Abstract
Objective
Robotic thoracic surgery provides another minimally invasive approach in addition to video-assisted thoracoscopic surgery (VATS) that yields less pain and faster recovery compared with open surgery. However, robotic incisions are generally placed more inferiorly, which may increase the risk of intercostal nerve injury that affects the abdominal wall. We hypothesized that a robotic approach causes greater ipsilateral rectus muscle atrophy compared with open and VATS approaches.
Methods
The cross-sectional area and density of bilateral rectus abdominis muscles were measured on computed tomography scans in patients who underwent lobectomy in 2018. The differences between the contralateral and ipsilateral muscles were compared between preoperative and 6-month surveillance scans. Changes were compared among the open, VATS, and robotic approaches through a mixed effects model after adjustments of correlation and covariates.
Results
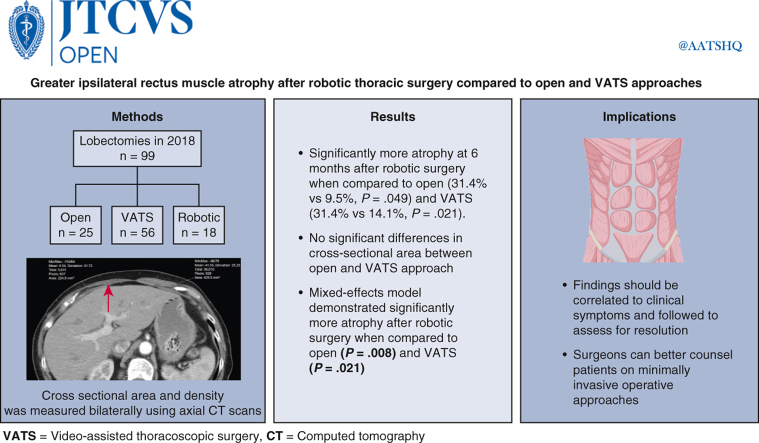
Of 99 lobectomies, 25 (25.3%) were open, 56 (56.6%) VATS, and 18 (18.1%) robotic. The difference between the contralateral and ipsilateral rectus muscle cross-sectional area was significantly larger at 6 months after robotic surgery compared with open (31.4% vs 9.5%, P = .049) and VATS (31.4% vs 14.1%, P = .021). There were no significant differences in the cross-sectional area between the open and VATS approach.
Conclusions
In this retrospective analysis, there was greater ipsilateral rectus muscle atrophy associated with robotic thoracic surgery compared with open or VATS approaches. These findings should be correlated with clinical symptoms and followed to assess for resolution or persistence.
Key Words: muscle atrophy, intercostal nerve injury, lobectomy, robot assisted, rectus abdominis
Graphical Abstract
Greater ipsilateral rectus muscle atrophy after robotic thoracic surgery compared with open and VATS approaches.
Measurement of rectus abdominis pre- and postoperatively using computed tomography scans.
Central Message.
Greater ipsilateral rectus muscle atrophy was associated with robotic thoracic surgery when compared with open or VATS approaches. Patients should be followed to assess for resolution of symptoms.
Perspective.
Robotic thoracic surgery was associated with greater ipsilateral rectus muscle atrophy when compared with open or VATS approaches. This finding could be due to the more inferior port placement affecting intercostal nerves that innervate the abdominal wall. Findings from this study will better allow surgeons to adequately counsel patients regarding minimally invasive operative approaches.
Robotic surgery offers a minimally invasive approach, in addition to video assisted thoracoscopic surgery (VATS), that yields less pain1 and a faster recovery time compared with open surgery.2 When compared with traditional approaches, robotic incisions are generally placed more inferiorly, which may increase the risk of intercostal nerve injury that affects the abdominal wall.3,4 This may lead to symptoms such as tingling, numbness, muscle atrophy, and the development of pseudohernias.5, 6, 7
The rectus abdominis muscle is innervated by the intercostal nerves T7 to T12 and damage to these nerves is less likely during a thoracotomy where the incisions are typically made between intercostal nerves T4 and T6.4,6, 7, 8 However, port placement during multiport VATS may damage the intercostal nerves that innervate the rectus abdominis.4 Previous studies have evaluated the effect of different surgical approaches on the development of this nerve damage by assessing nerve conductance and the development of pseudohernias in the rectus abdominis.5,9,10 One study found that among patients who underwent a thoracotomy, the greatest rate of total nerve conduction block at the intercostal nerve right above the incision site.8 This nerve damage was attributed to the use of a rib spreader during a thoracotomy and may help establish a cause of atrophy in the rectus abdominis. There have been other studies that have reported paralysis and atrophy of the rectus abdominis muscle during surgery.4,7,11 However, to our knowledge, there are no studies that have evaluated the degree of muscle loss between different surgical approaches.
The objective of this study is to compare the degree of muscular atrophy among open, VATS, and robotic approaches. We hypothesize that robotic surgery causes greater ipsilateral rectus muscle atrophy compared with open and VATS approaches.
Methods
Study Design
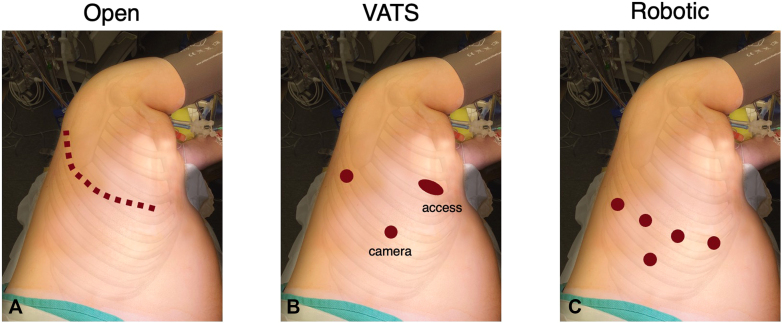
Patients who underwent lobectomy at our institution in 2018 were identified and classified by initial operative approach. Patients underwent a thoracotomy, VATS, or robotic-assisted approach. The approximate incision and port placement sites are depicted in Figure 1. In general, thoracotomies were performed in the fourth or fifth intercostal space; VATS access incisions were made in the fourth or fifth intercostal space anteriorly, whereas the camera and retraction incisions were made at about the seventh or eighth intercostal space; robotic incisions were made at about the seventh or eighth intercostal spaces, with the assistant port in the ninth intercostal space. For robotic cases, the specimens were usually extracted from the assistant port site. Those who underwent sternotomy, and those who did not have a 6-month follow-up scan available, were excluded. The study was approved by the Stanford University Institutional Review Board (No. 53467; December 2, 2019).
Figure 1.
Representative example of incision and port placement sites. A, Thoracotomy. B, Video-assisted thoracoscopic surgery. C, Robot-assisted thoracoscopic surgery approach.
Rectus Abdominis Muscle Measurements
Axial images from chest computed tomography (CT) scans were used to measure the rectus abdominis muscles. The upper bundle of the right and left rectus abdominis just inferior to the costal margin were manually traced using a polygon region of interest tool, and the cross-sectional area and radio density were recorded. Because CT scans of the chest include the upper abdomen, this upper bundle could be measured on our routine preoperative and surveillance scans. Matching locations were measured for comparisons between scans. The measurements were taken from scans at the following time points: preoperative, 6 months after surgery, and 12 months after surgery. In the majority of cases, these scans were performed as part of routine surveillance after treatment of primary lung cancer. A 1-month margin was provided on either side of the 6-month and 12-month time points. Measurements were taken for both the ipsilateral and contralateral sides. The reviewers were blinded to the surgical approach while measuring the rectus abdominis muscles. All measurements were made using Sectra PACS viewer IDS7 version 20.2.14 (Sectra Medical).
Statistical Analyses
The difference between the ipsilateral and contralateral cross-sectional area and density for the rectus abdominis muscle were calculated for each patient. Patients with extreme differences (more than 1.5 × interquartile range [IQR]) between the preoperative ipsilateral and contralateral cross-sectional area and density were excluded from the study.
Patient baseline characteristics were reported using frequencies and proportions for categorical variables and were compared using Pearson χ2 or Fisher exact test while continuous variables were reported as median and interquartile range (IQR) and compared using Kruskal-Wallis test.
The percent atrophy in the rectus abdominis muscle was calculated by using the difference between the preoperative and postoperative measurements, divided by the preoperative measurement. This was performed for both ipsilateral and contralateral sides, evaluating both cross-sectional area and density, at 6 months and 12 months after surgery. A positive value for percent atrophy indicates a decrease in the cross-sectional area or density of the muscle after surgery, whereas a negative value indicates an increase.
Descriptive analyses were first performed on the preoperative as well as 6- and 12-month postoperative cross-sectional area and density. Univariate analyses were performed on the percent atrophy to estimate the average changes from preoperative to postoperative. One sample t test was used to determine if percent atrophy of the cross-sectional area or density was significantly larger than 0.
To adjust correlations among repeated measurements from the same patient and reduce confounding from covariates, a mixed effects model was fitted by setting the patients as the random effect, including surgery laterality, initial surgical approach, any variables with P value < .2 in the modeling exploration, and their interaction as covariates. The adjusted estimations of differences in muscle atrophy among operative approaches were then obtained from the mixed effects model. Secondary analyses were performed to compare percent atrophy with 12-month postoperative cross-sectional area and density. Patients with missing 12-month scan data were excluded from the secondary analyses.
All analyses were performed in IBM SPSS Statistics version 26, and SAS version 9.4 (SAS Institute Inc). All tests of significance were 2-sided with the value of alpha for statistical significance of P < .05.
Results
We identified 108 patients who underwent lobectomy in 2018 and had both preoperative and 6-month follow up CT scan images available (Figure 2). Three patients were excluded due to a sternotomy approach. The preoperative difference in rectus muscle area and density were 3.0 ± 91.2 mm2 and 0.3 ± 10.2 Hounsfield units, respectively. Six patients were excluded for baseline rectus muscle asymmetry, considered as a difference > 1.5 × IQR between the preoperative ipsilateral and contralateral cross-sectional area and density; chart review showed that 1 patient had undergone prior thoracic surgery and 1 patient had prior chest trauma.
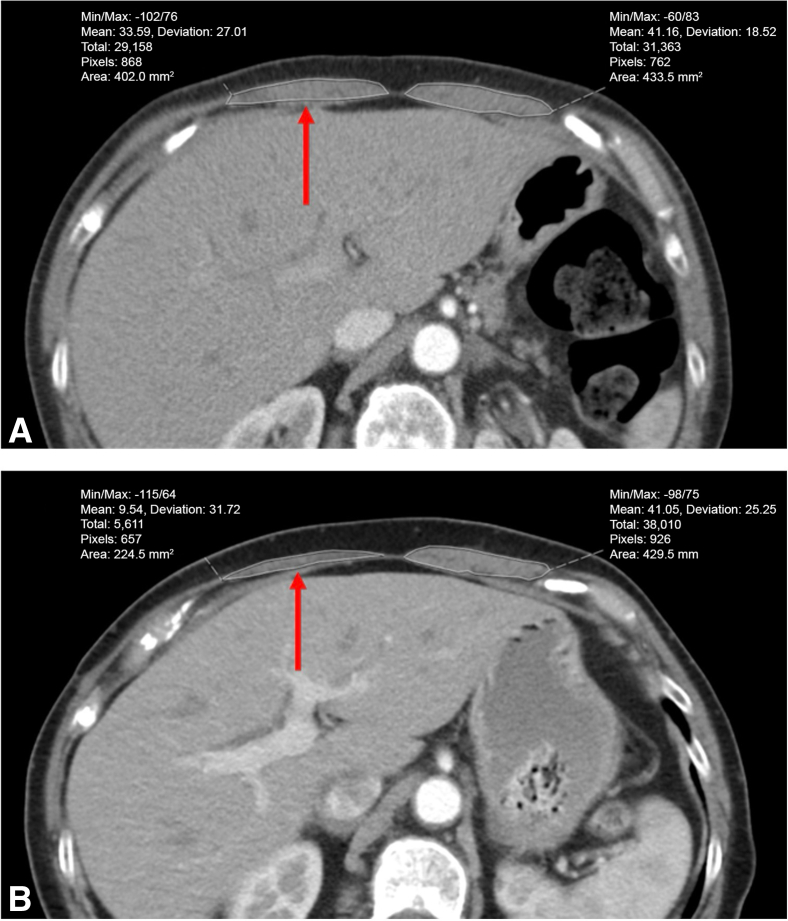
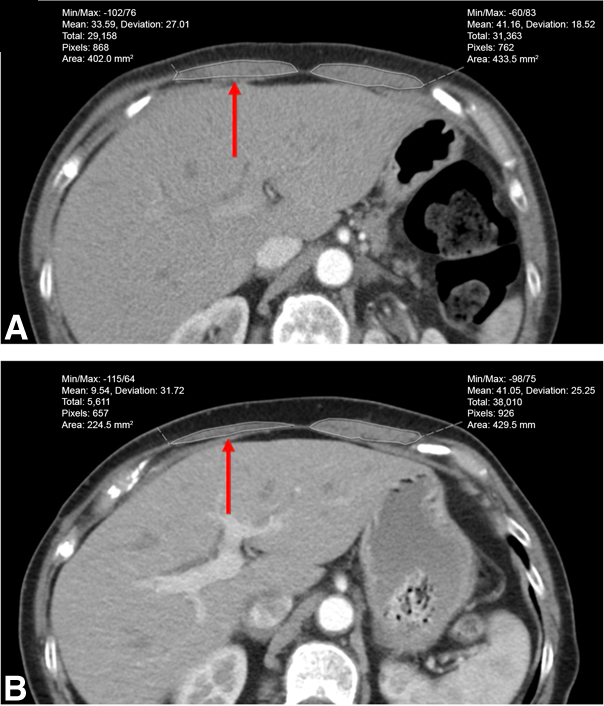
Figure 2.
Representative example of rectus abdominis measurement on axial chest computed tomography scans. A, Preoperative. B, Six months postoperative. There is 44% atrophy in the ipsilateral rectus muscle cross-sectional area on the right (red arrow), and only 1% atrophy in contralateral rectus muscle cross-sectional area on the left.
Of the 99 remaining patients, 56 (56.6%) patients underwent a VATS approach, 25 (25.3%) underwent an open approach, and 18 (18.1%) underwent a robotic approach (Table 1). One robotic and 2 VATS cases were converted to an open approach and classified as the original approach. There was no statistical difference in demographic characteristics, including age (P = .40), gender (P = .18), and preoperative body mass index (P = .36). Other patient characteristics, including length of stay (P < .01), length of surgical case (P < .001), disease indication (P = .01), cancer stage (P = .05), and tumor size (P = .032), were significantly different among the operative approaches (Table 1). Of the 99 patients in the study cohort, 77 (77.8%) patients had a 12-month scan. Among these, 43 (55.8%) underwent a VATS approach, 19 (24.7%) underwent an open approach, and 15 (19.5%) underwent a robotic approach.
Table 1.
Patient characteristics
| Demographic characteristic | Total (N = 99) | VATS (n = 56) | Open (n = 25) | Robot (n = 18) | P value |
|---|---|---|---|---|---|
| Age (y) | 67 (59-74) | 68 (62-74) | 65 (55-74) | 66 (62-74) | .40∗ |
| Gender | .18† | ||||
| Male | 43 (43.4) | 24 (42.9) | 14 (56.0) | 5 (27.8) | |
| Female | 56 (56.6) | 32 (57.1) | 11 (44.0) | 13 (72.2) | |
| Preoperative body mass index | 25.6 (22.9-30.6) | 24.9 (22.4-30.5) | 27.0 (23.4-33.0) | 26.2 (23.7-29.0) | .362∗ |
| Length of stay (d) | 4 (3-5) | 3 (3-4) | 4 (4-6) | 4 (3-5) | .002∗ |
| Length of surgical case (min) | 232 (188-308) | 206.5 (164.5-262) | 266 (227-343) | 317.5 (241-362) | <.001∗ |
| Disease indication | .012‡ | ||||
| Primary lung cancer | 81 (82.7) | 50 (89.3) | 17 (68.0) | 14 (82.4) | |
| Lung metastases | 14 (14.3) | 5 (8.9) | 8 (32.0) | 1 (5.9) | |
| Other | 3 (3.1) | 1 (1.8) | 0 (0.0) | 2 (11.8) | |
| Stage | .048‡ | ||||
| I | 59 (59.6) | 40 (71.4) | 9 (36.0) | 10 (55.6) | |
| II | 22 (22.2) | 10 (17.9) | 7 (28.0) | 5 (27.8) | |
| III | 3 (3.0) | 1 (1.8) | 2 (8.0) | 0 (0.0) | |
| N/A | 15 (15.2) | 5 (8.9) | 7 (28.0) | 3 (16.7) | |
| Tumor size (cm) | 2.5 (1.7-3.6) | 2.4 (1.5-3.6) | 3.5 (2.2-5.5) | 2.2 (1.8-2.9) | .032∗ |
Values are presented as median (interquartile range) or n (%). VATS, Video-assisted thoracoscopic surgery; N/A, not available.
P value from Kruskal-Wallis test.
P value from χ2 test.
P value from Fisher exact test.
Rectus Atrophy
For all 99 patients, the ipsilateral rectus cross-sectional area decreased from 508.4 mm2 preoperatively to 412.8 mm2 at 6 months after surgery, corresponding to a percent atrophy of 15.8% ± 0.29% (median, 16.3%; IQR, −4.9% to 37.0%; P < .001) (Table 2). For the 77 patients who had a 12-month scan available, the ipsilateral rectus cross sectional area decreased from 507.3 mm2 preoperatively to 440.0 mm2 at 12 months after surgery, corresponding to a percent atrophy of 10.9% ± 0.25% (median, 10.8%; IQR, −1.7% to 26.6%; P < .001). There was no significant difference in ipsilateral rectus density at 6 months or 12 months after surgery compared with preoperatively. There was no significant difference in contralateral rectus cross-sectional area or density at 6 months or 12 months after surgery compared with preoperatively.
Table 2.
Atrophy between preoperative and postoperative rectus area and density among all operative approaches
| Surgery laterality | Preoperative | Postoperative | Percent atrophy∗ | P value (atrophy ≠ 0) |
|---|---|---|---|---|
| 6-mo follow-up | ||||
| Area (mm2) | ||||
| Ipsilateral | <.001† | |||
| Mean ± SD | 508.4 ± 217.0 | 412.8 ± 210.2 | 15.8 ± 0.29 | |
| Median (IQR) | 469.3 (346.9 to 623.3) | 372.7 (247.4 to 546.1) | 16.3 (−4.9 to 37.0) | |
| Contralateral | .973 | |||
| Mean ± SD | 511.4 ± 223.1 | 494.1 ± 206.1 | −0.1 ± 0.25 | |
| Median (IQR) | 475.1 (340.4 to 644.7) | 470.7 (330.4 to 616.3) | 1.6 (−8.3 to 12.9) | |
| Density (HU) | ||||
| Ipsilateral | .273 | |||
| Mean ± SD | 30.6 ± 18.5 | 24.5 ± 19.0 | 16.8 ± 1.52 | |
| Median (IQR) | 33.9 (23.3 to 42.1) | 27.7 (16.7 to 37.5) | 17.3 (−10.8 to 40.7) | |
| Contralateral | .209 | |||
| Mean ± SD | 30.8 ± 10.0 | 32.2 ± 15.9 | −29.4 ± 2.31 | |
| Median (IQR) | 33.5 (19.4 to 44.1) | 35.5 (24.6 to 42.2) | 1.0 (−32.3 to 21.3) | |
| 12-mo follow-up | ||||
| Area (mm2) | ||||
| Ipsilateral | <.001∗ | |||
| Mean ± SD | 507.3 ± 209.3 | 440.0 ± 203.1 | 10.9 ± 0.25 | |
| Median (IQR) | 469.2 (351.0 to 618.8) | 373.0 (293.2 to 548.7) | 10.8 (−1.7 to 26.6) | |
| Contralateral | .972 | |||
| Mean ± SD | 505.3 ± 209.4 | 490.9 ± 219.3 | −0.1 ± 0.30 | |
| Median (IQR) | 476.5 (349.4 to 644.7) | 449.4 (349.1 to 605.7) | 3.4 (−5.3 to 13.6) | |
| Density (HU) | ||||
| Ipsilateral | .484 | |||
| Mean ± SD | 32.7 ± 15.4 | 28.9 ± 17.7 | −25.0 ± 3.10 | |
| Median (IQR) | 33.9 (24.9 to 42.1) | 31.1 (20.4 to 38.7) | 13.8 (−17.2 to 31.9) | |
| Contralateral | .130 | |||
| Mean ± SD | 32.4 ± 16.0 | 32.8 ± 15.3 | −37.8 ± 2.15 | |
| Median (IQR) | 34.0 (21.8 to 44.6) | 35.0 (24.6 to 42.6) | 1.3 (−25.4 to 22.1) |
IQR, Interquartile range; HU, Hounsfield units.
Atrophy = (preoperative – postoperative)/preoperative.
P value < .05, atrophy is significantly different from 0.
Ipsilateral Rectus Atrophy by Operative Approach
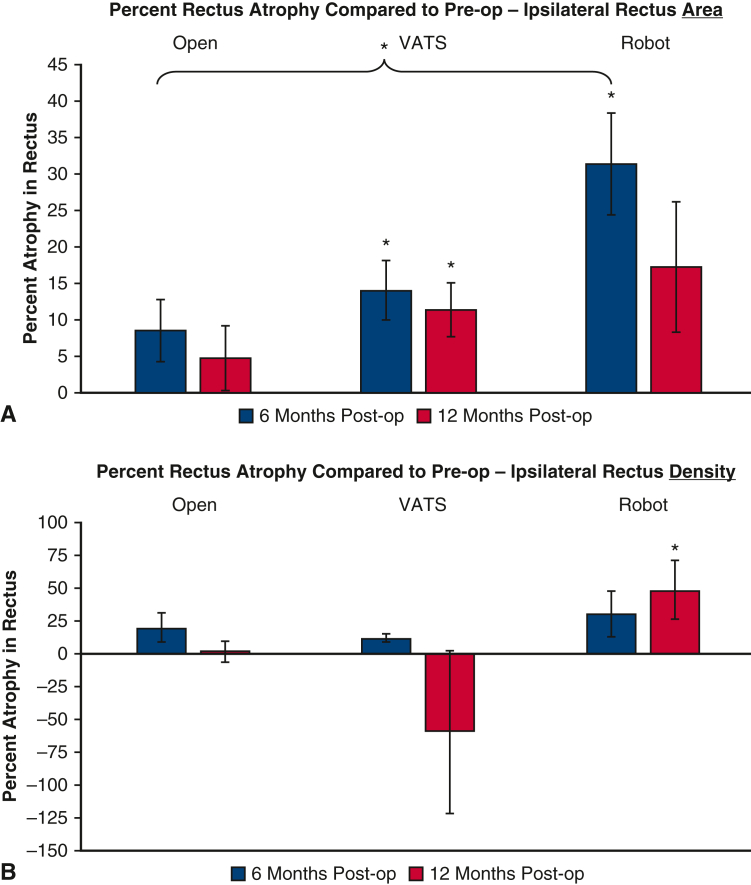
Patients who underwent a VATS approach demonstrated significant ipsilateral rectus atrophy by cross-sectional area of 14.1% ± 30.6% (P = .001; n = 56; median, 12.4; IQR, −6.8% to 31.4%) at 6 months, which decreased to 11.4% ± 24.0% (P = .003; n = 43; median, 8.7%; IQR, −0.8% to 29.8%) at 12 months (Figure 3, A). Patients who underwent a robotic approach demonstrated significant ipsilateral rectus atrophy by cross-sectional area of 31.4% ± 29.7% (P < .001; n = 18; median, 36.8%; IQR, 24.8% to 49.2%) at 6 months, which decreased to 17.3% ± 34.6% (P = .074; n = 15; median, 19.3%; IQR, 5.5% to 31.6%) at 12 months and was no longer significant. Patients who underwent an open approach demonstrated ipsilateral rectus atrophy by cross-sectional area of 8.6% ± 21.4% (P = .056; n = 25; median, 7.0%; IQR, −4.5% to 21.7%) at 6 months and 4.8% ± 19.4% (P = .30; n = 19; median, 4.5%; IQR, −9.6% to 12.3%) at 12 months, but these were not significant.
Figure 3.
Percent atrophy in the ipsilateral rectus abdominis muscle. A, Using cross-sectional area. B, Using density ratios, by operative approach. Pre-op, preoperative; VATS, video-assisted thoracoscopic surgery; post-op, postoperative. ∗P value < .05.
Patients who underwent a robotic approach demonstrated significant ipsilateral rectus atrophy by density of 48.2% ± 83.0% (P = .049; n = 14; median, 27.5%; IQR, 8.8% to 71.7%) at 12 months (Figure 3, B). There were no other operative approaches with significant ipsilateral rectus atrophy by density at 6 or 12 months.
Patents who underwent a robotic approach had greater ipsilateral rectus atrophy by cross-sectional area at 6 months compared with those who underwent an open approach (31.4% vs 8.5%; difference, 23%; 95% CI, 1%-45%; P = .039). There were no other differences in ipsilateral rectus atrophy by cross-sectional area or density when comparing other operative approaches.
Mixed Effects Modeling of Change in Rectus Area at 6 Months
Although tumor size and length of case were distributed differently amongst the 3 study groups, neither of these variables demonstrated any significant effect on relative atrophy (tumor size P value = .26, length of case P = .34 in the modeling exploration) and were not included in the final random effects model. After controlling for correlations and confounding in the mixed effects model, patients who underwent a robotic approach had significantly greater ipsilateral rectus atrophy by cross-sectional area compared with those who underwent an open approach (31.4% vs 8.5%; difference, 23%; 95% CI, 6%-40%; P = .008) and VATS approach (31.4% vs 14.1%; difference, 18%; 95% CI, 3%-33%; P = .021) at 6 months (Table 3). There was no significant difference in ipsilateral rectus atrophy by cross-sectional between VATS and open approaches at 6 months. There was no significant contralateral rectus atrophy by cross-sectional area in this model. See Figure 4 for a graphical abstract of the study.
Table 3.
Mixed effect model estimates of rectus area atrophy∗
| Difference in atrophy comparing operative approaches | Ipsilateral |
Contralateral |
||
|---|---|---|---|---|
| Estimated difference in atrophy† (95% CI) | P value | Estimated difference in atrophy† (95% CI) | P value | |
| Open (ref.) vs robot | −0.23 (−0.40 to −0.06) | .008‡ | 0.04 (−0.13 to 0.21) | .62 |
| Open (ref.) vs VATS | −0.05 (−0.18 to 0.07) | .40 | 0.06 (−0.07 to 0.19) | .40 |
| Robot (ref.) vs VATS | 0.18 (0.03 to 0.33) | .021‡ | 0.01 (−0.14 to 0.16) | .87 |
ref., Reference category; VATS, video-assisted thoracoscopic surgery.
Atrophy = (preoperative – postoperative)/preoperative.
Estimated difference in atrophy = (Atrophy)Approach, Comparative – (Atrophy)Approach, Reference. Negative value of estimated difference denotes greater atrophy.
P < .05.
Figure 4.
Graphical abstract. Greater ipsilateral rectus muscle atrophy after robotic thoracic surgery compared with open and video-assisted thoracoscopic surgery (VATS) approaches. CT, Computed tomography.
Discussion
Robotic surgery was associated with a greater decrease in the ipsilateral cross-sectional area of the rectus abdominis muscle at 6 months after surgery compared with both open and VATS approaches. By the 12-month surveillance scan, robotic surgery was associated with a decrease in the ipsilateral density of the rectus muscle. These results suggest that robotic surgery, when compared with other operative approaches, may result in disproportionate atrophy and nerve injury to the rectus abdominis muscle. As robotic lobectomy becomes more common, these findings will help surgeons counsel patients on postoperative recovery.
Intercostal nerve damage can develop due to the compression of the intercostal nerve during port placement for a VATS or robotic approach or the use of a retractor during an open approach.11,12 In many cases, the nerve conduction block due to nerve damage can persist even after the removal of the port or retractor.12 This nerve damage can lead to several postoperative complications, including numbness, pseudohernias, and chronic pain around the rectus abdominis. Additionally, previous studies have identified neuropathic pain as a major contributor to chronic postoperative pain.13,14 It is unclear why pseudohernias develop in some patients but not in others, and in different areas of the abdominal wall and flank. Furthermore, in a questionnaire study by Maguire and colleagues,15 40% of patients reported that postoperative pain limits their daily activities. This inactivity after surgery may lead to worsening skeletal muscle loss, which is associated with worse postoperative outcomes.16
Postoperative pseudohernias, also referred to as flank bulges, have been described across many specialties that employ flank incisions.17, 18, 19, 20 These pseudohernias are characterized by local nerve injury and muscle weakness without an accompanying hernia defect. However, there have only been a few studies that have evaluated nerve damage and its effect on rectus abdominis atrophy and pseudohernias following thoracic surgery.4,6,8,9,21 Before this study, rectus atrophy had not been described in any case series nor comparison. In previous studies, nerve damage was assessed by either using electrical stimulation to test the perception threshold before and after surgery or the Short-form McGill Pain Questionnaire.5,9 The findings from these studies found that nerve damage was more severe in a VATS approach than in an open approach.5,9 Our current study’s evaluation of rectus atrophy does not appear to show a similar pattern, given that our results demonstrated no significant differences between open and VATS approaches when assessing atrophy outcomes. We suspect the observed increase in atrophy among patients undergoing robotic surgery may be a result of the more inferior port placement, which occupies a larger number of rib spaces compared with VATS approaches—potentially injuring more nerves that serve the rectus abdominis. Additionally, patients with rectus nerve damage may also develop the flank bulge, making them more aware of the nerve damage. Our practice is aware of at least 2 patients whose right-sided pain and tenderness were substantial enough to prompt clinical evaluation for cholecystitis several months after robotic thoracic surgery.
This study has a few limitations. First, this is a single-institution retrospective study with a small sample size and a limited number of thoracic surgeons. Second, we do not know whether the degree of rectus muscle atrophy correlates to symptoms such as neuropathic pain or abdominal wall bulging. Further work on this topic is necessary to better understand the relationship between the degree of rectus atrophy and the development of clinical symptoms. Lastly, we have limited long-term follow-up scans, which limits our ability to determine if the atrophy eventually resolves. This is important to consider as nerve recovery can take as long as several months to years.
Conclusions
In this retrospective study, robotic thoracic surgery was associated with greater ipsilateral rectus muscle atrophy when compared with both open and VATS surgery. This could be a result of more inferior port placement affecting intercostal nerves that innervate the abdominal wall. Additional work should be done to determine the influence of alternative port placement on rectus atrophy. These findings should be correlated with clinical symptoms and followed to assess for resolution or persistence. Further evaluation of the incidence and attendant influences of this atrophy phenotype will better allow surgeons to adequately counsel patients regarding minimally invasive operative approaches.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
IRB Approval: The study was approved by the Stanford University Institutional Review Board (#53467).
References
- 1.Bendixen M., Jorgensen O.D., Kronborg C., Andersen C., Licht P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 2.Yan T.D., Black D., Bannon P.G., McCaughan B.C. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27(15):2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 3.Ashton R.C., Jr., Connery C.P., Swistel D.G., DeRose J.J., Jr. Robot-assisted lobectomy. J Thorac Cardiovasc Surg. 2003;126(1):292–293. doi: 10.1016/s0022-5223(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 4.Cho H.M., Sim H.J., Kim D.H., Lim M.H., Lee S.K. Paralysis of the rectus abdominis muscle after a video-assisted thoracoscopic surgery. Ann Thorac Cardiovasc Surg. 2018;24(1):40–42. doi: 10.5761/atcs.cr.17-00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki T., Sakai T., Tsuchiya T., et al. Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur J Cardiothorac Surg. 2011;39(6):1033–1039. doi: 10.1016/j.ejcts.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Patila T., Sihvo E.I., Rasanen J.V., Ramstad R., Harjula A., Salo J.A. Paralysis of the upper rectus abdominis muscle after video-assisted or open thoracic surgery: an underdiagnosed complication? Ann Thorac Surg. 2009;88(4):1335–1337. doi: 10.1016/j.athoracsur.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 7.Timmermans L., Klitsie P.J., Maat A.P., de Goede B., Kleinrensink G.J., Lange J.F. Abdominal wall bulging after thoracic surgery, an underdiagnosed wound complication. Hernia. 2013;17(1):89–94. doi: 10.1007/s10029-012-0971-9. [DOI] [PubMed] [Google Scholar]
- 8.Rogers M.L., Henderson L., Mahajan R.P., Duffy J.P. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg. 2002;21(2):298–301. doi: 10.1016/s1010-7940(01)01104-6. [DOI] [PubMed] [Google Scholar]
- 9.Maguire M.F., Latter J.A., Mahajan R., Beggs F.D., Duffy J.P. A study exploring the role of intercostal nerve damage in chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29(6):873–879. doi: 10.1016/j.ejcts.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Wu N., Yan S., Wang X., et al. A prospective, single-blind randomised study on the effect of intercostal nerve protection on early post-thoracotomy pain relief. Eur J Cardiothorac Surg. 2010;37(4):840–845. doi: 10.1016/j.ejcts.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Durham-Hall A., Wallis S., Butt I., Shrestha B.M. Abdominal wall pseudohernia following video-assisted thoracoscopy and pleural biopsy. Hernia. 2009;13(1):93–95. doi: 10.1007/s10029-008-0401-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R., Schwabe K., Kruger M., Haverich A., Krauss J.K., Alam M. Electro-physiological evidence of intercostal nerve injury after thoracotomy: an experimental study in a sheep model. J Thorac Dis. 2017;9(8):2461–2465. doi: 10.21037/jtd.2017.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Z., Li H., Zhang C., Qian X., Feng Z., Zhu S. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steegers M.A., Snik D.M., Verhagen A.F., van der Drift M.A., Wilder-Smith O.H. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9(10):955–961. doi: 10.1016/j.jpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Maguire M.F., Ravenscroft A., Beggs D., Duffy J.P. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29(5):800–805. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Takamori S., Toyokawa G., Okamoto T., et al. Clinical impact and risk factors for skeletal muscle loss after complete resection of early non-small cell lung cancer. Ann Surg Oncol. 2018;25(5):1229–1236. doi: 10.1245/s10434-017-6328-y. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee S., Nam R., Fleshner N., Klotz L. Permanent flank bulge is a consequence of flank incision for radical nephrectomy in one half of patients. Urol Oncol. 2004;22(1):36–39. doi: 10.1016/S1078-1439(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 18.Dakwar E., Le T.V., Baaj A.A., et al. Abdominal wall paresis as a complication of minimally invasive lateral transpsoas interbody fusion. Neurosurg Focus. 2011;31(4) doi: 10.3171/2011.7.FOCUS11164. [DOI] [PubMed] [Google Scholar]
- 19.Gardner G.P., Josephs L.G., Rosca M., Rich J., Woodson J., Menzoian J.O. The retroperitoneal incision. An evaluation of postoperative flank 'bulge'. Arch Surg. 1994;129(7):753–756. doi: 10.1001/archsurg.1994.01420310085015. [DOI] [PubMed] [Google Scholar]
- 20.van Ramshorst G.H., Kleinrensink G.J., Hermans J.J., Terkivatan T., Lange J.F. Abdominal wall paresis as a complication of laparoscopic surgery. Hernia. 2009;13(5):539–543. doi: 10.1007/s10029-009-0473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butensky A.M., Gruss L.P., Gleit Z.L. Flank pseudohernia following posterior rib fracture: a case report. J Med Case Rep. 2016;10(1):273. doi: 10.1186/s13256-016-1054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]