Abstract
Background
Clearance of human papillomavirus (HPV) among adolescent men who have sex with men (MSM) is not well studied. This study aimed to evaluate the clearance of HPV DNA and antibodies among adolescent MSM.
Methods
In our cohort study, we enrolled adolescent MSM in Melbourne between October 2010 and September 2013. At baseline, 3, 6, and 12 months, anal and penile swabs for HPV DNA and serum for HPV antibodies against genotypes 6, 11, 16, and 18, were collected. Definite clearance was defined as HPV DNA (same site) /antibodies for the same genotype undetected following a positive HPV DNA /antibodies test at baseline or month 3. Possible clearance was defined as HPV DNA (same site) /antibodies for the same genotype undetected at month 12 following a positive HPV DNA/antibodies test at month 6. Overall clearance was defined as either definite or possible clearance. The agreement between HPV DNA clearance and antibodies clearance was calculated.
Results
A total of 183 MSM were included (median age: 19 years, interquartile [IQR]: 18 to 20). At the anus, overall clearance rate was 21.6 (95 % confidence interval[CI]: 7.9 to 47.0), 44.8 (19.3 to 88.3), 51.9 (20.9 to 106.9) and 33.7 (7.0 to 98.5) per 1000 person months (PM) for HPV 6, 11, 16 and 18. At the penis, overall clearance rate was 64.5 (13.3 to 188.5), 71.3 (14.7 to 208.2), 96.5 (31.3 to 225.3) and 333.3 (8.4 to 1857.2) per 1000 PM for HPV 6, 11, 16 and 18. For antibodies, overall clearance rate was 22.2 (9.6 to 43.7), 18.8 (3.9 to 55.0), 10.8 (0.3 to 60.1) and 19.0 (2.3 to 68.8) per 1000 PM. Agreement between anal/penile HPV DNA clearance and antibodies clearance was low: kappa = -0.18 (95 % CI: −0.28 to 0.08)/-0.13 (−0.24 to −0.02), 0.04 (−0.29 to 0.36)/0.22 (−0.32 to 0.76), −0.10 (−0.27 to 0.08)/-0.14 (−0.37 to 0.10) and −0.14 (−0.28 to 0.01)/-0.14 (−0.33 to 0.06) for HPV 6, 11, 16 and 18, respectively.
Conclusion
Clearance rates of HPV DNA were low and varied by genotypes and anatomical sites among adolescent MSM. Antibodies against HPV were stable during the study period.
Keywords: Human papillomavirus (HPV), Natural history, Clearance, Immune response, Men who have sex with men
Introduction
Human papillomavirus (HPV) infection is the etiological factor for anogenital warts and HPV-related cancers [1]. HPV 16 and 18 cause approximately 90 % of anal cancers, and HPV 6 and 11 account for about 90 % of anogenital warts[2], [3]. Men who have sex with men (MSM) have high burdens of HPV infection, including HPV-related anal cancer[4], [5], [6]. A meta-analysis estimated the incidence rate of anal cancer to be 19 and 85 per 100 000 person years among HIV-uninfected and HIV-infected MSM[7], in comparison to only 1–2 per 100 000 person years in the general population[8]. The approaches for anal cancer prevention include programs for screening, diagnosis, and treatment, and follow-up of anal high-grade squamous intraepithelial lesions and external anogenital lesions, as well as HPV vaccination[9]. However, limited health resources and the lack of evidence-based screening guidelines largely hampered anal cancer preventional efforts targeting MSM.
Understanding the natural history parameters, namely the incidence, persistence, and clearance of HPV in MSM is vital to the development of anal cancer prevention strategies. Although HPV infection is common among MSM, most infections are cleared by the host without clinical symptoms, and only a small fraction of infections eventually develops into cancer[10]. During the clearing process, pro-inflammatory cytokines are released, and the signals for Langerhans cell and dendritic cell are activated and recruited[11]. Failure to develop effective host immune response may lead to persistent infection and increased probability of progression towards invasive cancer[12]. The understanding of the characteristics of type-specific HPV clearance after incident HPV infection is essential to determining screening strategies.
Previous studies on HPV natural history mostly focused on HPV DNA, which may only represent transient deposition[13], [14], [15]. Type-specific HPV antibodies are mostly the marker of previous exposure[16]. Studies in the pre-vaccine era examined the production, duration, and immunogenicity of HPV antibodies following natural infection[17], [18], [19]. Those studies found the presence and duration of antibodies against HPV were affected by many factors, including HPV genotypes, anatomical sites, and study populations [17], [20], [21], [22]. As HPV infection may not always result in seroconversion, HPV exposure may be underestimated when only antibodies are observed. Thereby, combining HPV DNA and serum results may provide a clearer picture of HPV exposure[22], [23].
When depicting HPV exposure, clearance of prevalent and incident infections was often mixed. Cross-sectional detection of HPV DNA may represent both recent (prone to clear) and existing (higher oncogenic potential) infections. Although a number of studies[13], [14], [24] reported on HPV clearance, participants included in those studies were mostly MSM who were more sexually experienced and had higher probability of repeated HPV exposures. As a result, incidence defined in those studies may be the reactivation of latent HPV infection rather than incident infection. The clearance of HPV among younger MSM who are less sexually experienced has not been well documented. We evaluated the clearance of type-specific anal/penile HPV DNA and HPV antibodies and their correlations among a cohort of adolescent MSM.
Materials and methods
Study population and design
The study population and design details were published elsewhere[22], [23], [25]. Briefly, men were recruited to participate in the Human Papillomavirus in Young People Epidemiological Research (HYPER) study between October 2010 and September 2012 through multiple sources oriented toward adolescent gay and bisexual men in Melbourne, Australia. Men aged 16–20 years were eligible for the HYPER study if they self-identified as being attracted to males, were HIV-negative, and had not received any doses of HPV vaccine. Participants did not need to have had sex with a male previously. Recruitment and all study procedures were conducted at the Melbourne Sexual Health Centre. Written informed consent was obtained from each participant before the study procedures commenced. Then, at baseline, 3, 6, and 12 months, specimens were obtained for HPV DNA testing using separate swabs from the anal canal and the penis. In addition, blood was obtained for HPV serum at each visit. Participants also completed a questionnaire at each visit which included details on sexual behaviors. After completing the study, participants were offered the 3-dose HPV vaccine (Gardasil, Merck, NJ) for free. As reimbursement, participants received AUD $15, $15, $20, and $50 for the first, second, third, and fourth visit, respectively.
Specimen collection
Participants were required to attend the Melbourne Sexual Health Centre at each visit. A research nurse collected an anal canal swab. After watching a demonstration video that recorded how to self-collect a penile sample, all participants conducted a self-collected swabbing of the penis. The anal and penile swab collecting techniques are published in detail elsewhere[22], [23], [25]. Blood for HPV serum was collected in 10 mL serum collection tubes (Greiner Bio-One International AG) and centrifuged at 4800 × g for 10 min to separate cells from serum. Serum samples were aliquoted and stored at −80 °C until testing.
HPV DNA detection
HPV DNA testing methods are published elsewhere[22], [23], [25]. The swabs were tested in the local HPV Labnet Reference Laboratory, Molecular Microbiology Department, Melbourne's Royal Women's Hospital. In brief, the extracted DNA was amplified with the L1 consensus primer PGMY09/11. The amplified product was hybridized with a biotin-labeled HPV L1 universal probe and captured on a streptavidin-coated plate (Roche Diagnostics, Mannheim, Germany). The bound hybrids were detected by the anti-digoxigenin peroxidase conjugate using the chromogenic substrate 3-ethylbenzothiazoline-6-sulphonic acid (ABTS). The samples with positive HPV L1 PCR shared primers were further genotyped by the HPV linear array genotyping assay (Roche Molecular Systems, Pleasanton, CA, USA). A total of 37 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 82v, and 89) were detected by HPV linear array genotyping assay. DNA samples that were negative for both β-globin and HPV were considered unqualified samples, and these samples were excluded from the analysis. This study included HPV genotypes 6, 11, 16, and 18 in analysis.
Serum detection
Details of serum detection methods were published elsewhere[19]. Serum samples were tested at PPD Vaccines and Biologics Lab. Antibodies against HPV genotypes 6, 11, 16, and 18 were measured using a Luminex competitive immunoassay[19]. Seropositivity was established based on milli Merck units per mL (mMU/mL). The cut-off value for HPV genotypes 6, 11, 16, and 18 seropositivity was 20 mMU/mL, 16mMU/mL, 20 mMU/mL, and 24 mMU/mL respectively[26].
Statistical analyses
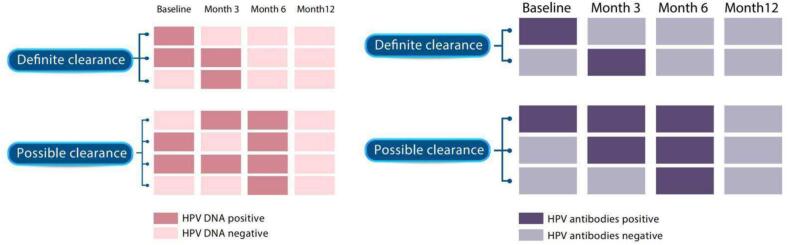
The sample size was based on the original study aim, which was to determine the prevalence of HPV at the baseline visit. The sample of 200 provided upper and lower 95 % confidence intervals (CIs) of between 2 % and 7 % around the expected proportion of men with any HPV DNA at baseline. Definite clearance of HPV DNA was defined as the same HPV type undetected from the same site following a positive HPV test at baseline or month 3. Possible clearance of HPV DNA was defined as the same HPV type undetected from the same site at month 12 following a positive HPV test at month 6. Definite clearance of HPV antibodies was defined as antibodies for the same HPV type undetected following a positive HPV test at baseline or month 3. Possible clearance of HPV antibodies was defined as antibodies for the same HPV type undetected from the same site at month 12 following a positive HPV test at month 6. Fig. 1 details specific scenarios for the clearance of HPV DNA and antibodies[23]. Only one clearance within one individual was allowed for a specific type of HPV DNA at a given anatomical site or a specific type of HPV antibodies. We considered each DNA or antibodies clearance as an independent event. We calculated the clearance rate of a specific type of HPV DNA at a given anatomical site or a specific type of HPV antibodies based on the number of clearance events divided by person months at risk. Corresponding 95 % confidence intervals (CI) were calculated presuming a Poisson distribution. The clearance of the HPV DNA and antibodies was evaluated by the Kaplan-Meier survival curves.
Fig. 1.
Definition of definite and possible clearance of anal and penile HPV 6, 11, 16, and 18 DNA and antibodies. Notes: HPV: human papillomavirus; Definite clearance of HPV DNA was defined as the same HPV type undetected from the same site following a positive HPV test at baseline or month 3. Possible clearance of HPV DNA was defined as the same HPV type undetected from the same site at month 12 following a positive HPV test at month 6. Definite clearance of HPV antibodies was defined as antibodies for the same HPV type undetected following a positive HPV test at baseline or month 3. Possible clearance of HPV antibodies was defined as antibodies for the same HPV type undetected from the same site at month 12 following a positive HPV test at month 6.
Logistic regression models were fitted to the data to identify correlates of HPV clearance among men who were tested positive for either anal or penile HPV DNA or antibodies at one or more occasions at month 0, 3 or 6. The clearance group included men who had definite or possible clearance of either anal or penile HPV DNA, or definite or possible clearance of HPV antibodies. The remaining were group into no clearance. Age at recruitment, educational level, number of male sex partner in lifetime, ever had sex with female, ever had chemical sex were included in the final multiple logistic regression model. We assessed the concordance between anal/penile HPV DNA clearance and HPV antibodies clearance for a given HPV genotype, using kappa scores. We performed all analyses in R 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria; appendix).
Ethics statement
This study was approved by the Alfred Hospital Research Ethics Committee (576/22). Written informed consent was obtained from each participant.
Results
Characteristics of the study population
The details of the participants' demographic characteristics and sexual practices were described elsewhere[27]. In summary, 200 MSM were recruited between October 2010 and September 2012. We included 183 MSM who consented for their data to be used in future research. The median age of participants was 19 years (IQR: 18 to 20), and 74.9 % (137/183) of men were in their final year of secondary school at the time of recruitment. The median age at first insertive or receptive anal sex was 17 years. At baseline, 183 participants provided an anal swab, a penile swab, and blood sample. At 3 months, 171 men provided an anal swab, a penile swab, and a blood sample. At 6 months, 167 men provided an anal swab, a penile swab, and blood sample. At 12 months, 159, 159, and 158 men provided an anal swab, a penile swab, and blood sample.
At baseline, 21, 17, 9, and 4 males were tested positive for HPV genotypes 6, 11, 16, and 18, respectively, at the anus. At baseline, 3, 4, 3, and 1 males were tested positive for HPV genotypes 6, 11, 16, and 18, respectively, at the penis. At baseline, 23, 12, 6, and 6 males were tested positive for antibodies of HPV 6, 11, 16, and 18.
Site-specific HPV DNA and antibodies clearance
The definite, possible and overall clearance of HPV 6, 11, 16, and 18 DNA and antibodies among participants are shown in Table 1. Men who had assessable samples had a total follow-up time of 1924 person months, 1930 person months, and 1992 person months for anal HPV DNA, penile HPV DNA, and HPV antibodies, respectively. Of the men who had assessable samples, for HPV 6, there were 28, 5, and 36 participants at risk of anal HPV DNA clearance, penile HPV DNA clearance, and antibodies clearance. For HPV 11, these figures were 22, 5, and 19. For HPV 16, these figures were 15, 6, and 9. For HPV 18, these figures were 12, 1, and 10.
Table 1.
Definite and possible clearance of HPV 6, 11, 16, and 18 DNA and antibodies among adolescent MSM.
| HPV Type | No. at risk |
No. Definite Clearance |
No. Possible Clearance |
No. Overall Clearance |
PM to Definite Clearance (median)# | PM to Possible Clearance (median) | PM to Overall Clearance (median) | PM at risk | Definite Clearance /1000 PM (95 %CI) |
Possible Clearance /1000 PM (95 %CI) |
Overall Clearance /1000 PM (95 %CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV6 | Anal DNA | 28 | 6 | 0 | 6 | 3.2 | /* | 3.2 | 277.9 | 21.6 (7.9 to 47.0) | /* | 21.6 (7.9 to 47.0) |
| Penile DNA | 5 | 2 | 1 | 3 | 3.1 | 5.8 | 3.4 | 46.5 | 43.0 (5.2 to 155.4) | 21.5 (0.5 to 119.8) | 64.5 (13.3 to 188.5) | |
| Antibodies | 36 | 3 | 5 | 8 | 9.3 | 11.9 | 9.3 | 360.7 | 8.3 (1.7 to 24.3) | 13.9 (4.5 to 32.3) | 22.2 (9.6 to 43.7) | |
| HPV11 | Anal DNA | 22 | 5 | 3 | 8 | 2.9 | 5.7 | 3.6 | 178.5 | 28.0 (9.1 to 65.4) | 16.8 (3.5 to 49.1) | 44.8 (19.3 to 88.3) |
| Penile DNA | 5 | 2 | 1 | 3 | 3.3 | 5.6 | 3.4 | 42.1 | 47.5 (5.8 to 171.6) | 23.8 (0.6 to 132.3) | 71.3 (14.7 to 208.2) | |
| Antibodies | 19 | 1 | 2 | 3 | 12.3 | 4.3 | 5.6 | 159.3 | 6.3 (0.2 to 35.0) | 12.6 (1.5 to 45.4) | 18.8 (3.9 to 55.0) | |
| HPV16 | Anal DNA | 15 | 3 | 4 | 7 | 3.2 | 6.2 | 6.0 | 134.9 | 22.2 (4.6 to 65.0) | 29.7 (8.1 to 75.9) | 51.9 (20.9 to 106.9) |
| Penile DNA | 6 | 1 | 4 | 5 | 2.7 | 6.6 | 5.6 | 51.8 | 19.3 (0.5 to 107.6) | 77.2 (21.0 to 197.7) | 96.5 (31.3 to 225.3) | |
| Antibodies | 9 | 0 | 1 | 1 | /* | 5.1 | 5.1 | 92.7 | /* | 10.8 (0.3 to 60.1) | 10.8 (0.3 to 60.1) | |
| HPV18 | Anal DNA | 12 | 1 | 2 | 3 | 3.7 | 6.0 | 5.5 | 89 | 11.2 (0.3 to 62.6) | 22.5 (2.7 to 81.2) | 33.7 (7.0 to 98.5) |
| Penile DNA | 1 | 1 | 0 | 1 | 3.0 | /* | 3.0 | 3.0 | 333.3 (8.4 to 1857.2) | /* | 333.3 (8.4 to 1857.2) | |
| Antibodies | 10 | 0 | 2 | 2 | /* | 7.6 | 7.6 | 105.0 | /* | 19.0 (2.3 to 68.8) | 19.0 (2.3 to 68.8) | |
Notes:
HPV: human papillomavirus; PM: person month; Definite clearance of HPV DNA was defined as the same HPV type undetected from the same site following a positive HPV test at baseline or month 3. Possible clearance of HPV DNA was defined as the same HPV type undetected from the same site at month 12 following a positive HPV test at month 6. Definite clearance of HPV antibodies was defined as antibodies for the same HPV type undetected following a positive HPV test at baseline or month 3. Possible clearance of HPV antibodies was defined as antibodies for the same HPV type undetected from the same site at month 12 following a positive HPV test at month 6.
Only individuals who had at least one positive result for DNA or antibodies at the baseline, 3 months, or 6 months contributed to the time at risk.
We calculated the clearance rate of a specific type of HPV DNA at a given anatomical site or a specific type of HPV antibodies based on the number of clearance events divided by PM at risk.
Among men at risk of clearance, nobody had possible clearance for anal HPV 6 DNA and penile HPV 18 DNA during the follow-up. Among men at risk of clearance, nobody had definite clearance for HPV 16 antibodies and HPV 18 antibodies. Thus, the PM to clearance and clearance rate could not be calculated.
We did not calculate IQR (Inter-Quartile Range) because the number of clearance events was less than four in most cases.
At the anus, overall clearance rate was 21.6 (95 % CI: 7.9 to 47.0), 44.8 (19.3 to 88.3), 51.9 (20.9 to 106.9) and 33.7 (7.0 to 98.5) per 1000 person months for HPV 6, 11, 16 and 18. At the penis, overall clearance rate was 64.5 (13.3 to 188.5), 71.3 (14.7 to 208.2), 96.5 (31.3 to 225.3) and 333.3 (8.4 to 1857.2) per 1000 person months for HPV 6, 11, 16 and 18. For antibodies, overall clearance rate was 22.2 (9.6 to 43.7), 18.8 (3.9 to 55.0), 10.8 (0.3 to 60.1) and 19.0 (2.3 to 68.8) per 1000 person months. The overall clearance rates of HPV 6 at the anus and penis were lower than those of HPV 11, 16 and 18. The overall clearance rates of HPV 6, 11, 16 and 18 at the penis were higher than those at the anus. For a given genotype, the overall clearance rate of antibodies was lower than that of DNA. For HPV DNA at the anus, the definite clearance rates of HPV 6, 11, 16 and 18 ranged from 11.2 (0.3 to 62.6) to 28.0 (9.1 to 65.4) person months. For HPV DNA at the penis, the definite clearance rates of HPV 6, 11, 16 and 18 ranged from 19.3 (0.5 to 107.6) to 333.3 (8.4 to 1857.2) person months. Definite clearance of HPV 16 or 18 antibodies was not detected.
Figure S1 provides Kaplan-Meier curves illustrating the proportion of men who cleared anal and penile HPV 6, 11, 16, and 18 DNA, and antibodies during the follow-up period. As seen in the figure, there was no significant difference between clearance of the specific HPV genotypes DNA at the anus and penis as well as antibodies for HPV 11, 16 and 18. However, when comparing clearance of HPV 6 DNA at the anus and penis as well as antibodies, the Kaplan-Meier curves was significantly different among three groups (log-rank P=0.020). The Kaplan-Meier curve of HPV 6 antibodies clearance was significantly different with that of HPV 6 DNA at the anus (log-rank P=0.008) but not significantly different with that of HPV 6 DNA at the penis (log-rank P=0.447). Furthermore, there was no significant difference between clearance of the HPV 6 DNA at anus and at penis (log-rank P=0.447).
Correlates of HPV clearance
Table S1 presents the distribution of demographic characteristics and sexual behaviors among adolescent MSM who occurred clearance, and did not clear for any genotype of HPV DNA at the anus/penis or antibodies during 12 months. The only significant determinant for not clear any genotype of HPV DNA at the anus/penis or antibodies during 12 months in multivariable analyses, was ever had sex with female (adjusted odd ratio [aOR] = 3.23 95 % CI: 1.11 to 9.44).
Concordance between HPV DNA and antibodies clearance
The concordance between type-specific HPV DNA and antibodies clearance is shown in Table 2. The agreement between anal/penile HPV DNA clearance and antibodies clearance was −0.18 (95 % CI: −0.28 to 0.08)/-0.13 (−0.24 to −0.02), 0.04 (−0.29 to 0.36)/0.22 (−0.32 to 0.76), −0.10 (−0.27 to 0.08)/-0.14 (−0.37 to 0.10) and −0.14 (−0.28 to 0.01)/-0.14 (−0.33 to 0.06) for HPV 6, 11, 16 and 18, respectively.
Table 2.
Type-specific concordance between HPV DNA clearance and antibodies clearance among adolescent MSM.
| Antibodies cleared | Antibodies did not clear | Kappa (95 %CI) | |||
|---|---|---|---|---|---|
| HPV 6 | Anal | Cleared | 0 (0.0 %) | 6 (13.6 %) | −0.18 (−0.28 to 0.08) |
| Did not clear | 8 (18.2 %) | 30 (68.2 %) | |||
| Penile | Cleared | 0 (0.0 %) | 3 (7.7 %) | −0.13 (−0.24 to −0.02) | |
| Did not clear | 8 (20.5 %) | 28 (71.8 %) | |||
| HPV 11 | Anal | Cleared | 1 (3.4 %) | 7 (24.1 %) | 0.04 (−0.29 to 0.36) |
| Did not clear | 2 (6.9 %) | 19 (65.5 %) | |||
| Penile | Cleared | 1 (4.8 %) | 2 (9.5 %) | 0.22 (−0.32 to 0.76) | |
| Did not clear | 2 (9.5 %) | 16 (76.2 %) | |||
| HPV 16 | Anal | Cleared | 0 (0.0 %) | 7 (35.0 %) | −0.10 (−0.27 to 0.08) |
| Did not clear | 1 (5.0 %) | 12 (60.0 %) | |||
| Penile | Cleared | 0 (0.0 %) | 5 (35.7 %) | −0.14 (−0.37 to 0.10) | |
| Did not clear | 1 (7.1 %) | 8 (57.1 %) | |||
| HPV 18 | Anal | Cleared | 0 (0.0 %) | 3 (15.8 %) | −0.14 (−0.28 to 0.01) |
| Did not clear | 2 (10.5 %) | 14 (73.7 %) | |||
| Penile | Cleared | 0 (0.0 %) | 1 (9.1 %) | −0.14 (−0.33 to 0.06) | |
| Did not clear | 2 (18.2 %) | 8 (72.7 %) |
Discussion
Low clearance rates of HPV DNA at the anus and penis as well as antibodies among adolescent men recently engaged in sexual activity were observed in this cohort study. We found that the clearance rates of HPV DNA varied by different HPV genotypes and anatomical sites of infection. The clearance rates of HPV 6 DNA at the anus and penis were lower than those of HPV 11, 16 and 18. The clearance rates of HPV DNA at the anus were lower than that at the penis for HPV 6, 11, and 18. For a given HPV genotype, the clearance rates of HPV DNA at the anus and penis were higher than that of HPV antibodies. However, the difference of clearance rates between different HPV genotypes and sites of infection did not reach a statistical significance.
Although some studies have examined the clearance of anal HPV DNA among MSM[14], [28], [29], [30], [31], [32], [33], these studies were conducted among diverse study populations, using different duration and follow-up intervals and varying in definition of clearance[34]. Thus, it is difficult to compare clearance rates between studies. Nonetheless, the clearance rates for anal HPV 6, 11, 16, and 18 DNA in our study were lower than those reported by other studies[33], [34], [35], [36], [37]. An international pooled analysis by Wei and colleagues reported the clearance rate for HPV 16 at the anus among HIV-negative MSM was 95.5 (95 %CI: 86.0 to 106.0) person months[37]. Poynten and colleagues found that the clearance rates for HPV 16 and HPV 18 at the anus among Australia MSM with a median age of 49 years (IQR: 43–56) were 13.83 (95 %CI: 10.46 to 18.32) and 27.13 (95 %CI: 20.19 to 36.45) person years[36]. Furthermore, these studies have shown that HPV 16 is the genotype with the lowest rate of clearance[36], [37]. In contrast, we found anal HPV 6 had the lowest clearance rate (21.6 per 1000 person months) among MSM recently engaged in sexual activity. It might be explained that the previous studies included older MSM who had more sexual experience and more cumulative sexual partners. Zhou and colleagues reported that HPV 6 DNA at the anus was the genotype with lowest clearance rate among MSM with a median age of 27.3 (IQR: 24.0 to 32.8) years[33]. These results indicated that HPV 6 may be one of the most persistent genotypes among young MSM recently engaged in sexual activity, which could lead to a higher disease burden of anal warts.
In this study, although the clearance rates at the penis were higher than those at the anus for HPV 6, 11, and 18, the difference did not reach a statistical significance. Only one previous study on a cohort of HIV-negative MSM in Netherlands simultaneously followed the anal and penile HPV changes [30]. Mooij and colleagues reported similar patterns to our study in terms of the difference between penile and anal HPV DNA clearance. Mooij and colleagues found that for a given genotype of HPV, the clearance rate at the penis were higher than that at the anus[30]. Although no clear explanation for the difference in clearance between anal and penile HPV infection could be derived, it could be hypothesized that infection in keratinized epithelium of the penile shaft clears faster than mucosal infection in the anal canal[38].
In the current study, we found the naturally acquired antibodies against HPV 6, 11, 16, and 18 were stable among young MSM recently engaged in sexual activity during 12 months follow-up. However, the study on the duration of naturally acquired antibodies against HPV among MSM was limited, as only one existing study followed the dynamic changes of naturally acquired HPV antibodies among MSM[39]. Albeit multiple studies evaluated the immune response following HPV infection among MSM[40], [41], [42], [43], [44], [45], [46], they failed to evaluate the absence of serum antibodies after the initial infection in the form of follow-ups by specific time intervals, which hampered further comparison between different studies in terms of duration of HPV antibodies. The study conducted by Beachler and his colleagues focusing on HPV 16 had a similar conclusion with respect to the duration of HPV antibodies[39]. Beachler and his colleagues found that 94 % (111/118) of cases seropositive for HPV 16 at baseline remained such a status at month 12, while 90 % remained so at month 48[39]. The duration of follow up in our study was only 12 months, which limited our ability to detect real serum conversion of antibodies in the long run. Prolonged follow-ups should be considered in further studies to evaluate the longitudinal patterns of antibody titers and to explore whether natural antibodies could protect against subsequent anal or penile HPV infection among adolescent MSM.
This study is the first longitudinal investigation to evaluate the type-specific HPV DNA clearance at anal and penile sites and antibodies clearance among MSM recently engaged in sexual activity. Nevertheless, several limitations should be taken into account. First, although our study is one of the few studies on the natural history of HPV among young MSM entering sexual activity, the relatively small sample size may render our study to be statistically underpowered to detect difference in HPV clearance comparing various anatomical sites and HPV subtypes. However just as Noel T Brewer, chair of the U.S. National HPV Vaccination Roundtable noted, these transmission estimates are by the best we have for adolescent MSM and the best data for this population that we will have any time soon[47]. Second, the comparatively shorter period of follow up and missed samples at ninth months of this study may limit our ability to detect all clearance events. Previous studies have shown that HPV DNA infection clears spontaneously within 4–24 months, while serum antibodies takes longer to have its negative conversion observed. This is one of the important possible reasons that led to the significantly lower clearance rates observed in our study as compared to other research studies. Third, in this kind of epidemiologic studies it is impossible to distinguish true HPV clearance from viral latency completely. In our study, we reported definite clearance rates based on two consecutive negatives. Thus, we may have greater potential to distinguish clearance from viral latency than studies used a single negative result as their definition of clearance. The last but not the least, because of the methods of recruitment and rigors of study participation, the cohort presented here is likely to be a select population. Therefore, the estimates of HPV clearance rates should be generalized with caution.
In conclusion, we dynamically observed changes in the HPV anal, penile DNA and serum antibodies. The results showed the clearance rates of HPV 6, 11, 16 and 18 DNA at the anus and penis among adolescent MSM were low, while serum antibodies among adolescent MSM were stable. Considering observed low clearance rates of HPV in adolescent MSM prophylactic HPV vaccines should be given priority to young MSM, ideally before their sexual debut.
Authors' contributions
HZ conceived and designed this study. TT did the preliminary data analysis, prepared the figures, and wrote the first draft of the manuscript with edits from HZ. LF, WB, XZ, YF, YG, YS, and DJ critically reviewed the paper. All authors have read and approved the final report. All authors have contributed to the interpretation of data and study findings.
Funding
This study was supported by the Natural Science Foundation of China Excellent Young Scientists Fund [82022064], Natural Science Foundation of China International/Regional Research Collaboration Project [72061137001], the 14-th Five-Year Plan Distinctive Program of Public Health and Preventive Medicine in Higher Education Institutions of Xinjiang Uygur Autonomous Region. All funding parties did not have any role in the design of the study or in the explanation of the data.
CRediT authorship contribution statement
Tian Tian: Writing – original draft, Formal analysis, Data curation. Leiwen Fu: Writing – review & editing. Bingyi Wang: Writing – review & editing. Xinyi Zhou: Writing – review & editing. Yi-Fan Lin: . Yanxiao Gao: Writing – review & editing. Yuwei Li: . Yinghui Sun: Writing – review & editing. Jianghong Dai: Writing – review & editing. Huachun Zou: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the following organizations and individuals for their support: Minus18, ALSO Foundation, YAK, the Action Centre, Family Planning Victoria, queer clubs at the University of Melbourne, RMIT, Monash University, Deakin University, Latrobe University, Victoria University, Joy FM, MCV, Rainbow Network Victoria, Gay, and Lesbian Health Victoria, CAN Resource Centre, Prahran Market Clinic, Tim Read, Lenka Vodstrcil, David Lee, Matiu Bush, Mark Chung, Helen Henzell, Helen Kent, Julie Silvers, staff at the Melbourne Sexual Health Centre, Ed Yap, David Towl, Greg Adkins, Amanda Grattan (MSD) and Merck Australia, TaNisha Evans, Sneha Kishnani, and Houda Abdo.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100551.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- 1.Serrano B., et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Alemany L., et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanofsky V.R., Patel R.V., Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5(6):25–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y., et al. Human papillomavirus prevalence among men who have sex with men in China: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2021;40(7):1357–1367. doi: 10.1007/s10096-021-04229-y. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza G., et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48(4):491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18(2):198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford G.M., et al. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int J Cancer. 2021;148(1):38–47. doi: 10.1002/ijc.33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bown E., et al. Cancers of the anal canal: diagnosis, treatment and future strategies. Future Oncol. 2014;10(8):1427–1441. doi: 10.2217/fon.14.23. [DOI] [PubMed] [Google Scholar]
- 9.Tinmouth J., et al. Progression from perianal high-grade anal intraepithelial neoplasia to anal cancer in HIV-positive men who have sex with men. Dis Colon Rectum. 2016;59(9):836–842. doi: 10.1097/DCR.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 10.Park I.U., Introcaso C., Dunne E.F. Human papillomavirus and genital warts: A review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2015;61(Suppl 8):S849–S855. doi: 10.1093/cid/civ813. [DOI] [PubMed] [Google Scholar]
- 11.Egawa N., et al. Human papillomaviruses; Epithelial tropisms, and the development of neoplasia. Viruses. 2015;7(7):3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebner C.M., Laimins L.A. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16(2):83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- 13.van Aar F., et al. Twelve-month incidence and clearance of oral HPV infection in HIV-negative and HIV-infected men who have sex with men: the H2M cohort study. BMC Infect Dis. 2014;14:668. doi: 10.1186/s12879-014-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yunihastuti E., et al. Incidence, clearance, persistence and factors related with high-risk anal HPV persistence in South-East Asian MSM and transgender women. AIDS. 2020;34(13):1933–1941. doi: 10.1097/QAD.0000000000002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliano A.R., et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377(9769):932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillner J. The serological response to papillomaviruses. Semin Cancer Biol. 1999;9(6):423–430. doi: 10.1006/scbi.1999.0146. [DOI] [PubMed] [Google Scholar]
- 17.Carter J.J., et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 18.Carter J.J., et al. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61(5):1934–1940. [PubMed] [Google Scholar]
- 19.Opalka D., et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10(1):108–115. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vriend H.J., et al. Patterns of human papillomavirus DNA and antibody positivity in young males and females, suggesting a site-specific natural course of infection. PLoS One. 2013;8(4):e60696. doi: 10.1371/journal.pone.0060696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B., et al. Human papillomavirus (HPV) 6, 11, 16, and 18 seroprevalence is associated with sexual practice and age: results from the multinational HPV Infection in Men Study (HIM Study) Cancer Epidemiol Biomarkers Prev. 2011;20(5):990–1002. doi: 10.1158/1055-9965.EPI-10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou H., et al. Antibody responses following incident anal and penile infection with human papillomavirus in teenage men who have sex with men. Int J Cancer. 2016;139(3):639–646. doi: 10.1002/ijc.30093. [DOI] [PubMed] [Google Scholar]
- 23.Zou H., et al. Site-specific human papillomavirus infection in adolescent men who have sex with men (HYPER): an observational cohort study. Lancet Infect Dis. 2015;15(1):65–73. doi: 10.1016/S1473-3099(14)70994-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., et al. Natural history of anal papillomavirus infection in hiv-negative men who have sex with men based on a markov model: A 5-year prospective cohort study. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.891991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou H., et al. Early acquisition of anogenital human papillomavirus among teenage men who have sex with men. J Infect Dis. 2014;209(5):642–651. doi: 10.1093/infdis/jit626. [DOI] [PubMed] [Google Scholar]
- 26.Dias D., et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12(8):959–969. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou H., et al. Sexual behaviors and risk for sexually transmitted infections among teenage men who have sex with men. J Adolesc Health. 2014;55(2):247–253. doi: 10.1016/j.jadohealth.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Silva R.J., et al. Persistence and clearance of HPV from the penis of men infected and non-infected with HIV. J Med Virol. 2011;83(1):127–131. doi: 10.1002/jmv.21950. [DOI] [PubMed] [Google Scholar]
- 29.Nadar H.J., et al. Incidence and clearance of penile high-risk human papillomavirus infection and their determinants among HIV-negative men who have sex with men. Sex Transm Dis. 2021;48(11):864–872. doi: 10.1097/OLQ.0000000000001455. [DOI] [PubMed] [Google Scholar]
- 30.Mooij S.H., et al. The effect of HIV infection on anal and penile human papillomavirus incidence and clearance: A cohort study among MSM. AIDS. 2016;30(1):121–132. doi: 10.1097/QAD.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 31.Donà M.G., et al. Anal human papillomavirus in HIV-uninfected men who have sex with men: incidence and clearance rates, duration of infection, and risk factors. Clin Microbiol Infect. 2016;22(12):1004.e1–1004.e7. doi: 10.1016/j.cmi.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Phanuphak N., et al. Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. J Acquir Immune Defic Syndr. 2013;63(4):472–479. doi: 10.1097/QAI.0b013e3182918a5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., et al. Incidence, persistence, and clearance of anal human papillomavirus among men who have sex with men in china: an observational cohort study. Pathogens. 2022;11(3) doi: 10.3390/pathogens11030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jongen V.W., et al. Estimating incidence rates of grouped HPV types: A systematic review and comparison of the impact of different epidemiological assumptions. Papillomavirus Res. 2019;8 doi: 10.1016/j.pvr.2019.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marra E., et al. Incidence and clearance of anal high-risk human papillomavirus infections and their determinants over 5 years among human immunodeficiency virus–negative men who have sex with men. Clin Infect Dis. 2018;68(9):1556–1565. doi: 10.1093/cid/ciy738. [DOI] [PubMed] [Google Scholar]
- 36.Poynten I.M., et al. HIV, immune dysfunction, and the natural history of anal high-risk human papillomavirus infection in gay and bisexual men. J Infect Dis. 2021;224(2):246–257. doi: 10.1093/infdis/jiaa723. [DOI] [PubMed] [Google Scholar]
- 37.Wei F., et al. Incidence and clearance of anal human papillomavirus infection in 16 164 individuals, according to HIV status, gender, and male sexuality: an international pooled analysis of 34 longitudinal studies. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gheit T. Mucosal and cutaneous human papillomavirus infections and cancer biology. Front Oncol. 2019;9:355. doi: 10.3389/fonc.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beachler D.C., et al. An examination of HPV16 natural immunity in men who have sex with men (MSM) in the HPV IN Men (HIM) study. Cancer Epidemiol Biomarkers Prev. 2018;27(4):496–502. doi: 10.1158/1055-9965.EPI-17-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beachler D.C., et al. Natural acquired immunity against subsequent genital human papillomavirus infection: A systematic review and meta-analysis. J Infect Dis. 2016;213(9):1444–1454. doi: 10.1093/infdis/jiv753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellsagué X., et al. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of patricia. J Infect Dis. 2014;210(4):517–534. doi: 10.1093/infdis/jiu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safaeian M., et al. Risk of HPV-16/18 infections and associated cervical abnormalities in women seropositive for naturally acquired antibodies: pooled analysis based on control arms of two large clinical trials. J Infect Dis. 2018;218(1):84–94. doi: 10.1093/infdis/jiy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosillon D., et al. Risk of newly detected infections and cervical abnormalities in adult women seropositive or seronegative for naturally acquired HPV-16/18 antibodies. Cancer Med. 2019;8(10):4938–4953. doi: 10.1002/cam4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson L., et al. Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis. 2014;210(3):448–455. doi: 10.1093/infdis/jiu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao X., et al. Naturally acquired HPV antibodies against subsequent homotypic infection: A large-scale prospective cohort study. Lancet Reg Health West Pac. 2021;13 doi: 10.1016/j.lanwpc.2021.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mooij S.H., et al. No evidence for a protective effect of naturally induced HPV antibodies on subsequent anogenital HPV infection in HIV-negative and HIV-infected MSM. J Infect. 2014;69(4):375–386. doi: 10.1016/j.jinf.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Brewer N.T., Calo W.A. HPV transmission in adolescent men who have sex with men. Lancet Infect Dis. 2015;15(1):8–9. doi: 10.1016/S1473-3099(14)71019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.