Abstract
Therapy-related acute myeloid leukemia (t-AML) often exhibits adverse (genetic) features. There is ongoing discussion on the impact of t-AML on long-term outcome in AML. Therefore, we retrospectively analyzed clinical and biological characteristics of 1133 AML patients (225 t-AML patients and 908 de novo AML patients) with a median follow-up of 81.8 months. T-AML patients showed more adverse genetic alterations, higher age and more comorbidities as compared to de novo AML. Median OS in intensively treated t-AML patients was 13.7 months as compared to 39.4 months in de novo AML (p < 0.001). With non-intensive therapy, OS did not differ significantly (p = 0.394). With intensive therapy, significant differences in favor of de novo AML were observed in the ELN intermediate I/II (p = 0.009) and adverse (p = 0.016) risk groups but not within favorable risk groups (APL p = 0.927, ELN favorable p = 0.714). However, t-AML was no independent risk factor for OS (p = 0.103), RR (p = 0.982) and NRM (p = 0.320) in the multivariate analysis. A limitation of our study is an ELN 2010 risk stratification due to a lack of more comprehensive molecular data according to ELN 2022. We conclude that therapeutic algorithms in t-AML, in particular with regard to allo-HSCT, should be guided by ELN genetic risk rather than classification as t-AML alone. Our data support the WHO and ICC 2022 classifications, which include t-AML as diagnostic qualifier rather than a separate subcategory.
Subject terms: Epidemiology, Medical research
Introduction
Therapy-related AML (t-AML) is a myeloid neoplasm that evolves secondary to cytotoxic therapy (chemotherapy and/or radiotherapy) for malignant or non-malignant diseases due to DNA damage in hematopoietic progenitor cells. Approximately 5–15% of adult AML patients are reported to have t-AML [1–4]. Chemotherapy and/or radiotherapy may cause mutations, leading to clonal hematopoiesis with subsequent selection of resistant clones during AML therapy. Especially alkylating agents and topoisomerase-II-inhibitors have been associated with mutagenic effects, typically with different latency periods [5, 6]. Over the past decades, increasing numbers of multidrug combinations in cancer therapy and improved long-term survival in cancer patients have led to an increasing incidence of t-AML [1, 7–10]. Owing to its secondary nature, t-AML is frequently associated with molecular aberrations and clonal complexity at baseline, adverse cytogenetics and preceding therapy-induced sequelae, which altogether may result in a poorer outcome as compared to de novo AML [1, 2, 11–17]. In the literature, there are partially conflicting results with regard to the role of t-AML as an independent prognostic factor. However, many studies are small or performed within particular subgroups only. Higher age and adverse genetics in t-AML can be interpreted as being secondary features in t-AML that may fully explain inferior survival. On the other hand, confounding variables such as preceding therapies with associated toxicities and possibly yet unknown t-AML-specific genetic features might cause increased comorbidity and resistance to therapy and thus lead to impaired overall survival. This aspect seems to be particularly relevant for patients, who are eligible for intensive therapy including allogeneic hematopoietic stem cell transplantation (allo-HSCT). In the current WHO and ICC classifications published in 2022, t-AML is included as a „diagnostic qualifier“ in addition to genetically and morphologically defined subgroups of AML (i.e. “AML with KMT2A mutation post cytotoxic therapy”) [18, 19]. However, there is an ongoing discussion as to whether t-AML per se is associated with an inferior clinical outcome and whether there are particular clinical or biological factors that determine the prognosis in t-AML [1, 2, 13, 14, 20–22]. To address these questions, we performed a large retrospective study in 1133 AML patients with the aim to compare clinical and biological baseline characteristics as well as OS, NRM and RR in t-AML versus de novo AML and to identify further risk factors in t-AML.
Patients and methods
Study design and endpoints
1133 patients aged ≥18 years with newly diagnosed AML were treated at our center within the past two decades (January 1st, 1995–June 30th, 2018) and were eligible for this retrospective, non-interventional study. In our database, 20% (n = 225) had t-AML and 80% (n = 908) had de novo AML. T-AML was defined as secondary to previous cytotoxic therapy for solid cancer, hematologic malignancy or autoimmune disease [18, 19, 23]. In both groups, baseline characteristics, risk factors, remission rates, OS, RR and NRM were analyzed and compared between t-AML and de novo AML. OS, RR and NRM were defined as clinical endpoints by applying the Cheson criteria [24, 25] and the response criteria of the European Society for Blood and Bone Marrow Transplantation. Patients were further stratified with regard to type of administered therapy (intensive vs. non-intensive) and age (<60 years vs. ≥60 years). In patients with intensive therapy, cytarabine- and daunorubicin-based chemotherapy was administered as first-line induction therapy. Subsequently, patients received cytarabine- +/- anthracycline-based consolidation therapy and/or allo-HSCT following either myeloablative or reduced intensity conditioning regimens. Non-intensive therapy consisted of best supportive care +/- oral or parenteral chemotherapy (primarily low-dose AraC, hypomethylating agents or hydroxyurea). None of the patients received CPX-351 and only two patients were treated with Venetoclax since these drugs had not been approved during the study period. The study design is depicted in Fig. 1. Bone marrow evaluation was conducted using cytology, flow cytometry, histology and immunohistochemistry. The 2010 European LeukemiaNet (ELN) classification was applied for the assessment of the remission status and patients were stratified accordingly. ELN intermediate risk group I and II were merged as “ELN intermediate I/II” since previous studies had shown a lack of discriminatory power between these risk groups, particularly in older patients [26]. According to ELN, CR was defined as complete remission with hematological recovery, CRi was defined as complete remission with incomplete hematological recovery and MLFS was referred to as morphologic leukemia free state [3]. For the analysis, CR, CRi and MLFS were summarized as overall response rate (ORR). Partial remission (PR) was defined by a decrease of bone marrow blasts by at least 50% to a blast percentage in the range of 5–25%. Primary refractory disease (RD) was defined as a lack of CR or CRi after two courses of intensive induction treatment [18]. Furthermore, patients were stratified with regard to their molecular/cytogenetic risk group according to the ELN 2010 classification [27], due to a lack of additional molecular data that are mandatory for the ELN 2022 classification. The patients’ general condition was measured by the Eastern Cooperative Oncology Group-performance score (ECOG) [28]. Comorbidity was assessed using the Charlson Comorbidity Index (CCI) [29]. The study was in line with the Declaration of Helsinki and was approved by the local ethics committee (EA4/026/23).
Fig. 1. Study design.
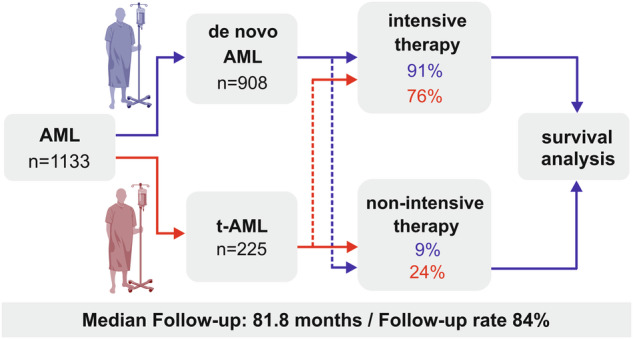
n number of patients, t-AML therapy-related AML.
Statistical analysis
Data curation and retrospective analysis were performed using SPSS Statistics, version 25 (IBM®, 2022, Armonk, NY). Baseline characteristics were analyzed using non-parametric tests, such as the Mann-Whitney U test in two subgroups, the Kruskal-Wallis-H-test in multiple subgroups or the Chi-Square test for nominal variables followed by post-hoc testing and Bonferroni adjustment [30]. Median follow-up was analyzed by using the reverse Kaplan–Meier method [31]. OS was analyzed using the Kaplan-Meier method. For comparisons in OS, the logrank test was applied. To define a hazard-ratio (HR), a univariate Cox regression model was applied. To determine the HR of independent risk factors, a multivariate Cox proportional hazards model was used. The latter included factors with a univariate significance level of p < 0.05. In order to define a hazard ratio (HR), some variables were transformed into categorical dichotomous data. To estimate the risk of relapse and non-relapse mortality, a multivariate cause-specific Cox proportional hazards model that included confounding factors with significant impact on relapse and survival was used based on an etiological approach. Within this model, death and relapse were defined as competing events and treated as censored observations [32]. In order to address possible multicollinearity between t-AML and ELN adverse risk that could have concealed the impact of t-AML on OS, subgroup analyses were performed within the different ELN risk groups. Furthermore, variance inflation factor (VIF) analysis for multicollinearity was performed for all factors included in the multivariate Cox regression model using survival as dependent variable. VIF-values of 1-2 were interpreted as lack of relevant collinearity, VIF-values > 2 and ≤ 4 were interpreted as mild collinearity, VIF-values > 4 should have warranted further investigation and VIF values ≥ 10 would have meant severe collinearity with subsequent exclusion of the factor which was not the case in our study (https://cran.r-project.org/web/packages/olsrr/vignettes/regression_diagnostics.html). A p-value of p < 0.05 was considered statistically significant. For graphical presentation, Graph Pad Prism 8 (GraphPad Software.Inc), Corel Draw Graphics Suite (Version 22, 2020) and BioRender.com (Fig. 1) were applied.
Results
Adverse baseline characteristics accumulate in t-AML
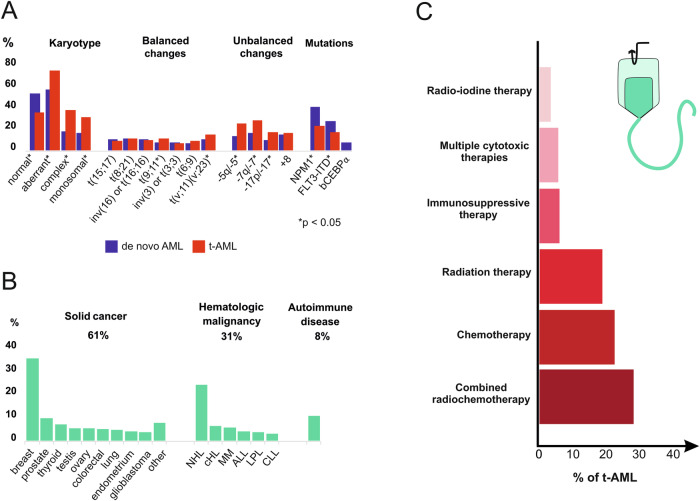
In the entire cohort (n = 1133), median follow up was 81.8 months. Of all patients included in this study, 20% had t-AML (n = 225) and 80% (n = 908) were diagnosed with de novo AML. 91% of de novo AML patients (n = 823/908) and 76% (n = 172/225) of t-AML patients were eligible for intensive therapy (p < 0.001). T-AML patients were more likely to be female, were significantly older and had more comorbidities as well as a higher frequency of unfavorable cytogenetic risk factors as compared to de novo AML patients, such as complex karyotypes, monosomal karyotypes and unbalanced translocations (Table 1, Fig. 2A). These differences led to a doubling of patients with adverse ELN 2010 risk features in our t-AML cohort. Median latency period from initial diagnosis to t-AML was 69.2 months. Of all t-AML patients, 61% (n = 138/225) had been diagnosed with solid cancer prior to development of AML with highest numbers for breast cancer (n = 74/225) and prostate cancer (n = 16/225). 31% of t-AML patients (n = 69/225) had hematological malignancies and 8% (n = 18/225) were diagnosed with an autoimmune disease (Fig. 2B). Active cancer was present in 16% of t-AML patients (n = 37/225). Combined radiochemotherapy had been administered in 33% of t-AML patients (n = 75/225), which was followed by chemotherapy and radiation therapy alone (Fig. 2C). For autoimmune diseases, patients had received either azathioprine, mitoxantrone, cyclophosphamide or methotrexate (MTX). MTX monotherapy had been administered in a few patients only (n = 8/225). Antecedent t-MDS was present in 25% of t-AML patients (n = 57/225). A detailed characterization of previous diseases and therapies is shown in Fig. 2 and Table 2.
Table 1.
Baseline characteristics in 1133 patients with t-AML vs. de novo AML.
| Characteristics | Entire cohort | t-AML | de-novo AML | p value |
|---|---|---|---|---|
| n (%) | 1133 | 225 (20) | 908 (80) | – |
| Eligible for intensive therapy, n (%) | 995 (88) | 172 (76) | 823 (91) | <0.001 |
| Sex (female), n (%) | 570 [50] | 144 [64] | 426 [47] | <0.001 |
| - intensive therapy | 504 [51] | 116 [67] | 388 [47] | <0.001 |
| - non-intensive therapy | 66 [48] | 28 [53] | 38 [45] | 0.451 |
| Age, years, median [IQR] | 56 [45–66] | 61 [52–69] | 55 [43–65] | <0.001 |
| - intensive therapy | 54 [43–63] | 57.5 [49–66] | 53 [42–62] | <0.001 |
| - non-intensive therapy | 74 [67–78] | 72 [65–79] | 74 [69–78] | 0.087 |
| ECOG-PS, median [IQR] | 1 [0-1] | 1 [0-1] | 1 [0-1] | 0.075 |
| - intensive therapy | 1 [0-1] | 1 [0-1] | 1 [0-1] | 0.524 |
| - non-intensive therapy | 1 [1-2] | 1 [1-2] | 1.5 [1-2] | 0.123 |
| CCI, median [IQR] | 0 [0-2] | 2 [2-3] | 0 [0-1] | <0.001 |
| - intensive therapy | 0 [0-2] | 2 [2-3] | 0 [0-1] | <0.001 |
| - non-intensive therapy | 2 [1–3] | 3 [2–4] | 1 [0–3] | <0.001 |
| Hemoglobin, g/dl, median [IQR] | 9.1 [7.7–10.2] | 8.9 [8.0–10.1] | 9.1 [7.6–10.3] | 0.781 |
| - intensive therapy | 9.1 [7.6–10.3] | 8.9 [7.9–10.2] | 9.1 [7.5–10.3] | 0.978 |
| - non-intensive therapy | 9 [8.1–10.0] | 9.3 [8.3–10.1] | 9.0 [8.1–9.9] | 0.523 |
| WBC, count/nl, median [IQR] | 9.7 [2.6–45.8] | 4.7 [2.0–24.3] | 12.5 [2.9–51.5] | <0.001 |
| - intensive therapy | 8.8 [2.6–45.2] | 4.4 [1.9–20.6] | 11.5 [2.8–50.9] | <0.001 |
| - non-intensive therapy | 13.3 [2.9–51.2] | 5.2 [2.2–34.8] | 22.7 [5.4–65.7] | 0.003 |
| PLT, count/nl, median [IQR] | 55 [28–106.7] | 53.5 [24.0–94.5] | 56.0 [30–108] | 0.272 |
| - intensive therapy | 55.0 [28–108] | 53.5 [25.3–93.5] | 56.0 [29.0–108.5] | 0.560 |
| - non-intensive therapy | 57.0 [30.0–97.2] | 56.5 [18–96.3] | 57.0 [33.0–98.0] | 0.264 |
n number of patients, IQR interquartile range, ECOG-PS Eastern cooperative oncology group performance score, CCI Charlson Comorbidity Index, ELN European LeukemiaNet, ECOG Eastern cooperative oncology group.
Fig. 2. Baseline characteristics in t-AML-patients.
A Karyotype and genetic alterations at baseline in t-AML vs. de novo AML. B Distribution of the most frequent primary diseases in the t-AML cohort, C Types of therapies that had been applied prior to primary diagnosis of t-AML. t translocation, inv inversion, NHL Non-Hodgkin lymphoma, cHL classical Hodgkin lymphoma, MM multiple myeloma, ALL acute lymphoblastic leukemia, LPL lymphoplasmacytic lymphoma, CLL chronic lymphocytic leukemia.
Table 2.
Primary diseases in 225 t-AML patients grouped for intensive and non-intensive therapy.
| Entity | Entire t-AML cohort | Intensive therapy | Non-intensive therapy | p value |
|---|---|---|---|---|
| n [%] | 225 [100] | 172 [76] | 53 [14] | |
| Solid cancer, n [%] | 138 [61] | 99 [58] | 39 [74] | 0.036 |
| Breast carcinoma | 74 [33] | 60 [35] | 14 [26] | |
| Prostate carcinoma | 16 [7] | 9 [5] | 7 [13] | |
| Thyroid carcinoma | 10 [4] | 7 [4] | 3 [6] | |
| Tumor of testicles | 6 [3] | 5 [3] | 1 [2] | |
| Ovarian carcinoma | 6 [3] | 4 [2] | 2 [4] | |
| Colorectal carcinoma | 5 [2] | 2 [1] | 3 [6] | |
| Lung carcinoma | 4 [2] | 1 [0.5] | 3 [6] | |
| Endometrial carcinoma | 3 [1] | 3 [2] | ─ | |
| Glioblastoma | 2 [1] | 1 [0.5] | 1 [2] | |
| Neuroendocrine tumor | 2 [1] | 1 [0.5] | 1 [2] | |
| Uterine sarcoma | 2 [1] | 2 [1] | ─ | |
| Ewing Sarcoma | 1 [0.5] | 1 [0.5] | ─ | |
| Liposarcoma | 1 [0.5] | 1 [0.5] | ─ | |
| Melanoma | 1 [0.5] | 1 [0.5] | ─ | |
| Nasopharyngeal carcinoma | 1 [0.5] | ─ | 1 [2] | |
| Osteosarcoma | 1 [0.5] | 1 [0.5] | ─ | |
| Squamous cell carcinoma of the skin | 1 [0.5] | ─ | 1 [2] | |
| Squamous cell carcinoma of the tonsil | 1 [0.5] | ─ | 1 [2] | |
| Carcinoma of the cervix | 1 [0.5] | ─ | 1 [2] | |
| Hematologic malignancies, n [%] | 69 [31] | 58 [34] | 11 [21] | 0.073 |
| B-cell Non-Hodgkin lymphoma | 48 [21] | 42 [24] | 6 [11] | |
| Classical Hodgkin Lymphoma | 8 [4] | 8 [5] | ─ | |
| Multiple Myeloma | 7 [3] | 4 [2] | 3 [6] | |
| Acute lymphoblastic leukemia | 3 [1] | 3 [2] | ─ | |
| Lymphoplasmacytic lymphoma | 2 [1] | 1 [0.5] | 1 [2] | |
| Chronic lymphocytic leukemia | 1 [0.5] | ─ | 1 [2] | |
| Autoimmune disease, n [%] | 18 [8] | 15 [9] | 3 [6] | 0.473 |
| Rheumatoid arthritis | 6 [3] | 5 [3] | 1 [2] | |
| Multiple sclerosis | 3 [1] | 3 [2] | ─ | |
| Psoriasis | 2 [1] | 2 [1] | ─ | |
| Autoimmune hemolytic anemia | 1 [0.5] | 1 [0.5] | ─ | |
| Other autoimmune diseases | 6 [3] | 4 [2] | 2 [4] |
Overall survival in t-AML subgroups: treatment modality and age
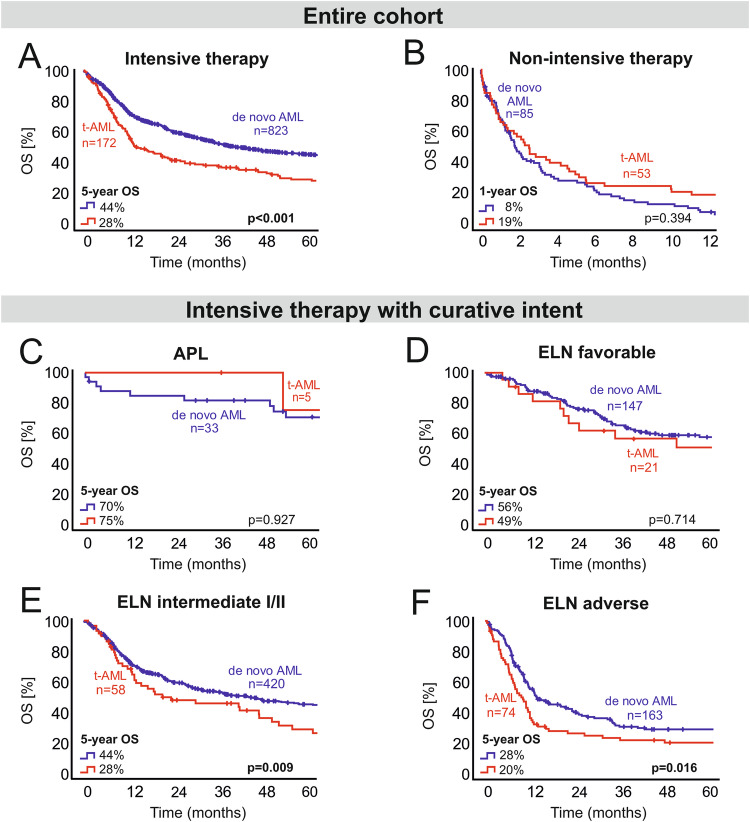
Median OS was 24 months in the entire cohort (95% CI 19.5–28.5) and 5-year OS was 36.6% respectively. In intensively treated patients, OS was significantly inferior (p < 0.001) in t-AML as compared to de novo AML (5-year OS 28% vs. 44%, median OS 13.7 months vs. 39.4 months, Fig. 3A). In patients with non-intensive therapy, OS was particularly poor in the vast majority of patients, both with t-AML and de novo AML. In this subgroup, 1-year OS was 19% in t-AML vs. 8% in de novo AML. Median OS was 2.6 months in t-AML vs. 1.8 months in de novo AML, respectively (p = 0.394, Fig. 3B). In patients with intensive therapy aged < 60 years, the inferior OS of t-AML was maintained. Comparing t-AML (n = 96/225) and de novo AML (n = 562/908) in this age group, we found that 5-year OS and median OS were 31% and 19.3 months in t-AML vs. 51% and 64.6 months in de novo AML (p < 0.001). In contrast, patients aged ≥ 60 years showed 5-year OS rates of 23% in t-AML vs. 30% in de novo AML with a median OS of 12.4 months in t-AML vs. 23.3 months in de novo AML (p = 0.066).
Fig. 3. OS in t-AML vs. de novo AML (logrank test).
A OS within the entire cohort of intensively treated patients: survival with t-AML is inferior to de novo AML. B OS within the entire cohort of non-intensively treated patients: no significant difference between t-AML and de novo AML. C OS of patients with APL: no significant difference between t-APL and de novo APL. D OS of patients with ELN 2010 favorable risk: no significant difference between t-AML and de novo AML. E OS of patients with ELN 2010 intermediate risk: survival with t-AML is inferior to de novo AML. F OS of patients with ELN 2010 adverse risk: survival with t-AML is inferior to de novo AML. n number of patients, OS overall survival, t-AML therapy-related AML, APL acute promyelocytic leukemia, ELN European LeukemiaNet.
ELN risk groups and overall survival in t-AML
To address the impact of genetic risk groups on the outcome of t-AML with intensive therapy, survival analysis was performed within the different risk groups according to ELN 2010 (Fig. 3C–F). Within the ELN intermediate I/II and adverse risk groups, survival of patients with t-AML was inferior to patients with de novo AML. In the ELN intermediate I/II risk group, 5-year OS was 28% with t-AML vs. 44% with de novo AML (median OS 21.3 vs. 41.3 months, p = 0.009). In the ELN adverse risk group, 5-year OS was 20% with t-AML vs. 28% with de novo AML (median OS 10.1 vs. 14.3 months, p = 0.016). In both of these ELN risk groups, t-AML patients were significantly older than patients with de novo AML. Median age was 60 vs. 53 years in ELN intermediate I/II risk patients (p = 0.001) and 58 vs. 55 years in the adverse ELN risk group (p = 0.029). Furthermore, t-AML patients had a tendency towards a lower ORR after induction therapy, so that standard consolidation therapy was less frequently completed. In ELN intermediate I/II risk patients, ORR was 73% with t-AML and 84% with de novo AML (p = 0.094). In ELN adverse patients, ORR was 63% in t-AML vs. 73% in de novo AML (p = 0.290). Within the ELN intermediate I/II risk group, consolidation chemotherapy was applied in 52% of patients with t-AML vs. 68% of patients with de novo AML. Within the ELN adverse risk group, only 35% of patients with t-AML received consolidation chemotherapy as compared to 50% in de novo AML. In contrast, there were no significant differences in OS between patients with t-AML and de novo AML in both the ELN favorable risk group (p = 0.714) and in acute promyelocytic leukemia (APL, p = 0.927). In the ELN favorable risk group, 5-year OS was 49% in t-AML vs. 56% in de novo AML (median OS 53.5 vs. 168.4 months, p = 0.714). In patients with t-APL, 5-year OS was 75 vs. 70% in de novo APL (median OS 107.9 vs. 198.3 months, p = 0.927). Patients within the ELN favorable risk group showed well balanced baseline characteristics, including age with a median age of 54 years in t-AML vs. 52 years in de novo AML (p = 0.878). Additionally, ORR and consolidation therapy rates were comparable in ELN favorable risk patients and patients with APL. In the ELN favorable risk group, ORR was 95% in t-AML vs. 92% in de novo AML (p = 1.000). In APL patients, ORR was 80% in t-APL vs. 94% in de novo APL (p = 0.362).
In first remission (after induction therapy), 38% of de novo AML patients and 37% of t-AML patients received allo-HSCT (p = 0.887). There was no significant difference in transplantation rates between t-AML and de novo AML within the different genetic risk groups. In the entire cohort, OS of t-AML patients who received allo-HSCT in first remission was significantly worse than OS of de novo AML patients (median OS 21.0 vs. 90.8 months; 5-years OS 39 vs. 55%, p = 0.016). This was accompanied by an accumulation of ELN adverse risk patients in the t-AML cohort (54% of t-AML patients vs 29% in de novo AML). Considering long-term outcome of patients transplanted in first remission, OS in t-AML was comparable to de novo AML within the different ELN risk groups (ELN favorable p = 0.196, ELN intermediate I/II p = 0.178, ELN adverse p = 0.510). In an additional survival analysis within the favorable ELN risk group (t-AML vs. de novo AML), patients receiving allo-HSCT in first remission were censored at the time point of allo-HSCT in order to exclude the influence of a possibly different transplantation strategy in this subgroup. In this additional analysis, the lack of difference in OS within the favorable ELN risk group was maintained (p = 0.745).
T-AML: specific clinical risk factors for overall survival
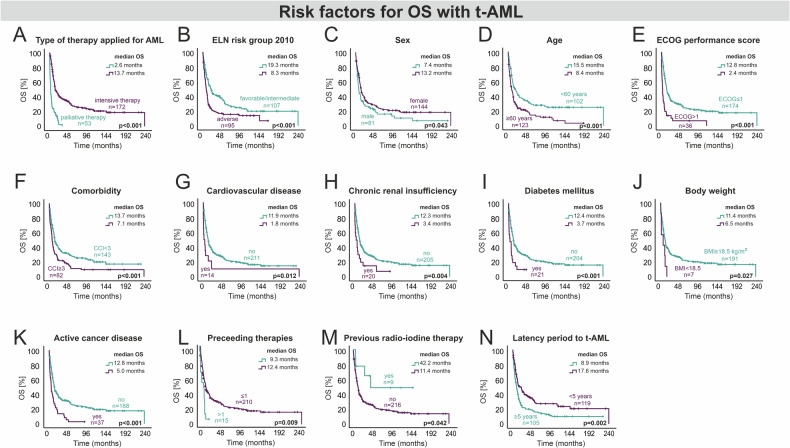
In order to identify risk factors which may contribute to the poor outcome in t-AML, t-AML patients were thoroughly analyzed (Fig. 4). As expected, active cancer disease was associated with a higher risk of death (HR 2.2). More than one preceding cytotoxic therapy had an adverse impact on OS (HR 2.1). In contrast, patients with an antecedent radioiodine therapy had a particularly favorable outcome (HR 0.4). However, patient numbers within these subgroups were rather small. The type of underlying disease (solid cancer vs. hematological malignancy vs. autoimmune disease) did not affect the OS significantly (p = 0.580). With regard to baseline characteristics at initial diagnosis of AML, t-AML patients with an ECOG score >1 (HR 2.3), an adverse ELN 2010 risk (HR 1.7), non-intensive therapy (HR 3.0), underweight (HR 2.7) and diabetes mellitus (HR 2.0) were at higher risk of death as confirmed by the multivariate analysis (Fig. 5).
Fig. 4. Baseline characteristics of t-AML patients with significant impact on OS (logrank-test).
A Type of AML therapy, B ELN adverse risk, C sex differences, D age-related survival difference, E ECOG performance score, F comorbidity, G cardiovascular disease, H chronic renal insufficiency, I diabetes mellitus, J body weight, K active cancer disease, L preceeding therapies, M previous radio-iodine therapy, N Latency period to t-AML. n Number of patients, OS overall survival, t-AML therapy-related AML, ELN European LeukemiaNet, ECOG Eastern Cooperative Oncology Group, CCI Charlson comorbidity index, BMI body mass index.
Fig. 5. Risk factors for overall survival (OS) in 225 patients with t-AML.
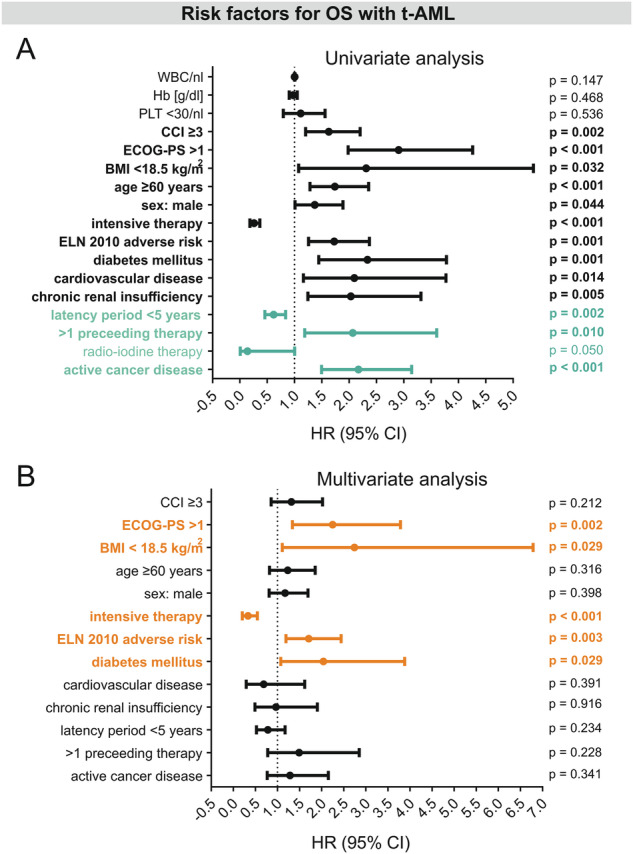
A Univariate Cox regression shows hazard ratio defined as risk of death from several risk factors in t-AML. Factors that were mainly determined by the previous disease are depicted in turquoise letters. B Multivariate Cox regression shows HR defined as risk of death from factors with univariate significance in t-AML and reveals ECOG-PS > 1, an adverse cytogenetic/molecular risk according to the ELN 2010 classification, intensive therapy, BMI < 18.5 kg/m2 and diabetes mellitus as independent risk factors for OS in t-AML (depicted in orange letters). WBC white blood cell count, Hb hemoglobin, PLT platelets, CCI Charlson comorbidity index, ECOG Eastern Cooperative Oncology Group, BMI body-mass index, ELN European LeukemiaNet, HR hazard ratio, CI confidence interval.
T-AML as a risk factor
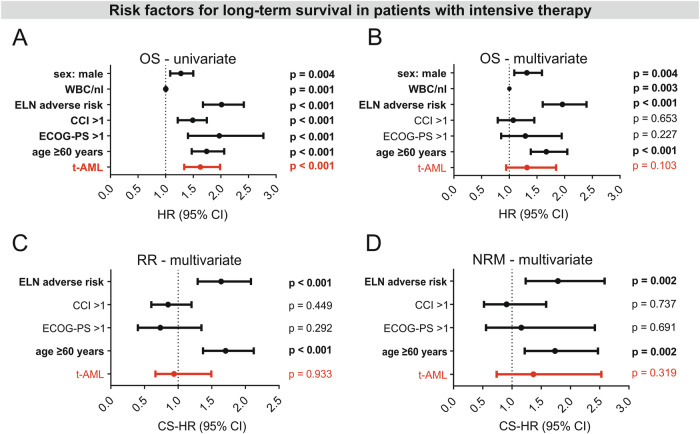
The important impact of baseline characteristics that was observed in the ELN subgroup analysis, was maintained in the multivariate analysis of patients with intensive therapy (Fig. 6). ELN 2010 adverse risk, higher age (60 years), high leukocyte counts, and male sex could be confirmed as independent risk factors for OS. In contrast, t-AML itself was no independent risk factor in the entire cohort, neither for OS (p = 0.103, HR 1.3) nor for RR (p = 0.933, HR 0.9) or NRM (p = 0.319, HR 1.4). Potential collinearity between adverse genetic risk and t-AML which could have concealed t-AML as independent risk factor in the multivariate analysis of the entire cohort was addressed by 1) VIF-analysis for t-AML and ELN adverse risk and 2) multivariate analysis within the different ELN 2010 risk groups to avoid genetic risk as confounding cofactor for t-AML. VIF values for collinearity in the model including all factors with univariate significance for OS were 1.085 for ELN 2010 adverse risk and 2.217 for t-AML, respectively. Furthermore, VIF values were < 2.0 for all other covariates in the model (age > 60 years, male sex, ECOG-PS > 1, leukocyte count) except for CCI > 1 (VIF 2.196). This indicated minimal but no severe collinearity between t-AML and CCI but no relevant collinearity between the other parameters. Multivariate analysis within the ELN risk groups confirmed t-AML to be no independent risk factor for OS, RR and NRM, although there was borderline significance for inferior OS of t-AML patients within the intermediate I/II risk group (p = 0.055, Supplementary Fig. 1).
Fig. 6. Risk factors for long-term survival in 995 AML patients with intensive therapy.
A Univariate Cox regression. B Multivariate Cox regression. C Cause-specific hazard ratios for non-relapse mortality in the presence of risk factors with univariate impact. D Cause-specific hazard ratios for risk of relapse in the presence of risk factors with univariate impact. T-AML is no independent risk factor in the multivariate analysis of intensively treated patients, neither for OS, nor for RR or NRM. OS overall survival, RR risk of relapse, NRM non-relapse mortality, t-AML therapy-related AML, CCI Charlson comorbidity Index, ECOG Eastern Cooperative Oncology Group, ELN European LeukemiaNet, HR hazard ratio, CI confidence interval.
Discussion
According to recent studies, t-AML has a frequency of up to 15% of all AML patients, with increasing incidence over the past decades [1, 3]. In our cohort, baseline characteristics, latency period and OS of t-AML patients were comparable to other studies [2, 9, 11, 22, 33]. In concordance with previous findings, the most frequent underlying malignancies were breast cancer and Non-Hodgkin lymphoma (NHL) in our cohort [2, 9, 19, 26, 34]. The high incidence of t-AML following breast cancer reflects the general population-based increase as well as advances in diagnosis and therapy, resulting in an improved long-term survival of breast cancer patients over the past decades [34, 35]. A possible explanation for the high percentage of t-AML following NHL might be frequent application of poly-chemotherapy together with increasing cure rates [35]. Preceding therapy-associated sequelae in t-AML may have an impact on comorbidities, which is mirrored by a higher CCI in our t-AML cohort.
The impact of t-AML as a risk factor has remained a matter of discussion over the past years due to partially conflicting results in different retrospective studies [1, 2, 13, 14, 20–23]. Our retrospective study shows that the inferior OS in t-AML mainly results from differences in clinical baseline characteristics and genetics. Although OS in t-AML is clearly inferior to de novo AML in general, long-term outcome of t-AML is comparable with de novo AML, if baseline characteristics and genetics are balanced. We hypothesize that the accumulation of adverse genetic and patient-associated risk factors in t-AML has an impact on long-term survival differences between t-AML and de novo AML patients. In our cohort, the differences in OS in favor of de novo AML reached beyond 15% after 5 years. This particularly affected patients who were younger than 60 years or were belonging to the ELN intermediate I/II and adverse risk groups that showed higher median age and signs of increased chemoresistance in terms of lower response rates. In these subgroups, it seems conceivable that inferior survival is mediated by additional genetic aberrations in t-AML that would have been detected by a more comprehensive molecular panel as proposed by the recent ELN 2022 recommendations, particularly within the intermediate I/II and adverse ELN risk groups [3]. Furthermore, “hidden” mutational and clonal complexity due to clonal selection over time is likely to affect OS in t-AML [12, 15–17, 36–39]. Interestingly, these differences resolved in intensively treated patients within the ELN favorable risk group and ─ in line with current literature ─ in APL patients [11, 40, 41] as well as in patients who were older than 60 years and had received non-intensive therapy. This may be explained by the fact that baseline characteristics in these subgroups were better balanced.
In this regard, several known risk factors with an adverse impact on OS in t-AML were confirmed in our multivariate analysis (ELN adverse risk, higher ECOG score and non-intensive therapy) [2, 11, 33, 42, 43]. In addition, underweight and diabetes were identified as independent risk factors in t-AML. Additionally, the univariate analysis showed an inferior OS in patients with more than one prior cytotoxic therapy and a trend towards better OS in patients with radio-iodine therapy. The latter group was mostly comprised of young and fit women who had been treated for thyroid cancer and thus were at risk of developing AML [2, 44–46]. According to the WHO, the role of radionucleotides in the pathogenesis of t-AML is unclear [18, 47] and our findings warrant further investigations in larger cohorts.
Despite the adverse outcome of t-AML patients as an entire group, t-AML per se was no independent risk factor, neither for OS nor for RR or NRM in intensively treated patients. Any difference in survival parameters between t-AML and de novo AML did not reach the level of significance in multivariate analysis although many adverse molecular features that are part of the ELN 2022 risk classification and are accumulated in t-AML were not available for our analysis. This lack of more comprehensive molecular data is a limitation of our study which is probably most relevant within the ELN 2010 intermediate I/II risk group. This subgroup comprises many AML patients with normal karyotype which would have been classified as “adverse risk” according to ELN 2022 due to additional molecular data including MDS-related abnormalities. Interestingly, in this subgroup, multivariate analysis showed borderline significance for t-AML (p = 0.055) as an independent prognostic factor (Supplemental Fig. 1). Nevertheless, we acknowledge that further, yet unknown factors might have an impact on survival in t-AML.
Besides classification and prognostication, the impact of t-AML becomes particularly important for therapeutic decision making in young/fit favorable risk t-AML patients, since the attribution of high-risk disease can lead to allo-HSCT in first CR in these patients. Instead, t-AML patients can be treated with consolidation chemotherapy when classified as favorable risk AML and showing MRD clearance at predefined timepoints [3]. Our study strongly suggests that ELN favorable risk t-AML patients (AML with NPM1mut or core binding factor AML) as well as patients with t-APL are not at a higher risk for relapse or death as compared to de novo AML patients and thus should not routinely undergo allo-HSCT in first CR. Our findings are in accordance with a recent Swedish registry-based study also showing that t-AML is no independent risk factor in patients belonging to the favorable ELN 2010 risk group (with and without APL), whereas it is associated with a higher risk of death in ELN 2010 intermediate I/II and ELN 2010 adverse risk t-AML patients [1]. Moreover, another recently published retrospective study in NPM1mut AML showed that NPM1mut t-AML and de novo NPM1mut AML have overlapping genetic features without differences in OS with regard to the multivariate analysis [48, 49].
Another clinically relevant question arising from these data is the most appropriate chemotherapy for t-AML patients within the favorable ELN risk group. On the one hand, a liposomal formulation of cytarabine and daunorubicin (CPX-351) has been shown to be superior to a standard cytarabine/anthracycline-based regimen in AML-MRC and t-AML. On the other hand, this survival advantage was predominantly demonstrated in a cohort of older AML patients aged > 60 years, containing only about 5% patients with favorable cytogenetics according to National comprehensive cancer network (NCCN) criteria and was most prominent within the subgroup that underwent allo-HSCT as consolidation treatment [50, 51]. Furthermore, consolidation chemotherapy in this trial was different from ELN standard recommendations since it did not contain repetitive cycles of intermediate dose cytarabine (ID AraC). Whether the outcome of consolidation therapy with CPX-351 is comparable or superior to ID AraC remains an open question, particularly in younger patients, and will assumingly be answered by currently active AML trials (AMLSG 30-18 trial/ NCT03897127) [52, 53].
In synopsis with current literature, our study strongly supports the view that risk stratification in t-AML, particularly with regard to the indication for allo-HSCT, should align with strategies used in de novo AML, both in intensive and non-intensive therapy. Additionally, our data support the current WHO and ICC 2022 classifications [18, 19], which consider t-AML as a “diagnostic qualifier” within the different AML subgroups rather than a separate subcategory.
Supplementary information
Acknowledgements
We thank Margrit Stodder, Alma Herneth and Annabel Sick for their administrative support in this project. Furthermore, we owe thanks to Sven Bischoff for his support with the statistical analysis. Parts of graphical elements used in Fig. 1 were created with BioRender.com.
Author contributions
SG, JI and JW designed the study. JI, SG, KA, LEB and JW collected, analyzed, and interpreted the data used in this study. JI, JS, JJ, MS, AF, LVG, KR, JK, PLC, VB, AVB, IWB, TB, UK, DH, LAB and JW performed clinical and diagnostic workup required for this study. JI, SG and JW wrote the manuscript draft. All authors critically revised the manuscript and approved the final version.
Funding
Jana Ihlow and Jens Schrezenmeier are participants in the BIH Charité Clinician Scientist Program funded by the Charité-Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH). In addition, we acknowledge support from the DKTK (joint-funding project RiskY-AML to LB) and the Open Access Publication Fund of Charité - Universitätsmedizin Berlin. Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical restrictions but are available from the corresponding author upon reasonable request.
Competing interests
JI: travel support from Medac. AF: honoraria from Pharmaphar; Ipsen; Lilly. JK: honoraria from Janssen, BMS/Celgene, Takeda; Astra Zeneca, Amgen; Sanofi Aventis; Abbvie. PLC: honoraria from Pfizer; Incyte; Novartis. UK: Honoraria from Novartis. LB: honoraria from AbbVie, Amgen, Astellas, BristolMyers Squibb, Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer, Roche, and Sanofi, as well as research support from Bayer and Jazz Pharmaceuticals. JW: honoraria from Abbvie, Pfizer, Novartis; Jazz Pharmaceuticals, Roche; Astellas; Amgen; Sanofi Aventis; Bristol Myers Squibb, SoBi, Otsuka. The authors declare no competing interests.
Ethics approval and consent to participate
The study is in line with the Declaration of Helsinki and was approved by the local ethics committee of the Charité-Universitätsmedizin Berlin (registration number EA4/026/23). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sophia Gross, Jana Ihlow.
Contributor Information
Jana Ihlow, Email: jana.ihlow@charite.de.
Jörg Westermann, Email: joerg.westermann@charite.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01140-5.
References
- 1.Nilsson C, Linde F, Hulegårdh E, Garelius H, Lazarevic V, Antunovic P, et al. Characterization of therapy-related acute myeloid leukemia: increasing incidence and prognostic implications. Haematologica. 2023;108:1015–25. 10.3324/haematol.2022.281233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–45. 10.1182/blood-2010-08-301713 [DOI] [PubMed] [Google Scholar]
- 3.Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. 10.1182/blood.2022016867 [DOI] [PubMed] [Google Scholar]
- 4.Westermann J, Bullinger L. Precision medicine in myeloid malignancies. Semin Cancer Biol. 2022;84:153–69. 10.1016/j.semcancer.2021.03.034 [DOI] [PubMed] [Google Scholar]
- 5.Andersen MK, Johansson B, Larsen SO, Pedersen-Bjergaard J. Chromosomal abnormalities in secondary MDS and AML. Relationship to drugs and radiation with specific emphasis on the balanced rearrangements. Haematologica. 1998;83:483–8. [PubMed] [Google Scholar]
- 6.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418–29. 10.1053/j.seminoncol.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004. 10.1182/blood-2012-08-448068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guru Murthy GS, Hamadani M, Dhakal B, Hari P, Atallah E. Incidence and survival of therapy related myeloid neoplasm in United States. Leuk Res. 2018;71:95–9. 10.1016/j.leukres.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90:208–14. 10.1002/ajh.23908 [DOI] [PubMed] [Google Scholar]
- 10.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33:3641–9. 10.1200/JCO.2014.60.0890 [DOI] [PubMed] [Google Scholar]
- 11.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–5. 10.1038/sj.leu.2403187 [DOI] [PubMed] [Google Scholar]
- 12.Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552–5. 10.1038/nature13968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jentzsch M, Grimm J, Bill M, Brauer D, Backhaus D, Goldmann K, et al. ELN risk stratification and outcomes in secondary and therapy-related AML patients consolidated with allogeneic stem cell transplantation. Bone Marrow Transpl. 2021;56:936–45. 10.1038/s41409-020-01129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmaelter AK, Labopin M, Socié G, Itälä-Remes M, Blaise D, Yakoub-Agha I, et al. Inferior outcome of allogeneic stem cell transplantation for secondary acute myeloid leukemia in first complete remission as compared to de novo acute myeloid leukemia. Blood Cancer J. 2020;10:26. 10.1038/s41408-020-0296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol. 2017;18:100–11. 10.1016/S1470-2045(16)30626-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76. 10.1182/blood-2014-11-610543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat genet. 2020;52:1219–26. 10.1038/s41588-020-00710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. 10.1038/s41375-022-01613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. 10.1182/blood.2022015850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant 2007;13:655–64. 10.1016/j.bbmt.2007.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelis FV, Atenafu EG, Gupta V, Kim DD, Kuruvilla J, Lipton JH, et al. Comparable outcomes post allogeneic hematopoietic cell transplant for patients with de novo or secondary acute myeloid leukemia in first remission. Bone Marrow Transpl. 2015;50:907–13. 10.1038/bmt.2015.59 [DOI] [PubMed] [Google Scholar]
- 22.AE ES, Chacim S, Ferreira I, Leite L, Moreira C, Pereira D, et al. Effect of therapy-related acute myeloid leukemia on the outcome of patients with acute myeloid leukemia. Oncol lett. 2016;12:262–8. 10.3892/ol.2016.4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–27. 10.1038/nrc.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised Recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9. 10.1200/JCO.2003.04.036 [DOI] [PubMed] [Google Scholar]
- 25.Iacobelli S, On behalf of the ESC. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2013;48:S1–37. 10.1038/bmt.2012.282 [DOI] [PubMed] [Google Scholar]
- 26.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buechner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. 10.1182/blood-2009-07-235358 [DOI] [PubMed] [Google Scholar]
- 28.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–56. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new Method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 30.Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educ. 1995;64:79–93. 10.1080/00220973.1995.9943797 [DOI] [Google Scholar]
- 31.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. [DOI] [PubMed]
- 32.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–70. 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fianchi L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, et al. Characteristics and outcome of therapy-related myeloid neoplasms: Report from the Italian network on secondary leukemias. Am J Hematol. 2015;90:E80–5. 10.1002/ajh.23966 [DOI] [PubMed] [Google Scholar]
- 34.Claerhout H, Lierman E, Michaux L, Verhoef G, Boeckx N. A monocentric retrospective study of 138 therapy-related myeloid neoplasms. Ann Hematol. 2018;97:2319–24. 10.1007/s00277-018-3462-y [DOI] [PubMed] [Google Scholar]
- 35.Erdmann F, Spix C, Katalinic A, Christ M, Folkerts J, Hansmann J, et al. Krebs in Deutschland für 2017/2018. Robert Koch-Institut; 2021. p. 172. [Google Scholar]
- 36.Gillis NK, Ball M, Zhang Q, Ma Z, Zhao Y, Yoder SJ, et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol. 2017;18:112–21. 10.1016/S1470-2045(16)30627-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voso MT, Pandzic T, Iskas M, Denčić-Fekete M, De Bellis E, Scarfo L, et al. Clonal hematopoiesis is associated with increased risk for therapy-related myeloid neoplasms in chronic lymphocytic leukemia patients treated with chemo(immuno)Therapy. Blood. 2020;136:19–20. 10.1182/blood-2020-140232 [DOI] [Google Scholar]
- 38.Duy C, Li M, Teater M, Meydan C, Garrett-Bakelman FE, Lee TC, et al. Chemotherapy induces senescence-like resilient cells capable of initiating AML recurrence. Cancer discov. 2021;11:1542–61. 10.1158/2159-8290.CD-20-1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl J Med. 2012;366:1090–8. 10.1056/NEJMoa1106968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayser S, Krzykalla J, Elliott MA, Norsworthy K, Gonzales P, Hills RK, et al. Characteristics and outcome of patients with therapy-related acute promyelocytic leukemia front-line treated with or without arsenic trioxide. Leukemia 2017;31:2347–54. 10.1038/leu.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun T, Cereja S, Chevret S, Raffoux E, Beaumont M, Detourmignies L, et al. Evolving characteristics and outcome of secondary acute promyelocytic leukemia (APL): A prospective analysis by the French-Belgian-Swiss APL group. Cancer. 2015;121:2393–9. 10.1002/cncr.29389 [DOI] [PubMed] [Google Scholar]
- 42.Ornstein MC, Mukherjee S, Mohan S, Elson P, Tiu RV, Saunthararajah Y, et al. Predictive factors for latency period and a prognostic model for survival in patients with therapy-related acute myeloid leukemia. Am J Hematol. 2014;89:168–73. 10.1002/ajh.23605 [DOI] [PubMed] [Google Scholar]
- 43.Sasaki K, Jabbour E, Cortes J, Kadia T, Garcia-Manero G, Borthakur G, et al. Outcome of patients with therapy-related acute myeloid leukemia with or without a history of myelodysplasia. Clin Lymphoma Myeloma Leuk. 2016;16:616–24. 10.1016/j.clml.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molenaar RJ, Sidana S, Radivoyevitch T, Advani AS, Gerds AT, Carraway HE, et al. Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid cancer. J Clin Oncol. 2018;36:1831–9. 10.1200/JCO.2017.75.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng CJ, Hu YW, Chen SC, Yeh CM, Chiang HL, Chen TJ, et al. Use of radioactive iodine for thyroid cancer and risk of second primary malignancy: a nationwide population-based study. J Natl Cancer Inst. 2016;108:djv314. [DOI] [PubMed]
- 46.Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–44. 10.1038/sj.bjc.6601319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 48.Othman J, Meggendorfer M, Tiacci E, Thiede C, Schlenk R, Dillon R, et al. Overlapping features of therapy-related and de novo NPM1-mutated AML. Blood. 2023;141:1846–57. 10.1182/blood.2022018108 [DOI] [PubMed] [Google Scholar]
- 49.Ustun C. Gold is gold even in mud: NPM1 mutations in T-AML. Blood. 2023;141:1784–5. 10.1182/blood.2022019295 [DOI] [PubMed] [Google Scholar]
- 50.Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36:2684–92. 10.1200/JCO.2017.77.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8:e481–91. 10.1016/S2352-3026(21)00134-4 [DOI] [PubMed] [Google Scholar]
- 52.Przespolewski A, Goldberg AD, Talati C, Fazal S, Vachhani P, Sanikommu SR, et al. Safety and efficacy of CPX-351 in younger patients (<60 years old) with secondary acute myeloid leukemia. Blood 2023;141:1489–93. 10.1182/blood.2022016678 [DOI] [PubMed] [Google Scholar]
- 53.Gaidzik VI, Mayr-Benedikter V, Weber D, Schrade A, Krauter J, Walz JS, et al. Higher dose of CPX-351 is associated with prolonged hematologic recovery: results from an interim safety analysis of the randomized, phase III AMLSG 30-18 trial. Blood. 2020;136:46–7. 10.1182/blood-2020-138738 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical restrictions but are available from the corresponding author upon reasonable request.