Abstract
Listeria monocytogenes is a rare and often fatal cause of pericarditis. This paper presents a patient with heart failure with preserved ejection fraction, CD4+ T-cell deficiency, and L monocytogenes constrictive pericarditis. This case highlights a rare association between heart failure and immunodeficiency, in addition to the multidisciplinary management of constrictive pericarditis.
Key Words: acute heart failure, constrictive, diastolic heart failure
Graphical Abstract
History of Presentation
A 59-year-old man presented to the hospital with 1 month of orthopnea, nonbloody diarrhea, malaise, and occasional chest pain. He denied fevers, chills, night sweats, or palpitations. His blood pressure was 112/63 mm Hg, heart rate was 60 beats/min, respiratory rate was 16 breaths/min, and oxygen saturation was 99% on room air. The physical examination was notable for distant heart sounds, S3 gallop, and 1+ bilateral lower extremity edema. Laboratory data demonstrated leukocytosis, thrombocytosis, hyponatremia, elevated pro-B-type natriuretic peptide, elevated alkaline phosphatase, decreased total protein, and hypoalbuminemia (Table 1).
Learning Objectives
-
•
To review the association of heart failure with protein-losing enteropathy and secondary immunodeficiency.
-
•
To understand the presentation, complications, and management of constrictive pericarditis.
Table 1.
Laboratory Values on Initial Presentation
| Normal Range | Result | |
|---|---|---|
| Hematology | ||
| White blood cell, K/mm3 | 4.5-11.0 | 16 |
| Hemoglobin, g/dL | 13.9-16.3 | 12.9 |
| Hematocrit, % | 41.0-53.0 | 40 |
| Platelet count, K/mm3 | 150-350 | 433 |
| Chemistry | ||
| Sodium, mmol/L | 135-148 | 124 |
| Potassium, mmol/L | 3.5-5.1 | 5.8 |
| Chloride, mmol/L | 96-109 | 86 |
| Anion gap, mmol/L | 7-16 | 11 |
| Urea nitrogen, mg/dL | 7-22 | 15 |
| Creatinine, mg/dL | 0.6-1.3 | 0.5 |
| Total protein, g/dL | 6.0-8.2 | 5.2 |
| Albumin, g/dL | 3.5-5.3 | 3.4 |
| Alkaline phosphatase, U/L | 30-120 | 324 |
| Aspartate amino trans, U/L | ≤37 | 40 |
| Alanine amino transferase, U/L | ≤40 | 42 |
| Total bilirubin, mg/dL | ≤1.2 | 0.7 |
| Erythrocyte sedimentation rate, mm/h | ≤20 | 52 |
| C-reactive protein, mg/dL | ≤0.5 | 19.1 |
| Clotting parameters | ||
| INR | 0.9-1.1 | 1.53 |
| Prothrombin time, seconds | 9.3-11.7 | 15.1 |
| APTT, seconds | 24.3-32.2 | 35.7 |
| Thyroid stimulating hormone, mIU/L | 0.4-4.5 | 1.08 |
| Vitamin B12, pg/mL | 232-1,245 | >2,000 |
| Homocysteine, μmol/L | ≤12.2 | 7.2 |
| Methylmalonate, nmol/L | 45-325 | 306 |
| Creatine kinase, total, U/L | 24-195 | 45 |
| hs-troponin, 0 hour, 1 hour, 3 hour, ng/L | ≤22 | 77, 72, 61 |
| Pro-B-type natriuretic peptide, pg/mL | 20-125 | 611 |
APTT = activated partial thromboplastin time; hs = high sensitivity; INR = international normalized ratio.
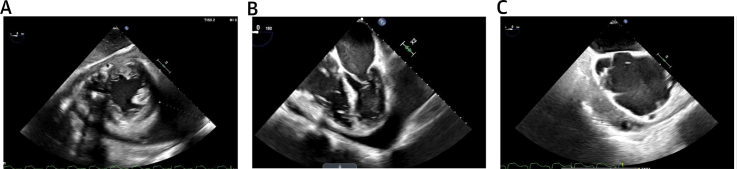
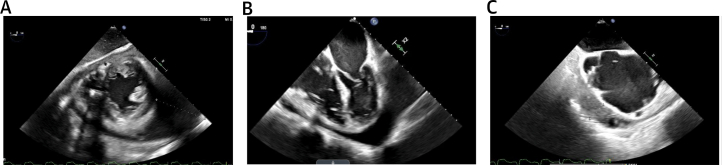
An electrocardiogram was notable for left axis deviation, atrial flutter, ventricular paced rhythm, and left bundle branch block. A transthoracic echocardiogram revealed a large circumferential pericardial effusion measuring 4.0 cm posteriorly without tamponade physiology. The patient underwent pericardiocentesis, during which 960 mL of serosanguinous fluid was drained, and he was discharged after resolution of his presenting symptoms. Subsequent pericardial fluid cultures grew Listeria monocytogenes, and the patient returned to the hospital for further treatment. A transesophageal echocardiogram was obtained to evaluate for endocarditis, which demonstrated a loculated, circumferential pericardial effusion measuring 6 cm, the largest at the apex and anterior regions of the heart (Figure 1). A pericardial window was pursued, but removal of the pericardial effusion was limited due to significant fibrotic material.
Figure 1.
Echocardiography
Transesophageal echocardiogram demonstrating a circumferential, loculated pericardial effusion on (A) transgastric mid-papillary short-axis view, (B) mid-esophageal 4-chamber view, and (C) mid-esophageal 2-chamber view.
Past Medical History
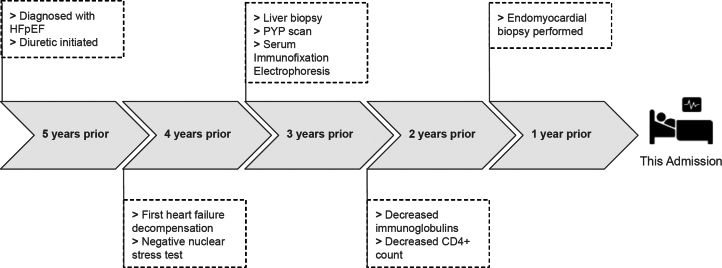
The patient’s medical history was notable for heart failure with preserved ejection fraction (HFpEF), complete heart block (CHB), atrial flutter, and atrial fibrillation. Review of outside medical records demonstrated an exhaustive work-up to determine the etiology of his HFpEF and CHB, comorbidities unusually seen in healthy individuals of 59 years of age (Figure 2). Prior evaluation included nuclear stress testing without inducible areas of ischemia and cardiac pyrophosphate scan without evidence of transthyretin amyloidosis. Right heart catheterization reported elevated right-sided filling pressures: right atrial pressure 14 mm Hg, right ventricular systolic/diastolic pressures 29/3 mm Hg, pulmonary arterial systolic/diastolic pressures 30/12 mm Hg, and pulmonary wedge pressure 11 mm Hg. Endomyocardial biopsy noted mild myocyte hypertrophy with negative Congo red staining for amyloid, and a liver biopsy had been performed with histologic findings supporting congestive hepatopathy. Review of laboratory data revealed a history of hypogammaglobulinemia (immunoglobulin G 234, immunoglobulin M <5, and immunoglobulin A 33), normal kappa and lambda free light chain ratio, and protein electrophoresis with immunofixation without evidence of clonality (Figure 2). Furthermore, a CD4+ T-cell count of 11, hypoproteinemia (total protein 4.6 g/dL), and hypoalbuminemia (albumin 2.5 g/dL) of unclear etiology were also noted 2 years prior to presentation. Ultimately, the patient’s HFpEF and CHB were noted to be idiopathic, and the patient developed worsening volume overload managed with escalating doses of diuretics.
Figure 2.
Clinical and Diagnostic Time Line
HFpEF = heart failure with preserved ejection fraction; PYP = pyrophosphate.
Differential Diagnosis
The differential diagnosis for the patient’s initial presentation of dyspnea included decompensated HFpEF, infection, constrictive pericarditis, and acute coronary syndrome. Broad differential diagnoses were entertained for the etiology of his immunocompromised state: infection, hematologic malignancies, autoimmune diseases, primary immunodeficiency syndromes, malnutrition, and lymphangiectasia.1
Investigations
The patient was started on intravenous ampicillin therapy, and blood cultures were obtained that grew L monocytogenes. An expanded hematologic work-up was pursued, given his known history of CD4+ T-cell deficiency and susceptibility to an opportunistic pathogen (Table 2). Notably, the patient’s CD4+ T-cell count was 18, and flow cytometry demonstrated mature T cells without any phenotypic abnormality. Additional microbiology work-up was pursued, and the following studies were negative: Epstein-Barr virus viral load, cytomegalovirus viral load, human herpesvirus-6 viral load, human immunodeficiency virus, human T-lymphotropic virus 1 and 2, hepatitis serologies, and mycobacterial and fungal cultures of the pericardial fluid (Table 2). Serologic work-up for autoimmune diseases and immunodeficiency syndromes was negative, and antibody titers of influenza, varicella, and rubeola demonstrated a robust immune reaction. Markers of malnutrition, including homocysteine and methylmalonic acid, were normal. Ultimately, infection, primary immunodeficiencies, hematologic malignancies, and malnutrition were ruled out as primary causes of the patient’s immunodeficiency.
Table 2.
Laboratory and Imaging Results
| Laboratory Data | ||
|---|---|---|
| Laboratory Test | Normal Results | Results |
| Hematology | ||
| Absolute CD4+ lymphocytes, mm3 | 458-1,344 | 26 |
| CD8+ lymphocytes, % | 10-36 | 22.4 |
| CD19+ lymphocytes, % | 6-19 | 2.3 |
| Serology | ||
| IgG, mg/dL | 610-1,616 | 529 |
| IgA, mg/dL | 61-348 | 99 |
| IgM, mg/dL | 35-242 | 41 |
| Free kappa light chains, mg/L | 7.0-27.0 | 65.4 |
| Free lambda light chains, mg/L | 5.6-26.3 | 55 |
| Kappa/lambda ratio | 0.26-1.65 | 1.19 |
| Influenza type A and B titers | 1:16, 1:8 | |
| Measles, rubeola, IgG, AU/mL | 288 | |
| Varicella IgG, CU | >4,000 | |
| Antinuclear antibody titer | 1/160 | |
| Microbiology | ||
| HIV-1/2 antigen/antibody screen | Nonreactive | |
| Hepatitis C antibody screen | Nonreactive | |
| Hepatitis B Core antibody, IgG + IgM | Nonreactive | |
| CMV viral load | Not detected | |
| EBV viral load | Not detected | |
| HHV-6 viral load | Not detected | |
| HTLV-I/II antibody screen | Nonreactive | |
| Alpha-1-antitrypsin, stool, mg/dL | <55 | 1,320 |
| Protein, urine, 24-h, mg/24 h | 0-100 | 384 |
| Imaging and Other Findings | |
|---|---|
| Imaging Test | Findings |
| Computed tomography scan of the chest | Pericardial effusion, moderate to large right pleural effusion. |
| Positron emission tomography scan | FDG uptake in pericardial space consistent with known infectious pericarditis, bilateral pleural effusions with adjacent atelectasis, and few mediastinal and left supraclavicular lymph nodes. |
| Flow cytometry (blood) | No definitive phenotypically abnormal population identified. Specimen with mixture of cell types. No increased blasts. Most lymphocytes are mature T cells, and the B cells comprise a mixture of kappa- and lambda-positive cells. T cells present comprise CD4+ and CD8+ cells with latter predominating (inverted CD4/CD8 ratio 0.2). |
CMV = cytomegalovirus; EBV = Epstein-Barr virus; FDG = fluorodeoxyglucose; HHV = human herpesvirus; HIV = human immunodeficiency virus; HTLV-I/II = human T-lymphotropic virus 1 and 2; Ig = immunoglobulin.
Evaluation of hypoproteinemia and hypoalbuminemia included quantification of urine protein and stool alpha 1-antitrypsin levels, and both proteinuria and protein excretion in the stool were observed (Table 2). The patient underwent an esophagogastroduodenoscopy, and biopsies of the duodenum demonstrated duodenal mucosa with ectatic lymphatics, negative Congo red stain for amyloid, and absence of intraepithelial lymphocytosis or villous blunting. Computed tomography imaging was negative for lymphadenopathy or other causes of lymphatic obstruction. Given the results of the diagnostic testing, the patient’s immunodeficiency was suspected to be due to protein-losing enteropathy secondary to increases in venous and lymphatic pressure from decompensated HFpEF.
Management
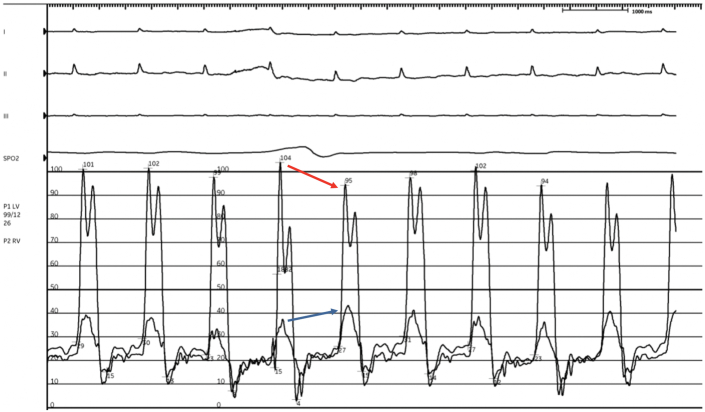
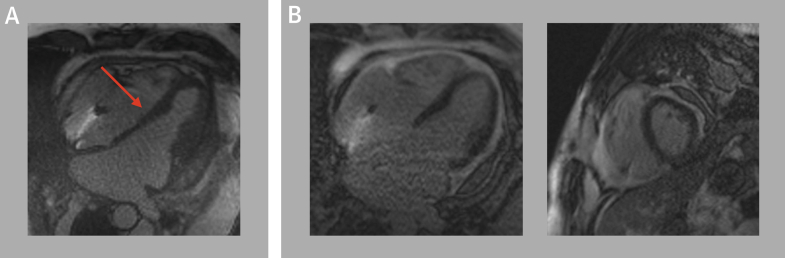
The patient’s listeriosis was managed with a 6-week course of intravenous ampicillin, and the patient was aggressively diuresed with intravenous loop diuretics to treat his decompensated heart failure. However, the patient’s course was complicated by worsening anasarca and dyspnea, with concern for constrictive physiology as the etiology of his worsening decompensation. Review of prior literature revealed that Listeria pericarditis can lead to inflammation and constriction associated with significant mortality.2,3 We thus pursued a simultaneous right and left heart catheterization that revealed elevated right-sided and left-sided filling pressures, a ventricular dip-plateau pattern or “square root” sign during early diastole, and near equalization of diastolic filling pressures: right atrial pressure 20 mm Hg, right ventricular systolic/diastolic pressures 40/20 mm Hg, pulmonary arterial systolic/diastolic pressures 40/20 mm Hg (mean 25 mm Hg), and pulmonary wedge pressure 22 mm Hg (Figure 3). The equalization of diastolic filing pressures led us to consider diagnoses such as restrictive pericarditis, restrictive cardiomyopathy, cardiac tamponade, and severe decompensated heart failure. Cardiac tamponade was thought to be unlikely because the dip-plateau pattern is normally absent given the impaired flow throughout diastole in tamponade. Constrictive pericarditis was distinguished from restrictive cardiomyopathy on hemodynamic tracings by ventricular interdependence manifesting as discordant right and left ventricular pressures changes with respiration. He underwent cardiac magnetic resonance that further demonstrated ventricular interdependence on inspiration, consistent with constrictive pericarditis, and the decision was made to pursue pericardiectomy (Figure 4).
Figure 3.
Right and Left Heart Catheterization Demonstrating Constrictive Physiology and Interventricular Dependence
Red arrow marks decrease in left ventricular pressure, and the blue arrow marks a rise in right ventricular pressure on inspiration.
Figure 4.
Cardiac Magnetic Resonance
Cardiac magnetic resonance demonstrating (A) evidence of intraventricular septal interdependence (red arrow) and (B) absence of late gadolinium enhancement in the myocardium.
Discussion
We report, to our knowledge, the first case of CD4+ T-cell immunodeficiency associated with decompensated HFpEF and L monocytogenes constrictive pericarditis. CD4+ T-cell lymphocytopenia can be caused by a myriad of etiologies including infection, malignancy, drugs, and autoimmune disorders. In this case, a broad differential diagnosis was considered, and our investigations were unrevealing. Idiopathic CD4+ T-cell lymphocytopenia is a rare phenomenon that has yet to be fully understood, and few cases have been reported.4,5 As the patient in this case, most patients present with complications, including neoplasia and infection, in their fourth decade of life.
Interestingly, the patient was concomitantly diagnosed with protein-losing enteropathy that is characterized by loss of proteins in the gastrointestinal tract, leading to hypoalbuminemia and hypogammaglobulinemia. The diagnosis is typically confirmed by direct measurement of elevated alpha-1 antitrypsin levels in the stool. Biopsy of the intestinal wall demonstrating intestinal lymphangiectasia is also supportive of the diagnosis. Chronic elevation of central venous pressure, intestinal wall inflammation, and damage to intestinal lymphatics have been proposed as mechanisms leading to loss of lymphatic fluid.6 Whereas common manifestations of protein-losing enteropathy include diarrhea and volume overload, secondary immunodeficiency due to loss of lymphatic fluid rich in immunoglobulins and proteins is a rare complication of protein-losing enteropathy. Cellular immunity is also impaired in protein-losing enteropathy and marked reductions in CD3+ and CD4+ T cells have been described.7,8 We thus considered an association between the patient’s CD4+ T-cell lymphopenia with his concomitant diagnosis of protein-losing enteropathy.
The reported cardiac causes of protein-losing enteropathy in the literature are most associated with the Fontan procedure in individuals with congenital heart disease.9,10 The Fontan procedure, where the inferior vena cava is connected to the pulmonary artery, has been used as a palliative measure for patients with a single ventricle that decreases the preload of the functional ventricle. However, complications of the procedure include increases in systemic venous procedure that can later lead to protein-losing enteropathy in this unique population. Isolated cases of protein-losing enteropathy secondary to heart failure and constrictive pericarditis, on the other hand, have been rarely described. The present case adds to the reported cases of heart failure leading to protein-losing enteropathy, manifesting as severe secondary cellular immunodeficiency and Listeria inflammatory pericarditis.
L monocytogenes is a gram-positive, intracellular pathogen that is commonly transmitted orally through contaminated foods such as unpasteurized milk products, raw vegetables, and uncooked deli meats. Listeriosis often presents as self-limited gastroenteritis. However, among those who are immunocompromised (eg, pregnant women, older adults, those with primary or secondary immunodeficiencies), listeriosis can manifest as a severe invasive infection associated with a high rate of mortality. Infection with L monocytogenes rarely presents as pericarditis, and to our knowledge only 8 cases have been documented, among which 4 of 8 cases (50%) led to death.2,3 The cases reported in the literature were associated with differing causes of immunodeficiency including corticosteroid use, human immunodeficiency virus, pregnancy, and hematologic malignancies. The present case represents a unique cause of immunodeficiency leading to Listeria pericarditis and pericardial effusion and demonstrates the life-threatening sequelae of an inflammatory pericarditis–constrictive pericarditis.
Follow-Up
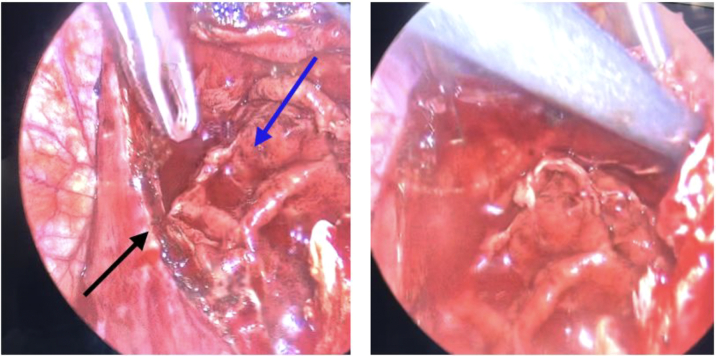
The patient underwent a successful phrenic-to-phrenic pericardiectomy (Figure 5). The pericardium was thickened predominantly over the right ventricle, and the posterior pericardium remained open from his prior pericardial window. A pocket of exudative, fibrinous material was observed in the pericardium surrounding the right ventricle, and the pocket was excised completely. After removal of the pericardium, intraoperatively, the patient’s right-sided filling pressures significantly improved. The patient recovered well postoperatively. After the operation, the patient underwent further intravenous diuresis and inotropic support was discontinued. Repeat CD4+ T-cell count revealed a significant improvement from 18 to 51 during the hospitalization, and the patient was eventually discharged to a subacute rehabilitation facility.
Figure 5.
Intraoperative Images From Pericardiectomy
Images from the phrenic-to-phrenic pericardiectomy that demonstrates thickened pericardium (black arrow) and exudative fibrinous material (blue arrow) overlying the right ventricle. Images courtesy of Dr Hamza Aziz.
Conclusions
The present case demonstrates that HFpEF and elevated filling pressures may be associated with protein-losing enteropathy and supports that significant cellular immunodeficiency, although rare, may be associated with the condition. Complications of protein-losing enteropathy include opportunistic infection, which manifested as Listeria bacteremia and constrictive pericarditis in this case.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to acknowledge Dr Hamza Aziz for providing intraoperative images of the patient's pericardiectomy.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Walker U.A., Warnatz K. Idiopathic CD4 lymphocytopenia. Curr Opin Rheumatol. 2006;18(4):389–395. doi: 10.1097/01.bor.0000231908.57913.2f. [DOI] [PubMed] [Google Scholar]
- 2.Findlater A.R., Haider S., Leto D. Listeria pericarditis in a lymphoma patient: case report and literature review. J Assoc Med Microbiol Infect Dis Can. 2020;5(3):182–186. doi: 10.3138/jammi-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delvallée M., Ettahar N., Loïez C., Decoene C., Courcol R., Wallet F. An unusual case of fatal pericarditis due to Listeria monocytogenes. Jpn J Infect Dis. 2012;65(4):312–314. [PubMed] [Google Scholar]
- 4.Vijayakumar S., Viswanathan S., Aghoram R. Idiopathic CD4 lymphocytopenia: current insights. ImmunoTargets Ther. 2020;9:79–93. doi: 10.2147/ITT.S214139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott-Algara D., Balabanian K., Chakrabarti L.A., et al. Idiopathic CD4+ T-cell lymphocytopenia is associated with impaired membrane expression of the chemokine receptor CXCR4. Blood. 2010;115(18):3708–3717. doi: 10.1182/blood-2009-02-202796. [DOI] [PubMed] [Google Scholar]
- 6.Ozen A., Lenardo M.J. Protein-losing enteropathy. N Engl J Med. 2023;389(8):733–748. doi: 10.1056/NEJMra2301594. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S., Keeton B.R., Salmon A.P., Vettukattil J.J. Acquired combined immunodeficiency associated with protein losing enteropathy complicating Fontan operation. Heart. 2003;89(10):1130. doi: 10.1136/heart.89.10.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller Ch, Wolf H., Göttlicher J., Zielinski C.C., Eibl M.M. Cellular immunodeficiency in protein-losing enteropathy. Dig Dis Sci. 1991;36(1):116–122. doi: 10.1007/BF01300099. [DOI] [PubMed] [Google Scholar]
- 9.Melenovsky V., Kubanek M., Kacer P. Protein-losing enteropathy in an adult with non-ischaemic cardiomyopathy: complete reversal by heart transplantation. ESC Heart Fail. 2018;5(5):842–845. doi: 10.1002/ehf2.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itkin M., Piccoli D.A., Nadolski G., et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol. 2017;69(24):2929–2937. doi: 10.1016/j.jacc.2017.04.023. [DOI] [PubMed] [Google Scholar]