-1α protein levels in WT cells exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) ± myxothiazol (1 μM) for 2 h. (B) HIF-1α protein levels in WT cells infected with null adenovirus (control) or adenovirus expressing myc-tagged GPX1 and subsequently exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) for 2 h.
DISCUSSION
The signal transduction pathways that regulate the stabilization of HIF-1α as well as the subsequent expression HIF-1 regulated genes are not fully understood. How cells sense the decrease in oxygen to activate signaling pathways resulting in the activation of HIF-1 remains unknown. Many signaling molecules have been implicated in the regulation of HIF-1, although most studies have relied on the use of pharmacological agents in cancer cell lines (17, 44). In the present study we provide genetic evidence, using cells from p38α−/− or Mkk3/6−/− knockout mice, that the p38 MAPK signaling cascade is necessary for the hypoxic activation of HIF-1. The reconstitution of p38α null cells with a p38α cDNA restores the ability to activate HIF-1 during hypoxia. This indicates that the inability of hypoxia to activate HIF-1 was due to a loss of p38α as opposed to an adaptation of p38α null cells in the absence of the kinase. The p38 MAPK activation during hypoxia was dependent on oxidant generation within mitochondrial complex III. Thus, p38 MAPK signaling provides a mechanistic link between the generation of oxidant production and the activation of HIF-1 during hypoxia (Fig. 7).
FIG. 7.
A signaling model for hypoxic activation of HIF-1. Based on our current findings we propose that hypoxia stimulates oxidant production within mitochondria. These oxidants interact with an unknown protein “X” in the cytosol to activate MKK3/6 and p38α MAPK. The activation of the p38 MAPK signaling pathway results in the decrease in hydroxylation of the HIF-1α protein at proline and asparagine residues resulting in the activation of HIF-1. By contrast, anoxia or iron chelators (DFO) during normoxia inhibit hydroxylation directly by limiting the availability of oxygen as a substrate and iron as a cofactor, respectively. Thus, anoxia or DFO does not require signaling pathways for the activation of HIF-1. Hypoxia and anoxia have distinct signaling mechanisms to activation HIF-1.
Presently there are two models that account for the activation of HIF-1. One model proposes that the hydroxylases, PHDs and FIH, serve as oxygen sensors that control HIF-1 activation during hypoxia (12, 27). According to this model, the hydroxylases reduce their activity directly as a function of declining oxygen levels resulting in a decrease in hydroxylation of proline and asparagine residues within the HIF-1α protein. The decrease in hydroxylation of the HIF-1α protein results in both the stabilization of the protein as well as recruitment of coactivators to induce gene expression. The fact that the hydroxylases could serve as oxygen sensors also suggests that there would be no requirement for signaling pathways to be initiated for the activation of HIF-1 during hypoxia. In order to fulfill the role of oxygen sensors the hydroxylases would have to have a Km in the hypoxic region. Recombinant prolyl hydroxylases have a Km of ambient air (20.9% O2) in vitro while asparaginyl hydroxylase (FIH) has a Km of 40% of ambient air in vitro, indicating that the hydroxylases decrease their enzymatic activity throughout the physiological range of PO2 (18). Therefore, if the hydroxylases were in fact the sensors, one would predict a continuous increase in the accumulation of HIF-1α protein as oxygen levels fall from 21% O2 to 0% O2. However, HIF-1α protein begins to accumulate around 5% O2, and its concentration increases as the oxygen levels approach anoxia (23). Thus, the Km of the hydroxylases is not compatible with the oxygen dependence of HIF-1α protein stabilization. Our current finding that p38 MAPK signaling is required for the activation of HIF-1 during hypoxia further suggests that the hydroxylases are not likely to be the sole regulators of HIF-1.
A second model proposes that the hydroxylases are only proximal regulators of the HIF-1α protein. According to this model there would be upstream regulators of the hydroxylases. Our present results are in agreement with this model. Loss of p38 MAPK signaling prevented both the hypoxic stabilization of HIF-1α protein as well as the transcriptional activity of the protein. The stabilization of HIF-1α protein is primarily regulated by hydroxylation of proline residues by PHDs while the transcriptional activity is regulated by asparagine hydroxylation by FIH. The activation of p38 MAPK signaling during hypoxia is likely to prevent PHDs as well as FIH from hydroxylating proline and asparagine residues. Our results are also consistent with previous studies indicating that signaling molecules are necessary for HIF-1α protein stabilization during hypoxia. These signaling pathways include but are not limited to the requirement of diacylglycerol kinase, small GTPases, and PI3-K/AKT (4, 17, 47, 48, 49). Many of these signal transduction molecules can activate p38 MAPK signaling pathways. Moreover, the transactivation potential of HIF-1α depends on phosphorylation of the conserved residue Threonine-796 (16). The modification of this residue increases the affinity of HIF-1α to the transcriptional coactivator CBP. Whether this modification does not allow FIH mediated hydroxylation at Asparagine 803 remains unknown. Also, p42/p44 MAPK can directly phosphorylate HIF-1α and increase the transcriptional activity of the protein (38). We interpret these findings to suggest that the hydroxylases are likely to have enough oxygen to carry out hydroxylation of HIF-1α protein throughout the physiological range but fail to hydroxylate HIF-1α protein as they approach oxygen levels in the hypoxic region due to modification by p38 MAPK signaling (Fig. 7).
Our results also indicate that cells utilize different mechanisms to activate HIF-1 during hypoxia compared with anoxia or exposure to iron chelators. The p38 MAPK signaling was not required for the activation of HIF-1 in cells exposed to anoxia or iron chelators under normoxia. Oxygen is required as a substrate and iron as a cofactor for the hydroxylation of the HIF-1α protein, and in the absence of oxygen this reaction will not occur. Therefore, HIF-1α protein will not be hydroxylated and targeted for ubiquitin mediated degradation under anoxia due to substrate limitation. The hydroxylases ultimately serve as oxygen sensors under anoxic conditions. Similarly, the hydroxylases are likely to be the only regulators of HIF-1 by iron chelators, such as DFO. Iron chelators can inhibit the hydroxylation reaction directly by limiting the availability of iron. Previous observations have indicated that hypoxia and anoxia or iron chelators have distinct mechanisms for the activation of HIF-1. For example, cells exposed to hypoxia fail to activate HIF-1 in the presence of mitochondrial inhibitors or in cells that lack mitochondrial DNA [rho0] cells). In contrast, cells exposed to anoxia retain their ability to activate HIF-1 even in the presence of mitochondrial inhibitors or in [rho0] cells (40). Furthermore, diphenylene iodonium (DPI), an inhibitor of a wide range of flavoproteins including mitochondrial complex I, prevents stabilization of HIF-1α protein and HIF-1 target genes at oxygen levels of 1% but not in the presence of iron chelators under normoxia (15).
A major finding of the present study is that ROS generated within mitochondrial complex III are required for the activation of both p38α MAPK and HIF-1. Previous studies have suggested that mitochondrial complex III can serve as an oxygen sensor and oxidant production from this complex serves as the major signaling molecule to initiate activation of HIF-1 during hypoxia (8, 9, 40). However, the mechanisms by which mitochondrial ROS would activate HIF-1 remains unknown. The current finding that p38 MAPK signaling is required for HIF-1 activation provides the first known link between mitochondrial oxidant production during hypoxia and HIF-1 activation. This premise is consistent with the observations that nonhypoxic stimuli such as chromium or arsenite generate oxidative stress resulting in a p38α MAPK-dependent activation of HIF-1. The physiological implication of ROS as a positive regulator of HIF-1 has been recently highlighted by the observation that cells deficient in JunD, a member of the AP-1 family of transcription factors, have increased ROS levels under normoxic conditions resulting in the accumulation of HIF-1α protein and induction of VEGF, a potent angiogenic factor (14). Thus, JunD can reduce tumorigenesis by reducing oxidative stress and HIF-1 activation. Furthermore, radiation-induced reoxygenation of hypoxic tumor cells results in the production of ROS, which activates HIF-1 to prevent radiation-induced endothelial cell death (33).
There are two important physiological implications of the present study. First, the requirement of p38 MAPK signaling for the hypoxic activation of HIF-1 may explain the resemblance seen in the phenotypes of the p38α, Mkk3/6, Hif-1α, and Hif-1β null mice. All these embryos die during midgestation due to multiple defects, including abnormal vascularization of the placenta (1, 2, 3, 5, 7, 21, 30, 34, 39, 46). In particular, both the Hif-1β knockout and p38α null mice display a complete loss of the labyrinth layer and significant reduction of the spongiotrophoblast layer in the developing placenta. Secondly, HIF-1 is up-regulated in most human cancers and is required for tumor progression by up-regulating its target genes, which are involved in angiogenesis, anaerobic metabolism, cell survival, cell invasion, and drug resistance (43). Here, we identify the p38 MAPK signaling pathway as a key upstream regulator of HIF-1 activity. Consequently, therapies targeted towards modulating the p38 MAPK signaling pathway may provide a basis for the development of new cancer therapies.
Acknowledgments
This work was supported in part by National Institutes of Health Grants GM60472-06, P01HL071643-01A4, American Heart Association Grant 0350054N to N.S.C., and National Institutes of Health Predoctoral Training Grant HL076139 to B.M.E.
We thank Greg Semenza for the GAL4-HIF-1α fusion construct and Richard A. Maurer for the GAL4-luciferase reporter construct.
REFERENCES
- 1.Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. [PubMed] [Google Scholar]
- 2.Adelman, D. M., M. Gertsenstein, A. Nagy, M. C. Simon, and E. Maltepe. 2000. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 14:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, M., L. Svensson, M. Roach, J. Hambor, J. McNeish, and C. A. Gabel. 2000. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191:859-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragones, J., D. R. Jones, S. Martin, M. A. San Juan, A. Alfranca, F. Vidal, A. Vara, I. Merida, and M. O. Landazuri. 2001. Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor 1 by low oxygen tension. J. Biol. Chem. 276:10548-10555. [DOI] [PubMed] [Google Scholar]
- 5.Brancho, D., N. Tanaka, A. Jaeschke, J. J. Ventura, N. Kelkar, Y. Tanaka, M. Kyuuma, T. Takeshita, R. A. Flavell, and R. J. Davis. 2003. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17:969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruick, R. K., and S. L. McKnight. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, E. Keshert, and E. Keshet. 1998. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485-490. [DOI] [PubMed] [Google Scholar]
- 8.Chandel, N. S., E. Maltepe, E. Goldwasser, C. E. Mathieu, M. C. Simon, and P. T. Schumacker. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 95:11715-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandel, N. S., D. S. McClintock, C. E. Feliciano, T. M. Wood, J. A. Melendez, A. M. Rodriguez, and P. T. Schumacker. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275:25130-25138. [DOI] [PubMed] [Google Scholar]
- 10.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 11.Duyndam, M. C., S. T. Hulscher, E. van der Wall, H. M. Pinedo, and E. Boven. 2003. Evidence for a role of p38 kinase in hypoxia-inducible factor 1-independent induction of vascular endothelial growth factor expression by sodium arsenite. J. Biol. Chem. 278:6885-6895. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke, D. R. Mole, M. Mukherji, E. Metzen, M. I. Wilson, A. Dhanda, Y. M. Tian, N. Masson, D. L. Hamilton, P. Jaakkola, R. Barstead, J. Hodgkin, P. H. Maxwell, C. W. Pugh, C. J. Schofield, and P. J. Ratcliffe. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43-54. [DOI] [PubMed] [Google Scholar]
- 13.Gao, N., B. H. Jiang, S. S. Leonard, L. Corum, Z. Zhang, J. R. Roberts, J. Antonini, J. Z. Zheng, D. C. Flynn, V. Castranova, and X. Shi. 2002. p38 Signaling-mediated hypoxia-inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J. Biol. Chem. 277:45041-45048. [DOI] [PubMed] [Google Scholar]
- 14.Gerald, D., E. Berra, Y. M. Frapart, D. A. Chan, A. J. Giaccia, D. Mansuy, J. Pouyssegur, M. Yaniv, and F. Mechta-Grigoriou. 2004. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 118:781-794. [DOI] [PubMed] [Google Scholar]
- 15.Gleadle, J. M., B. L. Ebert, and P. J. Ratcliffe. 1995. Diphenylene iodonium inhibits the induction of erythropoietin and other mammalian genes by hypoxia. Implications for the mechanism of oxygen sensing. Eur. J. Biochem. 234:92-99. [DOI] [PubMed] [Google Scholar]
- 16.Gradin, K., C. Takasaki, Y. Fujii-Kuriyama, and K. Sogawa. 2002. The transcriptional activation function of the HIF-like factor requires phosphorylation at a conserved threonine. J. Biol. Chem. 277:23508-23514. [DOI] [PubMed] [Google Scholar]
- 17.Hirota, K., and G. L. Semenza. 2001. Rac1 activity is required for the activation of hypoxia-inducible factor 1. J. Biol. Chem. 276:21166-21172. [DOI] [PubMed] [Google Scholar]
- 18.Hirsila, M., P. Koivunen, V. Gunzler, K. I. Kivirikko, and J. Myllyharju. 2003. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 278:30772-30780. [DOI] [PubMed] [Google Scholar]
- 19.Hudson, C. C., M. Liu, G. G. Chiang, D. M. Otterness, D. C. Loomis, F. Kaper, A. J. Giaccia, and R. T. Abraham. 2002. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22:7004-7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 21.Iyer, N. V., L. E. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. V. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, B. H., G. L. Semenza, C. Bauer, and H. H. Marti. 1996. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271:C1172-C1180. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, B. H., J. Z. Zheng, S. W. Leung, R. Roe, and G. L. Semenza. 1997. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 272:19253-19260. [DOI] [PubMed] [Google Scholar]
- 25.Kulisz, A., N. Chen, N. S. Chandel, Z. Shao, and P. T. Schumacker. 2002. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 282:L1324-L1329. [DOI] [PubMed] [Google Scholar]
- 26.Lando, D., D. J. Peet, J. J. Gorman, D. A. Whelan, M. L. Whitelaw, and R. K. Bruick. 2002. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16:1466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lando, D., D. J. Peet, D. A. Whelan, J. J. Gorman, and M. L. Whitelaw. 2002. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295:858-861. [DOI] [PubMed] [Google Scholar]
- 28.Li, Q., S. Sanlioglu, S. Li, T. Ritchie, L. Oberley, and J. F. Engelhardt. 2001. GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxidant Redox Signal. 3:415-432. [DOI] [PubMed] [Google Scholar]
- 29.Mahon, P. C., K. Hirota, and G. L. Semenza. 2001. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15:2675-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maltepe, E., J. V. Schmidt, D. Baunoch, C. A. Bradfield, and M. C. Simon. 1997. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386:403-407. [DOI] [PubMed] [Google Scholar]
- 31.Masson, N., C. Willam, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 20:5197-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 33.Moeller, B. J., Y. Cao, C. Y. Li, and M. W. Dewhirst. 2004. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 5:429-441. [DOI] [PubMed] [Google Scholar]
- 34.Mudgett, J. S., J. Ding, L. Guh-Siesel, N. A. Chartrain, L. Yang, S. Gopal, and M. M. Shen. 2000. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 97:10454-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porras, A., S. Zuluaga, E. Black, A. Valladares, A. M. Alvarez, C. Ambrosino, M. Benito, and A. R. Nebreda. 2004. p38alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol. Biol. Cell 15:922-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raingeaud, J., S. Gupta, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 37.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard, D. E., E. Berra, E. Gothie, D. Roux, and J. Pouyssegur. 1999. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 274:32631-32637. [DOI] [PubMed] [Google Scholar]
- 39.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroedl, C., D. S. McClintock, G. R. Budinger, and N. S. Chandel. 2002. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L922-L931. [DOI] [PubMed] [Google Scholar]
- 41.Semenza, G. L. 2000. HIF-1 and human disease: one highly involved factor. Genes Dev. 14:1983-1991. [PubMed] [Google Scholar]
- 42.Semenza, G. L. 2001. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1-3. [DOI] [PubMed] [Google Scholar]
- 43.Semenza, G. L. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 10:721-732. [DOI] [PubMed] [Google Scholar]
- 44.Shemirani, B., and D. L. Crowe. 2002. Hypoxic induction of HIF-1alpha and VEGF expression in head and neck squamous cell carcinoma lines is mediated by stress activated protein kinases. Oral Oncol. 38:251-257. [DOI] [PubMed] [Google Scholar]
- 45.Tacchini, L., P. Dansi, E. Matteucci, and M. A. Desiderio. 2001. Hepatocyte growth factor signaling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis 22:1363-1371. [DOI] [PubMed] [Google Scholar]
- 46.Tamura, K., T. Sudo, U. Senftleben, A. M. Dadak, R. Johnson, and M. Karin. 2000. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 47.Turcotte, S., R. R. Desrosiers, and R. Beliveau. 2003. HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J. Cell Sci. 116:2247-2260. [DOI] [PubMed] [Google Scholar]
- 48.Zhong, H., K. Chiles, D. Feldser, E. Laughner, C. Hanrahan, M. M. Georgescu, J. W. Simons, and G. L. Semenza. 2000. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60:1541-1545. [PubMed] [Google Scholar]
- 49.Zundel, W., C. Schindler, D. Haas-Kogan, A. Koong, F. Kaper, E. Chen, A. R. Gottschalk, H. E. Ryan, R. S. Johnson, A. B. Jefferson, D. Stokoe, and A. J. Giaccia. 2000. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 14:391-396. [PMC free article] [PubMed] [Google Scholar]