Abstract
Valve-in-valve transcatheter aortic valve replacement for failed Perceval sutureless valves has been shown to be safe and feasible. However, it is technically challenging and warrants understanding of potential risks and complications. We present a case of successful valve-in-valve implantation complicated by inadvertent wire passage outside of the Perceval frame.
Key Words: Perceval sutureless valve, transcatheter aortic valve replacement, valve-in-valve
Graphical Abstract
History of presentation
A 79-year-old woman presented with progressively worsening shortness of breath (NYHA functional class III) and lower extremity edema due to severe central bioprosthetic aortic regurgitation (AR) and mild paravalvular leakage (PVL).
Learning Objectives
-
•
To recognize the risk of and prevent inadvertent wire passage with ViV-TAVR in failing Perceval sutureless valves.
-
•
To underscore the need for adjunct prevention techniques including TEE guidance, passage of a partially inflated balloon, manipulation of a catheter in the LV, and routine assessment with fluoroscopy at extreme angles.
Past Medical History
The patient’s medical history included hypertension, hyperlipidemia, cardiomyopathy, heart failure with preserved ejection fraction, sick sinus syndrome, atrial fibrillation, permanent pacemaker, bilateral carotid stenosis, asthma with severe lung disease, hypothyroidism, and chronic kidney disease stage III. She underwent prior surgical aortic valve replacement (SAVR) due to severe aortic stenosis (2010), which required redo surgery using a Perceval sutureless 21-mm valve (LivaNova PLC) as well as surgical mitral valve replacement for mitral stenosis at the same time (May 2016). Later, the Perceval valve showed at least moderate PVL necessitating repair with 2 closure plugs (August 2016).
Differential Diagnosis
Differential diagnosis included bioprosthetic aortic stenosis, mitral regurgitation, and worsening of pre-existing heart failure.
Investigations
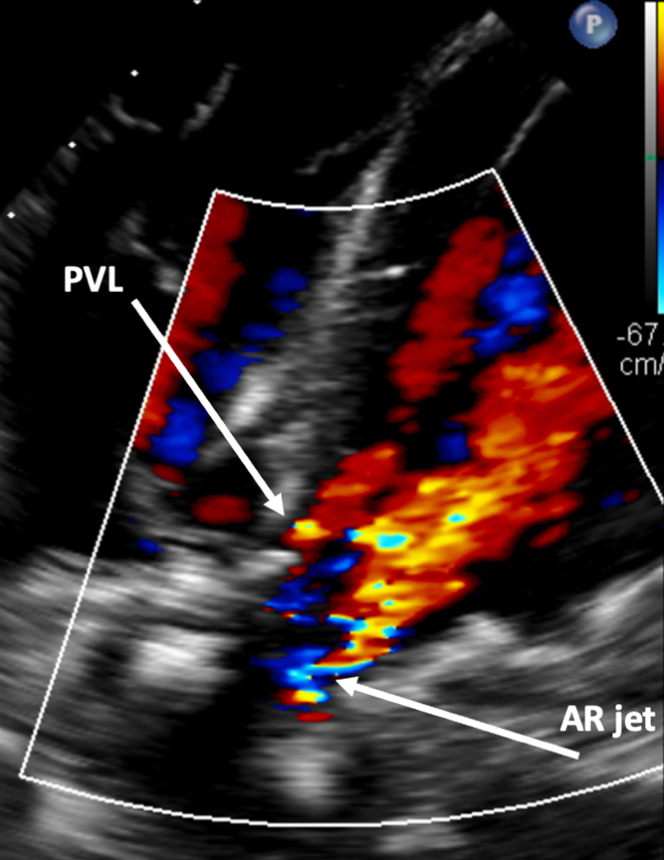
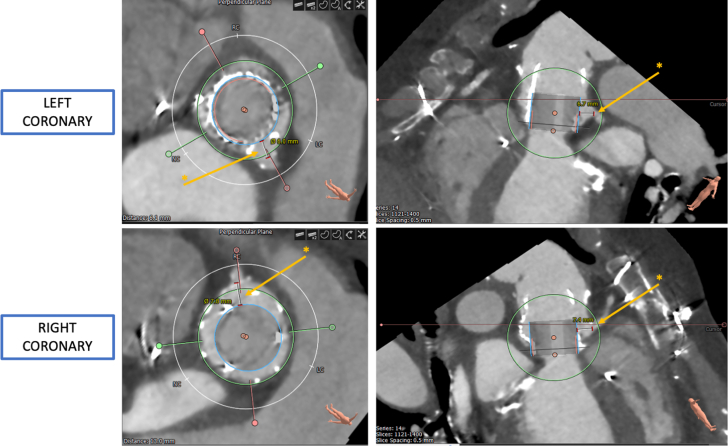
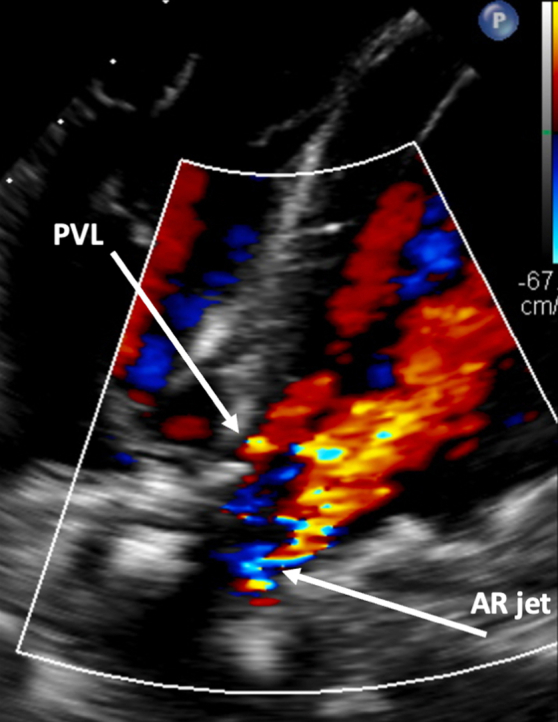
Transesophageal echocardiography (TEE) revealed severe central bioprosthetic AR due to poor coaptation along the neo right cusp with an eccentric, posteriorly directed regurgitant jet and mild PVL (Figure 1). Mean and peak aortic transvalvular gradients were 17.4 and 44.5 mm Hg, respectively, and left ventricular (LV) ejection fraction (LVEF) was 55% to 60%. The aortic valve area was 1.3 cm2 with a peak velocity of 3.3 m/s. Computed tomography angiography showed low-risk anatomy with adequate coronary height, no calcium in the left ventricular outflow tract, and minimal calcium at the sinotubular junction (Figure 2). Simulation of the transcatheter heart valve (THV) placement revealed distances between the virtual Edwards Lifesciences Sapien valve and the left and right coronary orifice of 6.7 and 7.4 mm, respectively (Figure 2).
Figure 1.
Preprocedural Transesophageal Echocardiography
Transesophageal echocardiography showing severe central bioprosthetic AR and mild PVL. AR = aortic regurgitation; PVL = paravalvular leak.
Figure 2.
Preprocedural Computed Tomography Angiography
Computed tomography angiography showing the distance between the virtual Edwards Sapien valve and the coronary orifices (asterisk).
The patient was deemed inoperable due to extreme surgical risk (STS score: 10%), age, frailty, comorbidities, and prior surgeries. Therefore, it was decided to perform a valve-in-valve transcatheter aortic valve replacement (ViV-TAVR). The study has been approved by the Institutional Review Board (AAA3154).
Management
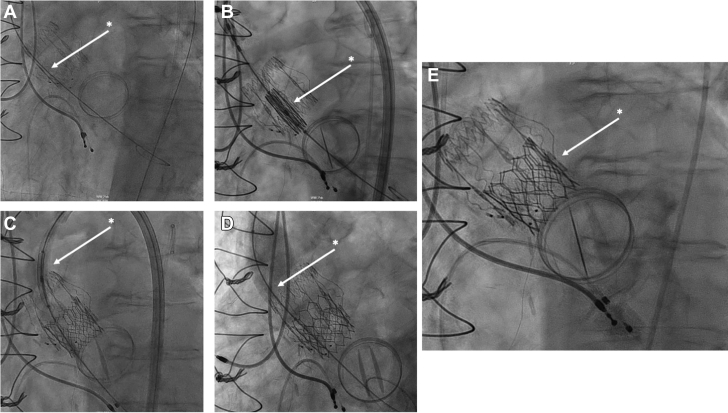
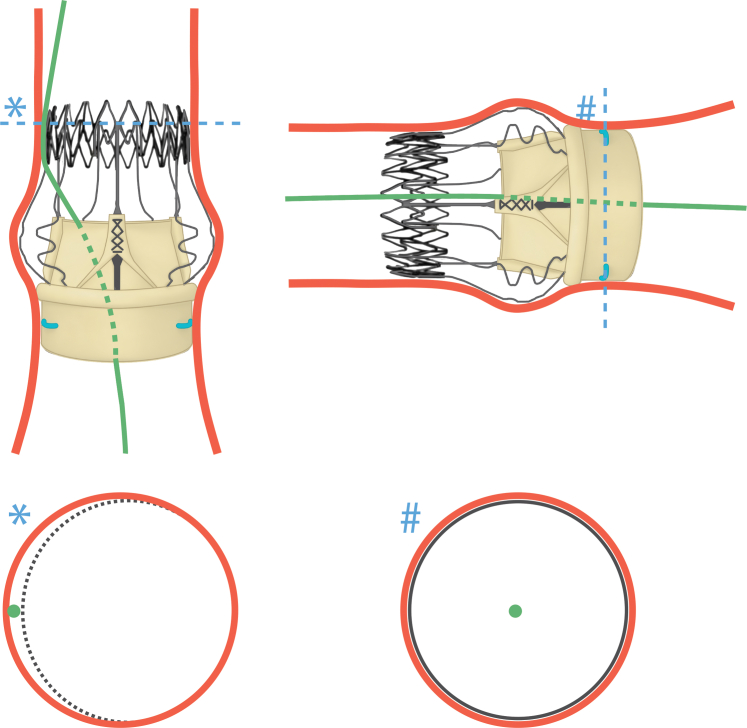
The patient was placed under sedation, and femoral access was obtained. Arterial and venous sheaths were placed, and a temporary pacer was inserted. A pigtail catheter was advanced to the aorta, and aortograms were made to determine the appropriate deployment angle. Next, 2 Perclose devices (Abbott Vascular) were deployed, and a 14-F Edwards delivery sheath was placed. Subsequently, the Perceval valve was crossed with a JR4 catheter and a J-wire. The location of the JR4 catheter was assessed in various extreme angles and believed to be inside the valve frame (Figure 3A). The catheter was removed, and a Sapien 3 Ultra 23-mm valve was advanced over the wire without resistance and deployed successfully with full volume (Figure 3B). The balloon was deflated and pacing stopped. However, the balloon could not be initially retracted. The location of the wire was found to be outside the frame and through a cell on the upper part of the frame (Figure 3C). A sequential balloon inflation followed to dilate the stent cell rather than forceful removal, which could cause THV embolization. This allowed for slow and careful withdrawal of the device. Figure 4 shows the wire path with proper passage at the aortic annulus explaining the successful THV deployment and with inadvertent passage at the level of the sinotubular junction explaining the initial failure to retract the balloon. The Perceval valve was then recrossed with a pigtail catheter and a J-wire. The position was again assessed in multiple angles. However, the Safari wire (Boston Scientific) was again found to be outside the frame when attempting to pass a balloon valvuloplasty for dilatation (Figure 3D). A decision was made to abort further attempts based on a risk/benefit assessment. Final root angiography showed good valve stability (Figure 3E). The valve position and function were confirmed on transthoracic echocardiography (TTE), with a final mean gradient of 20 to 24 mm Hg. No AR or PVL was reported. Wires and sheaths were removed, and the arteriotomy was closed. Video 1 summarizes the procedure.
Figure 3.
Intraprocedural Fluoroscopy
(A) Safari wire was inserted into the left ventricle through a JR4 catheter. Location was believed to be inside the Perceval frame (asterisk). (B) A Sapien 3 Ultra 23-mm valve was advanced over the Safari wire without issue (asterisk). (C) Deployment balloon would not retract through the Perceval frame. Sequential inflations were needed to expand frame cell and be removed slowly (asterisk). (D) Postdilation was attempted—the wire was found to be outside of the frame again (asterisk). Aborted. (E) Final root angiography. Good valve stability was noted (asterisk).
Figure 4.
Wire Passage Across Perceval Valve
Wire path (green) at 2 different angles with cross-sectional views at the level of the sinotubular junction (asterisk) and aortic annulus (hash mark) showing inadvertent and proper passage, respectively.
Discussion
SAVR using Perceval sutureless valves has been shown to be a feasible alternative to conventional prosthetic aortic valves.1,2 The major suggested benefit of decreased operative times due to ease of implantation makes them especially valuable in the setting of minimally invasive approaches and failing prosthetic valves requiring reoperation.3,4 With the growing number of sutureless valve implantations, there has also been an increase in patients presenting with dysfunction and failure of such valves.5 This has raised the question of long-term durability and optimal treatment in case of failure of these valves. The current treatment method of choice is surgical explantation and redo SAVR. However, in recent years, there has been a growing number of case reports and series describing the successful use of ViV-TAVR as an alternative in extreme-risk or inoperable patients.6, 7, 8, 9, 10 Even though the experience with ViV-TAVR in the setting of sutureless valve failure is expanding, there are still important technical challenges and possible complications that arise and warrant caution.
In this case report, we present a patient who underwent ViV-TAVR for a failing Perceval valve that was implanted 7 years before. Even though the THV implantation was successful, the procedure was complicated by an inadvertent wire passage outside of the Perceval frame. Initially, the wire appeared to be inside the frame. However, after the THV was implanted, the wire was found to be outside the frame and inside a cell, preventing the retraction of the deployment balloon. Sequential and repeated inflation of the balloon allowed for expansion of the cell and eventual retraction of the balloon. Even though the position of the wire relative to the frame was assessed extensively in various extreme angles on fluoroscopy, further measures to avoid this complication might be necessary. TEE rather than TTE guidance could be advantageous in reassuring intravalvular wire location. Testing proper wire positioning with the passage of a partially inflated balloon to cross the path and crossing with a pigtail catheter before THV deployment might be helpful. Lastly, manipulation of a catheter in the LV rather than a wire alone could aid in revealing improper wire location. This highlights the increased risk of inadvertent wire passage with Perceval valves that could potentially complicate THV deployment and balloon retraction. Furthermore, a possible future TAVR-in-TAVR to treat bioprosthetic degeneration warrants similar caution as the increased risk of inadvertent wire passage remains.
Follow-up
On postoperative day 1, TTE showed stable bioprosthesis position in absence of AR and PVL. Mean and peak aortic gradients were 26 and 46 mm Hg, respectively, and LVEF was 55% to 60%. The patient was discharged home on postoperative day 2. At 1-month follow-up, repeat TTE again revealed good bioprosthetic stability without AR and PVL. Mean and peak aortic gradients were 24 and 40 mm Hg, respectively, and LVEF was 55% to 60%. Follow-up at 1 year revealed stable gradients and the patient remained in NYHA functional class I without symptoms.
Conclusions
ViV-TAVR is a feasible option for the treatment of failing Perceval sutureless valves. However, special attention must be taken to avoid inadvertent wire passage outside of the frame, which can complicate THV deployment and balloon retraction.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Pre-procedure echocardiography/CTA, procedure summary, post-procedure echocardiography.
References
- 1.Santarpino G., Berretta P., Fischlein T., et al. Operative outcome of patients at low, intermediate, high and 'very high' surgical risk undergoing isolated aortic valve replacement with sutureless and rapid deployment prostheses: results of the SURD-IR registry. Eur J Cardiothorac Surg. 2019;56(1):38–43. doi: 10.1093/ejcts/ezy477. [DOI] [PubMed] [Google Scholar]
- 2.Fischlein T., Folliguet T., Meuris B., et al. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J Thorac Cardiovasc Surg. 2021;161(3):920–932. doi: 10.1016/j.jtcvs.2020.11.162. [DOI] [PubMed] [Google Scholar]
- 3.Berretta P., Andreas M., Carrel T.P., et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry) Eur J Cardiothorac Surg. 2019;56(4):793–799. doi: 10.1093/ejcts/ezz055. [DOI] [PubMed] [Google Scholar]
- 4.Dhanekula A.S., Nishath T., Aldea G.S., et al. Use of a sutureless aortic valve in reoperative aortic valve replacement. JTCVS Tech. 2022;13:31–39. doi: 10.1016/j.xjtc.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinelli M., Schwartz L., Spagnola J., et al. Early structural deterioration of a sutureless bioprosthetic aortic valve. Cardiol Res. 2020;11(2):113–117. doi: 10.14740/cr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangner N., Holzhey D., Misfeld M., et al. Treatment of a degenerated sutureless Sorin Perceval® valve using an Edwards SAPIEN 3. Interact Cardiovasc Thorac Surg. 2018;26(2):364–366. doi: 10.1093/icvts/ivx338. [DOI] [PubMed] [Google Scholar]
- 7.Sun X., Song Z., Soffer D., Pirris J.P. Transcatheter aortic valve-in-valve implantation for early failure of sutureless aortic bioprosthesis. J Card Surg. 2018;33(4):172–175. doi: 10.1111/jocs.13567. [DOI] [PubMed] [Google Scholar]
- 8.Misfeld M., Abdel-Wahab M., Thiele H., et al. A series of four transcatheter aortic valve replacement in failed Perceval valves. Ann Cardiothorac Surg. 2020;9(4):280–288. doi: 10.21037/acs-2020-surd-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Concistrè G., Gasbarri T., Ravani M., et al. Transcatheter aortic valve replacement in degenerated Perceval bioprosthesis: clinical and technical aspects in 32 cases. J Clin Med. 2023;12(19):6265. doi: 10.3390/jcm12196265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilalta V., Piñón P., García de Lara J., et al. Transcatheter aortic valve-in-valve replacement for failed sutureless aortic valves. JACC Cardiovasc Interv. 2023;16(1):122–124. doi: 10.1016/j.jcin.2022.09.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pre-procedure echocardiography/CTA, procedure summary, post-procedure echocardiography.