Abstract
Background/Aim
The soluble interleukin‐2 receptor (sIL‐2R) serve as a valuable biomarker for tumors in human patients, as its levels increase during the activation of T lymphocytes in clinical states such as inflammation, infection, and tumor. This study aimed to demonstrate that sIL‐2R levels can be also elevated in dogs with tumors and evaluate its applicability as a diagnostic and prognostic factor in canine cancer patients.
Patients and Methods
Serum was collected from 6 healthy dogs and 34 dogs with solid tumors. The concentration of sIL‐2R was measured using a commercial enzyme‐linked immunosorbent assay kit.
Results
The median sIL‐2R concentration was significantly higher in dogs with solid masses than in healthy dogs (117.3 vs 68.33 pg/ml, p = 0.016). The highest median sIL‐2R concentration was found in dogs with malignant tumors, followed by those with benign tumors, and healthy dogs (119.6 vs 93.74 vs 68.33 pg/ml, respectively). In dogs with malignant tumors, the mortality rate was significantly higher in the group with high sIL‐2R levels than in the group with low sIL‐2R levels. Dogs with solid tumors, particularly those with malignant tumors, had higher concentrations of sIL‐2R than healthy dogs. Among dogs with malignant tumors, a correlation between sIL‐2R concentration and mortality rate was confirmed.
Conclusion
Serum sIL‐2R levels may be used to detect malignant tumors and serve as a prognostic factor in dogs with malignant tumors.
Keywords: dog, malignant tumour, soluble interlukin‐2 receptor, tumour biomarker
This study found that serum soluble interleukin‐2 receptor (sIL‐2R) levels are significantly higher in dogs with solid tumours compared to healthy dogs, with the highest levels in those with malignant tumours. Additionally, dogs with malignant tumours and high sIL‐2R levels had a higher mortality rate than those with lower levels. Thus, sIL‐2R concentration can be used as a diagnostic and prognostic marker for malignant tumours in dogs.

1. INTRODUCTION
Cancer is a leading cause of death in companion animals. In the United States, more than 165 million companion animals are diagnosed with cancer annually, and at least 4 million dogs and cats each are diagnosed with cancer annually (Withrow et al., 2013). Many spontaneous tumour types are found in companion animals, including mammary tumours, osteosarcoma, hemangiosarcoma, lung cancer, skin cancer, prostate cancer and gastrointestinal cancers (Pinho et al., 2012).
In veterinary medicine, it is difficult to diagnose cancer at an early stage; performing a biopsy, even after the cancer is detected, is challenging because of the cost and risk associated with anaesthesia. Therefore, biomarkers identified in the blood, urine and other body fluids of veterinary cancer patients will play an important role in tumour diagnosis, staging, prognosis and treatment‐response monitoring (Brawley & Kramer, 2005; Colombe et al., 2022). In addition, as it is difficult to elicit symptoms directly in animals, the detection of asymptomatic cancer using biomarkers has the potential to improve morbidity and reduce cancer‐related mortality in animals with advanced disease (Henry, 2010).
Interluekin‐2 (IL‐2) plays an important role in generating and regulating the immune response (DA et al., 1976). IL‐2 binds to a specific plasma membrane receptor, interleukin‐2 receptor (IL‐2R), on newly synthesized T lymphocytes (Morris & Waldmann, 2000), and comprises three components: the alpha, beta and gamma chains (Murakami, 2004). A portion of the alpha chain of IL‐2R is released from the affinity membrane receptor and detected in the bloodstream as serum soluble IL‐2R (sIL‐2R) (Murakami, 2004). In human medicine, sIL‐2R concentration is known to be elevated in patients with most types of lymphoid neoplasms (Goto et al., 2005); solid tumours and reactive conditions (Lissoni et al., 1990), such as severe inflammation, infections and immune‐mediated diseases (Correia et al., 2002; Heaney & Golde, 1998; Tartour et al., 2001; Witkowska, 2005). In particular, the blood level of sIL‐2R is elevated in almost all types of solid cancers, including digestive tract (Murakami et al., 2002; Wang et al., 2000), reproductive system (Gebauer et al., 1999; Klein et al., 1995), endocrine system (Mariotti et al., 1994), lung (Yano et al., 1996) and head and neck tumours (Murakami, 2004; Tartour et al., 1997).
The goal of this study was to identify serum factors that are significantly elevated in veterinary patients with solid tumours, which could be used for diagnosis, prognosis and eventually as markers of treatment response.
2. MATERIALS AND METHODS
2.1. Patient selection and diagnosis
Serum and plasma samples were collected from 6 healthy dogs and 34 dogs with solid tumours. The healthy group comprised five dogs that visited our animal hospital as blood donors and one dog that underwent a medical examination. Physical, blood and urine examinations were conducted in all six dogs and the results were normal, with no underlying diseases or medications being taken. The tumour group consisted of 34 dogs diagnosed with various types of solid tumours. The diagnosis of solid tumours was based on clinical signs, physical examination, blood examination and imaging (radiography and ultrasonography); 19 dogs had tumours confirmed by histological examination, 8 dogs had tumours confirmed by cytology through find needle aspiration (FNA), and 4 dogs had tumours confirmed by the BRAF test. Seven of the eight dogs that underwent fine needle aspiration (FNA) testing were examined by a pathologist who examined the cell morphology and assessed the degree of malignancy to make a diagnosis. The remaining one was diagnosed by a local animal hospital that sent the FNA sample to other pathologist. These confirmatory tests classified 6 dogs as having benign tumours and 25 dogs as having malignant tumours. In the remaining three dogs, the type of tumour could not be confirmed because of limited access owing to the location of the tumour or restricted examination upon the owner's request. However, a presumptive diagnosis of high malignancy was made based on clinical symptoms, imaging and a short median survival time (MST), and these dogs were included in the malignant tumour group.
2.2. Serum or plasma sample collection
Serum and plasma samples were collected with the owner's consent at our animal hospital from 2022 to 2023. Serum or plasma was collected from the jugular or cephalic vein using a 5‐mL serum separating tube (Vacutainers SST tube, Becton Dickinson) and heparin tube (Heparin tube 0.5 mL, FUJIFILM), respectively. The tubes were centrifuged at 4000 rpm for 2–3 min, and the serum or plasma was stored at −70°C until use.
2.3. Enzyme‐linked immunosorbent assay (sIL‐2R)
The sIL‐2R concentration was measured using a canine‐specific commercial enzyme‐linked immunosorbent assay kit (MyBioSource Inc.; Cat# MBS011881), according to the manufacturer's protocol. Briefly, the serum and plasma samples and standard solutions were incubated at 37°C with biotin antibody and horseradish peroxidase–avidin. Tetramethylbenzidine substrate was added, and the mixture was incubated at 37°C for 30 min. The optical density was measured at 450 nm using an Epoch microplate spectrophotometer (BioTek Instruments), and the sIL‐2R protein concentration was estimated by back‐calculation according to the standard curve.
2.4. Statistical analysis
The Mann–Whitney U test was used to compare the difference in the concentration of sIL‐2R between the control and solid tumour groups and the difference in death and survival in the malignant tumour group. The Kruskal–Wallis test was used to compare the control, benign and malignant tumour groups. The survival percentages of dogs with high and low sIL‐2R concentrations were analysed using Kaplan–Meier curves. The correlation between sIL‐2R and survival time was analysed using the Pearson R‐test and the p values and correlation coefficients were calculated. A p value <0.05 indicated a statistically significant difference. All statistical analyses were conducted using GraphPad Prism software (version 9.3.1; GraphPad Inc.).
3. RESULTS
3.1. Patient selection
The results for the healthy and tumour groups are presented in Table 1. This experiment involved 34 dogs with solid tumours and 6 healthy dogs. In the solid tumour group, 28 dogs had malignant tumours, including 3 dogs with provisional diagnoses in whom a definitive diagnostic test was not conducted; the remaining 6 had benign tumours. The median ages of the dogs in the malignant and benign groups were 11 years (mean, 11.6 years; range, 5–17 years) and 12 years (mean, 11.5 years; range, 7–14 years), respectively. In the malignant group, there were 17 (60.7%) female spayed dogs and 11 (39.3%) male castrated dogs, and in the benign group, there were 3 (50%) female spayed dogs, 2 (33.3%) male castrated dogs and 1 (16.6%) intact female dog. Among the 25 dogs with a definitive diagnosis in the malignant tumour group, 14 (56%) were diagnosed with carcinoma, 6 (24%) with sarcoma and 5 (20%) with other types (mast cell tumour, n = 2; melanoma, n = 2 and carotid body tumour, n = 1). Among the three dogs with only provisional diagnoses included in the malignant tumour group, the first dog had a cardiac tumour in the right atrium, developed a persistent hemorrhagic pleural effusion that required continuous thoracentesis and blood transfusions, had a suspected cardiac hemangiosarcoma and died 56 days after the diagnosis; the second dog had a suspected nasal adenocarcinoma, developed hypercalcaemia and died 65 days after the diagnosis; and the last dog had suspected prostate tumour on ultrasound, was confirmed to have severe anaemia with haematuria and died 27 days after the diagnosis.
TABLE 1.
Signalment and characteristics of 34 dogs with solid tumour and 6 control dogs.
| Type of tumour | Number | Age (years) (median, range) | Sex | Body weight (kg) (median, range) | Breed |
|---|---|---|---|---|---|
| Malignant tumour | |||||
| Apocrin gland adenocarcinoma | 1 | 5 | MC | 22.6 | Siberian husky |
| Buccal osteosarcoma | 1 | 14 | MC | 11.65 | Schnauzers |
| Cardiac tumour a | 1 | 15 | MC | 3.36 | Schnauzers |
| Carotid body tumour | 1 | 10 | FS | 5.28 | Poodle |
| Cholangiocarcinoma | 1 | 13 | MC | 4.67 | Mixed |
| Hepatic hemangiosarcoma | 2 | 10.5 (10–11) | MC (2) | 12.25 (11.3–13.2) | Spitz (2) |
| Hepatocellular carcinoma | 2 | 13 (12–14) | MC (1), FS (1) | 3.06 (2.34–3.79) | Maltese (1), Yorkshire terrier (1) |
| Intestinal adenocarcinoma | 1 | 10 | MC | 4.98 | Cocker spaniel |
| Mammary gland adenocarcinoma | 1 | 11 | FS | 9.08 | Schnauzers |
| Mast cell tumour | 2 | 10.5 (10–11) | FS (2) | 4.06 (4.03–4.09) | Poodle (1), Maltese (1) |
| Melanoma | 2 | 13.5 (11–16) | MC (1), FS (1) | 14.17 (3.94–24.2) | Labrador retriever (1), Yorkshire terrier (1) |
| Nasal tumour a | 1 | 12 | FS | 3.98 | Mixed |
| Parathyroid carcinoma | 2 | 12.5 (11–14) | MC (2) | 9.33 (3.86–14.8) | Maltese (1), Beagle (1) |
| Prostatic carcinoma | 2 | 14 (11–17) | MC (2) | 4.86 (3.72–6) | Poodle (1), Maltese (1) |
| Prostatic tumour a | 1 | 17 | MC | 5.18 | Pekingese |
| Pulmonary carcinoma | 1 | 7 | FS | 11.7 | Cocker spaniel |
| Renal cell carcinoma | 1 | 10 | MC | 3.67 | Maltese |
| Splenic hemangiosarcoma | 2 | 8.5 (8–9) | MC (1), FS (1) | 39.85 (27.2–52) | Standard poodle (1), Labrador retriever (1) |
| Subcutaneous hemangiosarcoma | 1 | 12 | FS | 8.16 | Maltese |
| Urinary bladder transitional cell carcinoma | 2 | 13 (12–14) | FS (2) | 4.07 (3.2–4.95) | Chihuahua (1), Maltese (1) |
| Benign tumour | |||||
| Gastric adenoma | 1 | 14 | MC | 3.1 | Maltese |
| Gastric leiomyoma | 1 | 11 | FS | 2.76 | Maltese |
| Hepatocellular adenoma | 1 | 13 | FS | 2.75 | Maltese |
| Mammary gland adenoma | 1 | 7 | F | 2.3 | Yorkshire terrier |
| Thyroid adenoma | 1 | 11 | MC | 9.9 | French bulldog |
| Urinary bladder polyp | 1 | 13 | FS | 2.61 | Maltese |
| Healthy | 6 | 4.5 (2–10) | MC (4), FS (2) | 27.7 (4–35.2) | Labrador retriever (1), Golden retriever (1), Maltese (1), Doberman (1), Pit bull terrier (1), Kane corso (1) |
This dogs were tentatively diagnosed to malignant tumour because of their clinical signs and imaging examination seen malignancy and their short MST.
3.2. Concentration of sIL‐2R in control and dogs with solid tumour
The sIL‐2R concentration was 77.63 (62.3–119.6) pg/mL in 6 healthy dogs and 150.55 (64.3–575.94) pg/mL in 34 dogs with solid tumours. There was a significant difference between the control and solid tumour groups, with a p value 0.016 (Figure 1).
FIGURE 1.
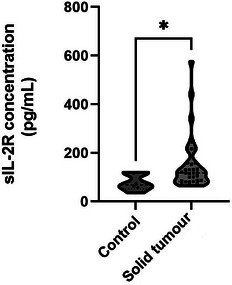
Soluble interluekin‐2 receptor (sIL‐2R) concentration in control dogs and dogs with solid masses. The sIL‐2R concentration was significantly higher in dogs with solid masses than in the control group (median concentration, 117.3 vs. 68.33 pg/mL, p = 0.016).
3.3. Elevation sIL‐2R in malignant tumour dogs compared to benign tumour
Within the solid tumour group, the sIL‐2R concentration of the 28 dogs with malignant tumours was 159.4 (64.3–575.95) pg/mL and that in dogs with benign tumours was 93.74 (64.3–158.42) pg/mL, showing a numerical difference but no statistical significance. In contrast, the sIL‐2R concentrations in the malignant tumour and control groups showed a statistically significant difference, with a p value of 0.045. The sIL‐2R concentrations in the benign tumour and the control groups showed a numerical difference, but no statistical significance (Figure 2).
FIGURE 2.
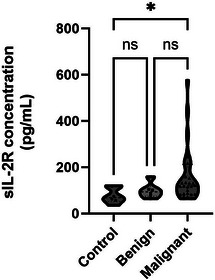
Difference in soluble interluekin‐2 receptor (sIL‐2R) concentrations in benign and malignant tumours in dogs. When the solid tumour group was classified into malignant and benign, there was a significant difference in the sIL‐2R concentration between the malignant tumour and the control groups (median concentration, 119.6 vs. 68.33 pg/mL, p = 0.045). Although not statistically significant, the malignant tumour group had the highest median concentration, followed by the benign tumour group and control group (119.6 vs. 93.74 vs. 68.33 pg/mL).
3.4. Analysis of correlation of sIL‐2R and survival time
To divide high and low sIL‐2R concentration groups in dogs with malignant tumours, 114 pg/mL, which is the upper limit of the 95% confidence interval for the control group, was used as the cut‐off value. When dogs in the malignant tumour group were divided into low and high sIL‐2R concentration subgroups based on a cut‐off value of 114 pg/mL, there were 18 dogs in the high sIL‐2R subgroup and 10 dogs in the low sIL‐2R subgroup. Dogs with higher serum sIL‐2R levels had shorter survival times (Figure 3). The overall survival at 300 days was 80% (8/10) in dogs with low serum sIL‐2R concentrations; the survival rate decreased to 16.6% (3/18) in dogs with high serum sIL‐2R concentrations; and the MST for the 15 deaths of high serum sIL‐2R concentration group was 43 days.
FIGURE 3.
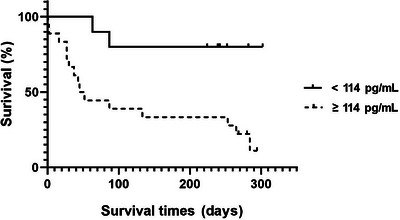
When the dogs with malignant tumours were divided into high and low soluble interluekin‐2 receptor (sIL‐2R) concentration subgroups based on a cut‐off value of 114 pg/mL, Kaplan–Meier survival plots for dogs with malignant tumours showed that the overall survival at 300 days was 80% (8/10) in dogs with low serum sIL‐2R concentrations; survival decreased to 16.6% (3/15) in patients with high serum sIL‐2R concentrations (114 pg/mL was selected as the cut‐off value of sIL‐2R because it corresponded to the upper limit of the 95% confidence interval of the control group).
Of the 17 malignant tumour dogs that died, 11 died in the hospital or were reported dead by their owners, but 6 were presumed dead because they did not return to the hospital afterwards. Of the 11 dogs that certainly died, 10 died of tumour complications and 1 died at home with the cause of death unknown. The survival time for the six presumed deceased dogs was calculated by extrapolating from the date of sample collection to the date of no follow‐up monitoring.
Moreover, a significant correlation (p = 0.0498) was observed between sIL‐2R concentration and survival time, with a correlation coefficient (r) of −0.3741; the goodness‐of‐fit (R 2) was 0.1400 (Figure 4).
FIGURE 4.
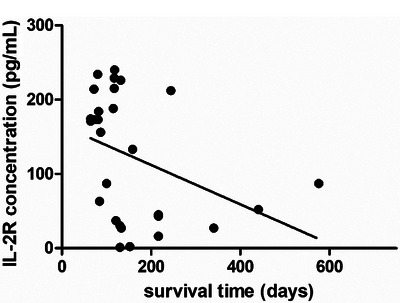
The correlation analysis between soluble interluekin‐2 receptor (sIL‐2R) and days after in malignant tumour dogs (r = −0.3741, goodness‐of‐fit R 2 = 0.1400, p = 0.0498).
4. DISCUSSION
In human medicine, serum sIL‐2R levels reflect the total number of activated T lymphocytes present in cancerous tissue or in organs to which the cancer has metastasized (Bien & Balcerska, 2008). As a result, elevated serum sIL‐2R levels are commonly observed in most malignant tumours compared to the levels in normal controls (Nakase et al., 2005; Orditura et al., 1998). Many studies have used this parameter not only for diagnosis but also for assessing disease stage and monitoring disease progression during post‐treatment follow‐up (Tartour et al., 2001). However, in veterinary medicine, there is little research on the relationship between sIL‐2R and tumours. As sIL‐2R reflects the activity of T lymphocytes, there are some studies on using sIL‐2R for the diagnosis or prognosis of tumours of the haematopoietic system, such as lymphoma (Im et al., 2023; Mizutani et al., 2020). However, there is only one study on solid tumours where sIL‐2R concentrations correlated with a mast cell tumour in dogs (Meyer et al., 2013).
In this study, sIL‐2R levels were significantly higher in the solid tumour group than in the control group. In addition, when comparing the concentration of sIL‐2R in the malignant and benign tumour groups within the solid tumour group, the malignant tumour group had a significantly higher concentration, whereas the benign tumour group and the normal group did not show a significant difference. Therefore, there may be an association between the malignancy of the tumour and concentration of sIL‐2R. Thus, it may be possible to use sIL‐2R concentrations for the diagnosis of tumours in dogs as well as humans. Furthermore, sIL‐2R levels can also be used to diagnose malignancy in cases where a solid tumour is identified by physical examination or imaging and advanced tests such as cytology or histopathology are difficult. However, there was no significant difference according to the type of malignancy whether sarcoma or carcinoma (Figure S2) and further research is required.
In addition, when patients with malignant tumours were classified into low and high sIL‐2R concentration subgroups with a cut‐off value of 114 pg/mL, the mortality rate was significantly higher in the high sIL‐2R concentration subgroup. In the subgroup with high sIL‐2R concentrations, 15 of the 18 dogs died and their MST was only 43 days. In contrast, in the subgroup with low sIL‐2R concentrations, 8 of the 10 dogs survived, indicating that even in dogs with the same malignancy, the prognosis varies depending on the sIL‐2R concentration. Therefore, there was a clear correlation between the sIL‐2R concentration and prognosis, confirming that the sIL‐2R concentration can be used as a prognostic factor.
In one patient, serial measurements showed that sIL‐2R concentrations increased as the dog approached the date of death. A dog was diagnosed with hepatic hemangiosarcoma, and its survival time was 178 days after the diagnosis; sIL‐2R concentrations were measured on days 80 and 171 after the diagnosis and were confirmed to be 132.1 and 216.4 pg/mL, respectively, with higher sIL‐2R concentrations closer to the date of death. When the first sIL‐2R level was measured, the dog received chemotherapy with alternating doses of doxorubicin and carboplatin every 3 weeks. Subsequently, the dog's condition deteriorated; chemotherapy was discontinued on day 118 after the diagnosis; and a second sIL‐2R concentration was measured a week before death, at which time she was not receiving chemotherapy (Figure S1). Although more cases need to be studied, we confirmed the possibility of using sIL‐2R as a prognostic factor for response to chemotherapy in one dog.
There are many studies on the increased concentration of sIL‐2R in malignant tumours, but the underlying biological mechanism of increased sIL‐2R levels in serum and other body fluids is not clearly defined (Bien & Balcerska, 2008; Rimoldi et al., 1993). In solid tumours, the mechanism of elevated sIL‐2R is known to be more complex than in haematopoietic tumours (Bien & Balcerska, 2008). There are hypotheses that propose an increase in IL‐2R expression due to the proliferation of activated normal lymphocytes in response to tumour growth (Rimoldi et al., 1993), as well as the hypothesis suggesting that the release of sIL‐2R is attributed to activated lymphocytes infiltrating the tumour tissue, rather than an increase in lymphocytes in peripheral blood (Trentin et al., 1994). Regardless of which hypothesis causes sIL‐2R to be elevated, the concentration of sIL‐2R shows a strong correlation with T lymphocyte activity. Similarly, in veterinary medicine, the elevation of sIL‐2R concentration is believed to reflect the activity of T lymphocytes in tumour patients (Pawlaczyk & Sobieska, 2006), which can be used for the diagnosis of tumours, evaluation of treatment response and prognosis assessment, similar to its applications in human medicine (Boyano et al., 2000; Kawashima et al., 2000; Rutkowski et al., 2002).
This study is the first to show that sIL‐2R concentrations are significantly increased in dogs with solid tumours and revealed a clear correlation among sIL‐2R concentrations, tumour malignancy and survival time. Although the number of cases was limited, we confirmed that sIL‐2R can be used to assess treatment responsiveness and prognosis. However, further research is required to gather more data and validate these findings.
AUTHOR CONTRIBUTIONS
Hyun NamKung: Conceptualization; data curation; writing original draft. Su‐Min Park: Conceptualization; data curation; investigation; supervision; writing review and editing. Jae‐Hyeon Im: Data curation; methodology. Ga‐Hyun Lim: Data curation; formal analysis; methodology. Min‐Ok Ryu: Supervision; writing editing. Kyoung‐Won Seo: Supervision. Hwa‐Young Youn: Conceptualization; project administration; supervision.
CONFLICT OF INTEREST STATEMENT
All authors associated with this document have disclosed any potential conflicts of interest.
FUNDING INFORMATION
None.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data. The study was approved by the Institutional Animal Care and Use Committee (IACUC) (approval number SNU‐220120‐3‐3).
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/vms3.70033.
Supporting information
Figure S1 In one patient with hepatic hemangiosarcoma, serial measurements showed an increase in sIL‐2R concentrations as the dog approached the date of death. The dog's survival time was 178 days after diagnosis. sIL‐2R concentrations were measured at 132.1 pg/mL on day 80 and 216.4 pg/mL on day 171 after diagnosis, indicating higher levels closer to the date of death. When the first sIL‐2R level was measured, the dog was receiving chemotherapy with alternating doses of doxorubicin and carboplatin every 3 weeks. However, the dog's condition deteriorated, and chemotherapy was discontinued on day 118. The second sIL‐2R concentration was measured a week before death, at which time the dog was no longer receiving chemotherapy.
Figure S2 Comparison of sIL‐2R between sarcoma and carcinoma tumour dogs. There was no significant difference in sIL‐2R levels between dogs with sarcoma and dogs with carcinoma (median concentration 117.3 vs. 116.6 pg/mL, p = 0.773).
ACKNOWLEDGEMENTS
We are very thankful to the Research Institute for Veterinary Science of Seoul National University and the BK21 PLUS Program for Creative Veterinary Science Research.
NamKung, H. , Park, S.‐M. , Im, J.‐H. , Lim, G.‐H. , Ryu, M.‐O. , Seo, K.‐W. , & Youn, H.‐Y. (2024). Evaluation serum soluble interleukin 2 receptor with diagnosis and prognosis in canine solid tumour: 34 cases. Veterinary Medicine and Science, 10, 1–7. 10.1002/vms3.70033
Hyun NamKung and Su‐Min Park are co‐first authors and contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- Bien, E. , & Balcerska, A. (2008). Serum soluble interleukin 2 receptor α in human cancer of adults and children: A review. Biomarkers, 13(1), 1–26.. [DOI] [PubMed] [Google Scholar]
- Boyano, M. D. , Garcia‐Vázquez, M. D. , López‐Michelena, T. , Gardeazabal, J. , Bilbao, J. , Cañavate, M. L. , Galdeano, A. G. , Izu, R. , Díaz‐Ramón, L. , Raton, J. A. , & Díaz‐Pérez, J. L. (2000). Soluble interleukin‐2 receptor, intercellular adhesion molecule‐1 and interleukin‐10 serum levels in patients with melanoma. British Journal of Cancer, 83(7), 847–852.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley, O. W. , & Kramer, B. S. (2005). Cancer screening in theory and in practice. Journal of Clinical Oncology, 23(2), 293–300.. [DOI] [PubMed] [Google Scholar]
- Colombe, P. , Béguin, J. , Benchekroun, G. , & Le Roux, D. (2022). Blood biomarkers for canine cancer, from human to veterinary oncology. Veterinary and Comparative Oncology, 20(4), 767–777.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia, O. , Delgado, L. , Roujeau, J.‐C. , Le Cleach, L. , & Fleming‐Torrinha, J. A. (2002). Soluble interleukin 2 receptor and interleukin 1α in toxic epidermal necrolysis: A comparative analysis of serum and blister fluid samples. Archives of Dermatology, 138(1), 29–32.. [DOI] [PubMed] [Google Scholar]
- Da, M. , Ruscetti, F. W. , & Gallo, R. (1976). Selective in vitro growth of T lymphocytes from normal human bone marrows. Science, 193(4257), 1007–1008.. [DOI] [PubMed] [Google Scholar]
- Gebauer, G. , Rieger, M. , Jäger, W. , & Lang, N. (1999). Prognostic relevance of soluble interleukin‐2 receptors in patients with ovarian tumors. Anticancer Research, 19(4A), 2509–2511.. [PubMed] [Google Scholar]
- Goto, H. , Tsurumi, H. , Takemura, M. , Ino‐Shimomura, Y. , Kasahara, S. , Sawada, M. , Yamada, T. , Hara, T. , Fukuno, K. , Goto, N. , Okuno, M. , Takami, T. , Seishima, M. , & Moriwaki, H. (2005). Serum‐soluble interleukin‐2 receptor (sIL‐2R) level determines clinical outcome in patients with aggressive non‐Hodgkin's lymphoma: In combination with the International Prognostic Index. Journal of Cancer Research and Clinical Oncology, 131, 73–79.. [DOI] [PubMed] [Google Scholar]
- Heaney, M. L. , & Golde, D. W. (1998). Soluble receptors in human disease. Journal of Leukocyte Biology, 64(2), 135–146.. [DOI] [PubMed] [Google Scholar]
- Henry, C. J. (2010). Biomarkers in veterinary cancer screening: Applications, limitations and expectations. The Veterinary Journal, 185(1), 10–14.. [DOI] [PubMed] [Google Scholar]
- Im, J. H. , Park, S. M. , An, J. H. , Kim, T. H. , Chae, H. K. , Oh, Y. I. , Seo, K. W. , & Youn, H. Y. (2023). Evaluation of serum interleukin 2 receptor and beta‐2‐microglobulin as prognostic factors for canine lymphoma: A pilot study. Veterinary and Comparative Oncology, 21(2), 184–190.. [DOI] [PubMed] [Google Scholar]
- Kawashima, O. , Kamiyoshihara, M. , Sakata, S. , Endo, K. , Saito, R. , & Morishita, Y. (2000). The clinicopathological significance of preoperative serum‐soluble interleukin‐2 receptor concentrations in operable non‐small‐cell lung cancer patients. Annals of Surgical Oncology, 7, 239–245.. [DOI] [PubMed] [Google Scholar]
- Klein, B. , Levin, I. , Kfir, B. , Mishaeli, M. , Shapira, J. , & Klein, T. (1995). The significance of soluble interleukin‐2, soluble interleukin‐2 receptors, soluble ICAM‐1 and β2‐microglobulin in breast cancer patients. Tumor Biology, 16(5), 290–296.. [PubMed] [Google Scholar]
- Lissoni, P. , Barni, S. , Rovelli, F. , Viviani, S. , Maestroni, G. J. , Conti, A. , & Tancini, G. (1990). The biological significance of soluble interleukin‐2 receptors in solid tumors. European Journal of Cancer and Clinical Oncology, 26(1), 33–36. [DOI] [PubMed] [Google Scholar]
- Mariotti, S. , Barbesino, G. , Caturegli, P. , Marinò, M. , Manetti, L. , Fugazzola, L. , Pacini, F. , & Pinchera, A. (1994). Serum soluble interleukin 2 (IL‐2) receptor (sIL‐2R) in differentiated thyroid carcinoma. Journal of Endocrinological Investigation, 17(11), 861–867.. [DOI] [PubMed] [Google Scholar]
- Meyer, A. , Gruber, A. , & Klopfleisch, R. (2013). All subunits of the interleukin‐2 receptor are expressed by canine cutaneous mast cell tumours. Journal of Comparative Pathology, 149(1), 19–29.. [DOI] [PubMed] [Google Scholar]
- Mizutani, N. , Goto‐Koshino, Y. , Kurata, K. , Fujiwara‐Igarashi, A. , Sakaguchi, M. , Asada, M. , Ohno, K. , & Tsujimoto, H. (2020). Measurement of the concentration of serum soluble interleukin‐2 receptor alpha chain in dogs with lymphoma. Veterinary Immunology and Immunopathology, 225, 110054.. [DOI] [PubMed] [Google Scholar]
- Morris, J. C. , & Waldmann, T. A. (2000). Advances in interleukin 2 receptor targeted treatment. Annals of the Rheumatic Diseases, 59(Suppl 1), i109–i114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, S. (2004). Soluble interleukin‐2 receptor in cancer. Frontiers in Bioscience‐Landmark, 9(5), 3085–3090. [DOI] [PubMed] [Google Scholar]
- Murakami, S. , Sakata, H. , Tsuji, Y. , Okubo, K. , Hamada, S. , & Hirayama, R. (2002). Serum soluble interleukin‐2 receptor as a predictor of lymph node metastasis in early gastric cancer. Digestive Surgery, 19(1), 9–14.. [DOI] [PubMed] [Google Scholar]
- Nakase, K. , Tsuji, K. , Tamaki, S. , Tanigawa, M. , Ikeda, T. , Miyanishi, E. , & Shiku, H. (2005). Elevated levels of soluble interleukin‐2 receptor in serum of patients with hematological or non‐hematological malignancies. Cancer Detection and Prevention, 29(3), 256–259.. [DOI] [PubMed] [Google Scholar]
- Orditura, M. , De Vita, F. , Roscigno, A. , Auriemma, A. , Infusino, S. , & Catalano, G. (1998). Soluble interleukin‐2 receptor and soluble CD8 antigen levels in serum from patients with solid tumors. International Journal of Molecular Medicine, 2(1), 75–84.. [DOI] [PubMed] [Google Scholar]
- Pawlaczyk, M. , & Sobieska, M. (2006). A correlation between acute phase proteins and cytokines in patients suffering from mycosis fungoides. Acta Dermatovenerologica Alpina Pannonica et Adriatica, 15(3), 107–112. [PubMed] [Google Scholar]
- Pinho, S. S. , Carvalho, S. , Cabral, J. , Reis, C. A. , & Gärtner, F. (2012). Canine tumors: A spontaneous animal model of human carcinogenesis. Translational Research, 159(3), 165–172.. [DOI] [PubMed] [Google Scholar]
- Rimoldi, D. , Salvi, S. , Hartmann, F. , Schreyer, M. , Blum, S. , Zografos, L. , Plaisance, S. , Azzarone, B. , & Carrel, S. (1993). Expression of IL‐2 receptors in human melanoma cells. Anticancer Research, 13(3), 555–564.. [PubMed] [Google Scholar]
- Rutkowski, P. , Kaminska, J. , Kowalska, M. , Ruka, W. , & Steffen, J. (2002). Cytokine serum levels in soft tissue sarcoma patients: Correlations with clinico‐pathological features and prognosis. International Journal of Cancer, 100(4), 463–471.. [DOI] [PubMed] [Google Scholar]
- Tartour, E. , Deneux, L. , Mosseri, V. , Jaulerry, C. , Brunin, F. , Point, D. , Validire, P. , Dubray, B. , Fridman, W. H. , & Rodriguez, J. (1997). Soluble interleukin‐2 receptor serum level as a predictor of locoregional control and survival for patients with head and neck carcinoma: Results of a multivariate prospective study. Cancer: Interdisciplinary International Journal of the American Cancer Society, 79(7), 1401–1408. [PubMed] [Google Scholar]
- Tartour, E. , Mosseri, V. , Jouffroy, T. , Deneux, L. , Jaulerry, C. , Brunin, F. , Fridman, W. H. , & Rodriguez, J. (2001). Serum soluble interleukin–2 receptor concentrations as an independent prognostic marker in head and neck cancer. The Lancet, 357(9264), 1263–1264. [DOI] [PubMed] [Google Scholar]
- Trentin, L. , Zambello, R. , Bulian, P. , Cerutti, A. , Milani, A. , Pirone, E. , Nitti, D. , Agostini, C. , & Semenzato, G. (1994). Functional role of IL‐2 receptors on tumour‐infiltrating lymphocytes. British Journal of Cancer, 69(6), 1046–1051.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.‐S. , Chow, K.‐C. , Li, W.‐Y. , Liu, C.‐C. , Wu, Y.‐C. , & Huang, M.‐H. (2000). Clinical significance of serum soluble interleukin 2 receptor‐α in esophageal squamous cell carcinoma. Clinical Cancer Research, 6(4), 1445–1451.. [PubMed] [Google Scholar]
- Withrow, S. J. , Vail, D. M. , & Page, R. L. (2013). Withrow and MacEwen's small animal clinical oncology‐E‐book. Elsevier Health Sciences. [Google Scholar]
- Witkowska, A. M. (2005). On the role of sIL‐2R measurements in rheumatoid arthritis and cancers. Mediators of Inflammation, 2005(3), 121–130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, T. , Fukuyama, Y. , Yokoyama, H. , Takai, E. , Tanaka, Y. , Asoh, H. , & Ichinose, Y. (1996). Interleukin‐2 receptors in pulmonary adenocarcinoma tissue. Lung Cancer, 16(1), 13–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 In one patient with hepatic hemangiosarcoma, serial measurements showed an increase in sIL‐2R concentrations as the dog approached the date of death. The dog's survival time was 178 days after diagnosis. sIL‐2R concentrations were measured at 132.1 pg/mL on day 80 and 216.4 pg/mL on day 171 after diagnosis, indicating higher levels closer to the date of death. When the first sIL‐2R level was measured, the dog was receiving chemotherapy with alternating doses of doxorubicin and carboplatin every 3 weeks. However, the dog's condition deteriorated, and chemotherapy was discontinued on day 118. The second sIL‐2R concentration was measured a week before death, at which time the dog was no longer receiving chemotherapy.
Figure S2 Comparison of sIL‐2R between sarcoma and carcinoma tumour dogs. There was no significant difference in sIL‐2R levels between dogs with sarcoma and dogs with carcinoma (median concentration 117.3 vs. 116.6 pg/mL, p = 0.773).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


