Corresponding Author

Key Words: aortic stenosis, surgical aortic valve replacement, transcatheter aortic valve replacement
Valve-in-valve (ViV) transcatheter aortic valve replacement (TAVR) has revolutionized the management of failed bioprosthetic valves, providing a less invasive option than redo surgical aortic valve replacement (SAVR). ViV procedures are typically straightforward in stented bioprosthetic valves, owing to the sutured stent frame, which provides a reliable anchor and well-established measurements such as the true internal diameter (ID) and leaflet height relative to the stent frame. However, the inherent design of sutureless and stentless surgical valves can pose unique challenges during ViV-TAVR.
In this issue of JACC: Case Reports, Reisinger et al1 present a compelling case of ViV-TAVR in a 79-year-old woman with a failing Perceval sutureless valve, previously implanted during redo-SAVR for severe aortic stenosis and subsequent bioprosthetic valve failure. The patient, deemed inoperable because she posed an extreme surgical risk, underwent ViV-TAVR with a Sapien 3 Ultra valve. The procedure was complicated by inadvertent wire passage outside the Perceval frame, requiring careful management to avoid further complications. This case underscores several critical aspects of ViV-TAVR in sutureless valves. In this editorial, we examine the complexities and technical nuances of TAVR in sutureless and stentless valves, drawing insights from recent studies and our clinical experience.
Sutureless Valves
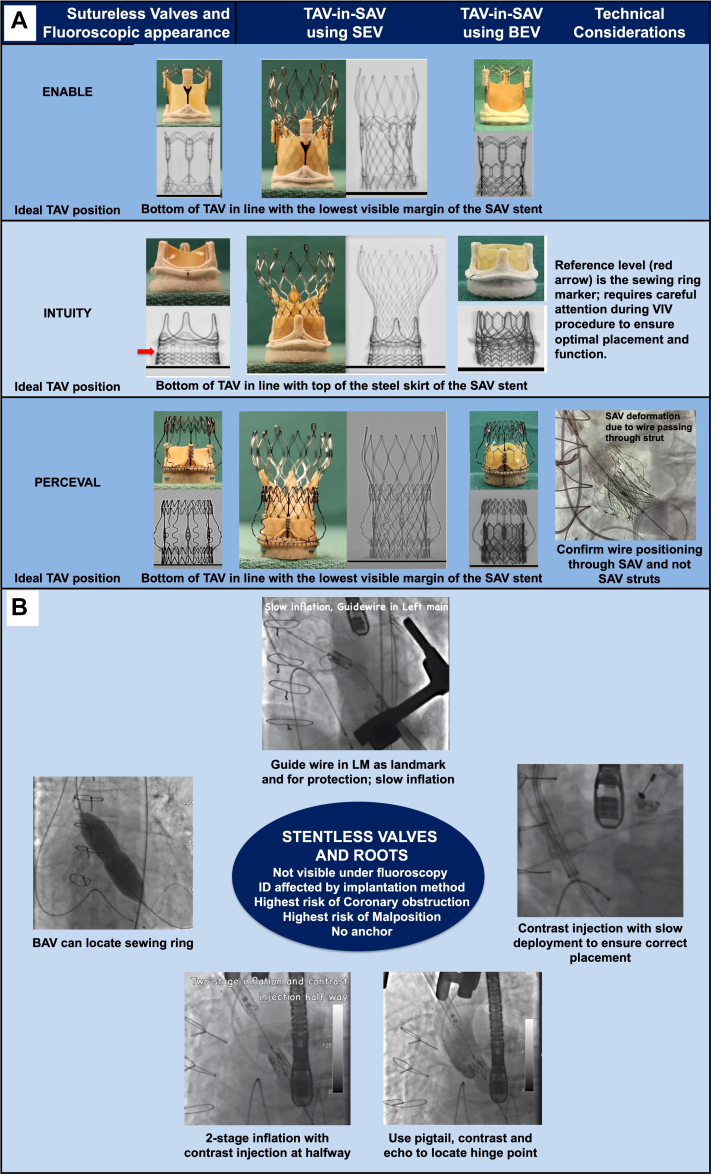
Sutureless valves, such as the Intuity (Edwards Lifescience) and Perceval (LivaNova) valves, have become popular for their ability to reduce procedural times and improve patient outcomes, particularly in those requiring SAVR with concomitant procedures.2 These valves are positioned between stented and sutured valves, offering a good frame to anchor the transcatheter heart valve (THV). The Intuity valve, similar to the Magna Ease, uses a familiar implantation technique; however, precise positioning is crucial. Unlike other valves in which the lower part of the skirt serves as the implant reference level, the Intuity valve's reference level is the sewing ring marker (Figure 1A). This unique feature requires careful attention to detail during ViV-TAVR to ensure optimal placement and function. Another important consideration, particularly for ViV-TAVR in small Intuity valves, is their fracturability, which must be taken into account to optimize hemodynamic performance and postoperative residual gradients.
Figure 1.
Technical Considerations for Valve-in-Valve TAVR
(A) Sutureless and (B) stentless bioprosthetic surgical valves are shown and discussed. BAV = balloon aortic valvuloplasty; BEV = balloon-expandable valve; ID = internal diameter; LM = left main; SAV = surgical aortic valve; SEV = self-expanding valve; TAVR = transcatheter aortic root replacement; VIV = valve-in-valve.
The Perceval valve, resembling a THV, presents unique challenges during ViV-TAVR owing to its taller profile and true sutureless design, which requires meticulous planning to avoid wire entanglement and ensure proper seating. During a ViV-TAVR with the Perceval valve, it is crucial to ensure that the wire crossing the valve into the left ventricle has not passed through the valve struts (Figure 1A). Confirmation in 2 views is necessary before the THV is inserted. Another key concern is the risk of coronary obstruction, given that the absence of a stent frame can lead to leaflet entrapment. Additionally, when the Perceval valve is associated with paravalvular leak (PVL) after ViV-TAVR, plugging the leak may not be as effective because there are no sutures to hold the plug in place on either side, which can lead to the opening up of the neighboring area. Despite these challenges, the fibrosis induced by the sutures used for implantation in sutureless valves may limit distensibility and provide anchorage for THVs, potentially simplifying the ViV procedure.
Stentless Valves
Stentless valves, such as the Freedom Solo, Freestyle, and O’Brien valves, present unique challenges for ViV-TAVR because of the absence of radiopaque markers and the need for different sewing techniques. The lack of fluoroscopic landmarks and the proximity to the coronary ostia can make positioning and sizing of THVs difficult. Additionally, the mechanism of failure in stentless valves, typically cusp perforation or prolapse, further complicates the ViV procedure, inasmuch as identifying the annular plane for correct THV positioning can be difficult.3,4
Stentless valves come in 2 distinct types: valves and roots. Valves are always used with an “inclusive technique,” meaning that sutures are placed inside the native root. This results in 2 unique issues: the anatomic true ID is reduced as the valve reduces the annular area, and the leaflets end up very close to the coronary ostia. This is more pronounced in pericardial stentless valves, such as the Freedom Solo, than in porcine stentless valves, such as the Freestyle or O'Brien valves. If a stentless root is used, the coronary ostia are usually reimplanted, with the right coronary artery positioned higher than the left coronary artery (LCA). Another important consideration is where to size and anchor the THV within stentless roots, inasmuch as typically there is a zone of implantation rather than a clear annular level, and sometimes the annulus can be narrower than the left ventricular outflow tract.
Technical Considerations for ViV-TAVR in Stentless Valves
The risk of malposition, embolization, and coronary obstruction during ViV-TAVR is greater in stentless valves than in stented valves, underscoring the importance of careful planning and precise execution.5,6 Proper prosthesis positioning is essential to reduce the risk of complications, particularly in stentless valves, where fluoroscopic markers and aortic regurgitation may be absent. THV sizing and type selection also play a significant role in ensuring secure anchorage and minimizing complications.
Mitigating risk of coronary obstruction
The risk of coronary obstruction is higher in stentless valves than in stented valves because of the absence of a stent frame.5,6 Leaflets in stented valves are usually sutured within the stent frame, leaving a gap between them and the coronary ostia even when pushed out with a THV. By contrast, the leaflets in stentless valves can be easily pushed to block the coronary ostia, increasing the risk of coronary obstruction after ViV-TAVR. In addition to established coronary risk mitigation strategies such as the chimney/snorkel stent technique and the bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) technique, procedural strategies for reducing the risk of coronary occlusion during ViV-TAVR in stentless valves may include using a smaller-diameter or underfilled balloon-expandable THV, implanting the THV more deeply, and considering recapturable self-expanding devices that can be deployed partially to allow for assessment of coronary flow before complete release.
Optimizing THV positioning
The absence of radiopaque markers in stentless valves poses challenges for THV positioning during ViV-TAVR. Compared with stented valves, stentless valves have a higher risk of THV embolization and migration, underscoring the need for precise positioning, sizing, and secure anchorage of the THV.6 Techniques such as placing a pigtail catheter at the base of a leaflet, using multiple injections of contrast material, and positioning a wire in the left main coronary artery can assist in THV positioning (Figure 1B). Slow deployment of the prosthesis and continuous injection of contrast material at the aortic root level can aid in achieving accurate deployment and minimizing complications. Balloon verification for the waist on the table can also help guide the level of implantation. The fibrosis induced by the sutures used for implantation in stentless valves may offer anchorage for THVs, reducing the risk of malposition and embolization.
Sizing considerations in stentless valves
THV sizing in stentless valves is crucial for a successful ViV-TAVR. Whereas the ID and true ID of stentless roots can be used as a reference, the final ID of stentless valves will be influenced by the suturing technique and the diameter of the native root in which it is implanted. Homografts, which are historically one of the most frequently used stentless valves, pose a challenge in sizing because the final dimensions can vary from the initial size. Complementing measurements with computed tomography or 3-dimensional transesophageal echocardiography can help determine the appropriate THV size intraoperatively. Oversizing the THV is often necessary for adequate anchoring, especially in cases of regurgitation and torn leaflets.
Conclusions
TAVR in stentless and sutureless surgical valves presents unique challenges that require careful consideration and technical expertise. However, with meticulous planning, precise positioning techniques, and the use of appropriate THV sizing, successful outcomes can be achieved in ViV-TAVR in sutureless and stentless valves. Further research and innovation in valve design and procedural techniques are needed to overcome the challenges associated with ViV-TAVR and improve outcomes for patients with failing surgical bioprosthetic valves.
Funding Support and Author Disclosures
Dr Bapat has served as a consultant for Medtronic, Edwards Lifesciences, Abbott, Anteris, 4C Medical, and Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Reisinger M., James E., Kachel M., Kodali S.K., George I. Perceval ViV-TAVR: perception isn’t always reality. JACC Case Rep. 2024;29(16) [Google Scholar]
- 2.Flynn C.D., Williams M.L., Chakos A., et al. Sutureless valve and rapid deployment valves: a systematic review and meta-analysis of comparative studies. Ann Cardiothorac Surg. 2020;9(5):364–374. doi: 10.21037/acs-2020-surd-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bapat V., Attia R., Redwood S., et al. Use of transcatheter heart valves for a valve-in-valve implantation in patients with degenerated aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg. 2012;144(6):1372–1379. doi: 10.1016/j.jtcvs.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 4.Aurigemma C., Burzotta F., Vergallo R., et al. Transcatheter aortic valve implantation to treat degenerated surgical bioprosthesis: focus on the specific procedural challenges. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.895477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro H.B., Rodés-Cabau J., Blanke P., et al. Incidence, predictors, clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018;39(8):687–695. doi: 10.1093/eurheartj/ehx455. [DOI] [PubMed] [Google Scholar]
- 6.Choi C.H., Cheng V., Malaver D., et al. A comparison of valve-in-valve transcatheter aortic valve replacement in failed stentless versus stented surgical bioprosthetic aortic valves. Catheter Cardiovasc Interv. 2019;93(6):1106–1115. doi: 10.1002/ccd.28039. [DOI] [PMC free article] [PubMed] [Google Scholar]