Abstract
DNA immunization of macaques with the SF162ΔV2 envelope elicited lymphoproliferative responses and potent neutralizing antibodies. The animals were depleted of their CD8+ T lymphocytes and then challenged intravenously with SHIV162P4. Compared to unvaccinated animals, the vaccinated macaques had lower peak viremia levels, rapidly cleared plasma virus, and showed delayed seroconversion.
DNA immunization stimulates both the cellular and humoral arms of the immune system (9, 16–18) and elicits immune responses capable of preventing infection of animals by slowly replicating viruses, such as human immunodeficiency virus type 1 (HIV-1) in chimpanzees (3). However, when the challenge virus replicates efficiently in the host, such as simian immunodeficiency virus (SIV) or SIV/HIV (SHIV) in macaques, the DNA-elicited immune responses offer only partial protection (4, 10, 14). To increase the potency of these responses, especially the development of high anti-HIV or -SIV envelope antibody titers, follow-up administration of soluble viral envelope proteins, viral particles or recombinant vaccinia-based viruses expressing the HIV or SIV envelope is required (1, 2, 8, 12–14). This bimodal method of immunization elicits responses capable of protecting rhesus macaques from infection by highly replication-competent SHIV (8, 14). It is unclear, however, whether the recorded protection was mediated by the cellular and/or humoral antiviral responses elicited during DNA immunization. By evaluating and comparing the respective antiviral protective roles of these two types of responses, we hope to develop more effective DNA immunization protocols.
Two rhesus macaques (H445 and J408) were immunized both intradermally and intramuscularly at weeks 0, 4, and 8 with a DNA vector (5, 22) (2 mg of total DNA each time) expressing the SF162ΔV2 gp140 envelope with an intact gp120-gp41 cleavage site (21). The DNA construct was codon optimized for high expression in mammalian cells. At week 27, the animals were immunized one additional time with DNA and with the CHO-produced, purified oligomeric SF162ΔV2 gp140 protein (100 μg) mixed with the MF-59C adjuvant. At week 38, the animals were immunized one additional time with the adjuvanted protein alone.
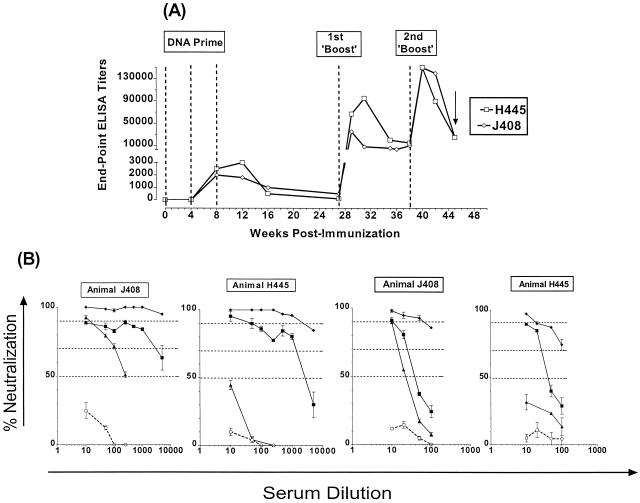
The development of binding antibodies was evaluated by enzyme-linked immunosorbent assay (ELISA) methodologies (20). Antibodies were detectable following the second DNA immunization, and their titers did not increase following the third DNA immunization (Fig. 1A). During the following 5 months, the titers decreased gradually, but were always detectable. The first “boost” increased the titers by approximately 1 to 2 log10 from the peak value recorded following the third DNA immunization. The titers gradually decreased and leveled off during the following 11 weeks, at which point the animals received a second boost, which further increased the antibody titers. Neutralizing antibodies (NA) were evaluated by using the activated peripheral blood mononuclear cell (PBMC) target assay (19), using preimmunization sera to correct for nonspecific neutralization (Fig. 1B). Following the third DNA immunization, the NA titers in animal H445 were lower than those in animal J408, even though the binding antibody titers were similar between the two animals. The NA titers against both SF162ΔV2 and SF162 increased significantly during the subsequent boosts. Vaccine-specific proliferative responses were recorded in both animals. Stimulation indices (SIs) of 5 and 10 were recorded following the first boost in animals J408 and H445, respectively. The second boost increased the potency of these responses in animal H445 (SI of 25), but not in animal J408 (SI of 5).
FIG. 1.
Generation of anti-HIV envelope antibodies during immunization. (A) Binding antibodies. The envelope-specific titers of binding antibodies in animals J408 and H445 throughout our immunization schedule were determined against the vaccine, i.e., the purified oligomeric SF162ΔV2 gp140 protein. Dashed lines indicate the time of immunization and the arrow indicates the time of viral challenge. (B) Neutralizing antibodies. The presence of neutralizing antibodies against the homologous SF162ΔV2 virus (left panels) and the parental SF162 viruses (right panels) was determined at various time points during the immunization schedule: ○, prebleeds; ▴, 1 month past the third DNA immunization; ■, 2 weeks following the 1st boost; and ⧫, 2 weeks following the 2nd boost.
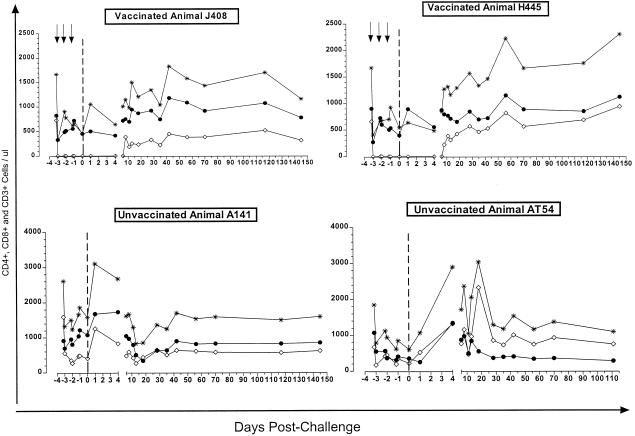
To evaluate the protective role of the anti-HIV envelope antibodies elicited by our vaccine, we depleted the CD8+ T lymphocytes from the vaccinated animals prior to viral challenge (Fig. 2). CD8 depletion was achieved by three intravenous administrations of the anti-CD8 MAb OKT8F (2 mg/kg of body weight) at daily intervals (7). CD8+ T lymphocytes remained undetectable for approximately 10 days. Concomitantly, we recorded a decrease in the total number of circulating CD3+ T cells. This indicates that the recorded depletion of CD8+ T cells from the periphery is due to their actual elimination. Although we did not evaluate CD8 depletion from the lymph nodes, it was previously demonstrated that a concomitant depletion of CD8+ T cells from the periphery and lymph nodes occurs when anti-CD8 monoclonal antibodies (MAbs) are introduced into the blood circulation of macaques (11, 15).
FIG. 2.
Depletion of CD8+ T lymphocytes. CD8+ T lymphocytes were depleted from the vaccinated animals by bolus injection of the anti-CD8 MAb OKT8F (arrows). The numbers of circulating CD4+ (solid symbols), CD8+ T (open symbols), and total CD3+ T lymphocytes (asterisks) from vaccinated and unvaccinated animals was determined in samples collected at various points prior to and following SHIV162P4 challenge (dashed line).
One day following the last administration of OKT8F, the immunized and two unimmunized naive animals were challenged intravenously with 100 50% tissue culture infective doses of a cell-free stock of the SHIV162P4 virus (6). This isolate was neutralized by 50 and 90% by sera (1:5 dilution) collected at the day of challenge from animals H445 and J408, respectively.
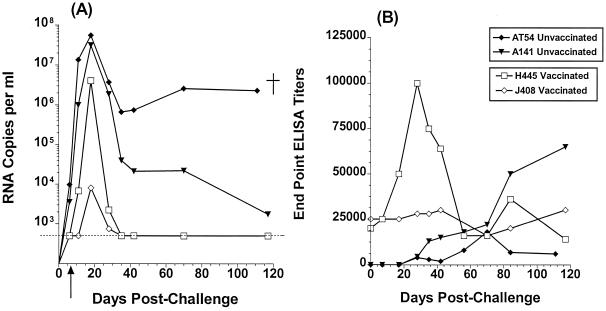
Both vaccinated and unvaccinated animals became infected; however, differences in the peak viral load levels and viral set points were noted between the two groups (Fig. 3A). Eleven days postchallenge, plasma viremia in the vaccinated animal H445 was lower by 2 and 4 log10 compared to that of the unvaccinated animals A141 and AT54, respectively, while the vaccinated animal J408 was aviremic. At peak viremia, plasma viral levels in the vaccinated animals were 1 to 4 log10 lower than those in the unvaccinated animals. Following peak viremia, an initial rapid decrease followed by a more gradual decrease in plasma viral loads was recorded in the unvaccinated animal A141, while sustained high viral loads were recorded in the second unvaccinated animal, AT54. A very rapid decrease to undetectable levels was recorded in both vaccinated animals within 35 days postchallenge.
FIG. 3.
Viral load and generation anti-HIV envelope antibody titers following SHIV162P4 exposure. (A) Viral load expressed as number of RNA copies per milliliter of plasma. Dashed lines indicate the detection limit of this assay (<500 copies per ml). †, animal AT54 was euthanized 111 days postchallenge following the development of simian AIDS. The arrow indicates the time at which CD8+ T lymphocytes reappeared in the periphery of the vaccinated animals. (B) The generation of anti-HIV envelope antibodies following SHIV162P4 challenge was monitored by SF162ΔV2 gp140-based ELISA methodology. The end point ELISA titers are presented.
Concomitant with the appearance of plasma viremia in the vaccinated animal H445, a rapid increase (by approximately fivefold) of the anti-HIV envelope antibody titers was monitored (Fig. 3B). Subsequently, as the viral load in this animal decreased to undetectable levels, the antibody titers gradually decreased to prechallenge titers. In contrast, the antienvelope antibody titers did not increase in the second vaccinated animal, J408, which had the lowest levels of peak plasma viremia. In the unvaccinated animals, anti-HIV envelope antibodies became detectable approximately 30 days postchallenge. Although their titers increased over time in animal A141 they remained weak and eventually declined prior to death in animal AT54.
The two unvaccinated animals seroconverted to SIV p27gag and pol 31 proteins within 2 weeks postchallenge, while the two vaccinated animals remained seronegative for the first 17 weeks postchallenge (data not shown). Also, although virus was recoverable from rhesus macaque PBMCs collected from the unvaccinated animals at 18, 42, and 48 days postchallenge, it was only recoverable at day 18 from the vaccinated animals (data not shown). Finally, in contrast to the two vaccinated animals and the unvaccinated animal A141, which have remained healthy so far, the second unvaccinated animal, AT54, died from simian AIDS 16 weeks postchallenge.
We believe that the anti-HIV envelope antibodies elicited by our vaccination, although not capable of completely eliminating all the infectious SHIV162P4 viral particles introduced in the blood circulation during challenge, reduced their number, so that fewer numbers of CD4+ T lymphocytes became initially infected in the vaccinated than the unvaccinated animals. This in turn resulted in lower peak levels of plasma viremia during acute infection in the vaccinated animals. The observation that the lowest levels of peak plasma viremia were recorded in the vaccinated animal J408, whose serum had the strongest neutralizing activity against SHIV162P4 at the day of challenge, suggests that neutralizing antibodies played an important protective role during the first 7 days postchallenge. However, in addition to neutralizing antibodies, envelope-specific antibodies without neutralizing activity may have been elicited by our vaccine and may also have contributed to viral clearance. The fact that strong anamnestic anti-HIV envelope responses were developed in animal H445 immediately following SHIV challenge indicates that antibodies contributed to the rapid viral clearance to undetectable levels. However, because the CD8+ lymphocytes reappeared in the periphery of the vaccinated animals 7 days postchallenge, they may also have contributed to this rapid viral clearance.
We should point out, however, that in addition to protective antibody responses, our vaccination might have elicited CD4+ T-cell-mediated protective responses, which most likely were present during CD8 depletion. It would be important to assess whether DNA vaccination elicits such responses.
In summary, these pilot studies highlight the important protective role of non-CD8-mediated DNA-vaccine-induced anti-HIV envelope responses and strongly suggest that efforts to develop an effective anti-HIV vaccine should not only aim at eliciting strong cytotoxic T-lymphocyte responses.
Acknowledgments
This study was supported in part by NIH grant AI 42670-01 (L.S.).
We are grateful to T. Mercalino from R. W. Johnson Pharmaceutical Research Institute for providing the anti-CD8 MAb OKT8F and to Jenny Booth at the Bayer Reference Testing Laboratory for performing the b-DNA assays. We thank A. Ly, Y. Liang, S. Hilt, and A. Fong for technical assistance. We acknowledge Cecilia Cheng-Mayer and Janet Harouse (ADARC) for many helpful discussions throughout this study and for providing the SHIV162P4 virus.
REFERENCES
- 1.Agadjanyan M G, Trivedi N N, Kudchodkar S, Bennett M, Levine W, Lin A, Boyer J, Levy D, Ugen K E, Kim J J, Weiner D B. An HIV type 2 DNA vaccine induces cross-reactive immune responses against HIV type 2 and SIV. AIDS Res Hum Retrovir. 1997;13:1561–1572. doi: 10.1089/aid.1997.13.1561. [DOI] [PubMed] [Google Scholar]
- 2.Barnett S W, Klinger J M, Doe B, Walker C M, Hansen L, Duliege A M, Sinangil F M. Prime-boost immunization strategies against HIV. AID Res Hum Retrovir. 1998;14(Suppl. 3):S299–S309. [PubMed] [Google Scholar]
- 3.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbet L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 4.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, Williams W V, Weiner D B. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J Med Primatol. 1996;25:242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapman B S, Thayer R M, Vincent K A, Haigwood N L. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;19:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harouse J M, Gettie A, Tan R C, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 7.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M-E, Lekutis C, Alroy M, Freed D L, Lord C I, Handt L K, Liu M A, Shiver J W. Potent protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M A, Yasutomi Y, Davis M-E, Perry H C, Freed D C, Letvin N L, Shiver J W. Vaccination of mice and nonhuman primates using HIV-gene-gun-containing DNA. Vol. 48. Basel, Switzerland: Karger; 1996. [DOI] [PubMed] [Google Scholar]
- 10.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richmond J F L, Lu S, Santoro J C, Weng J, Hu S-L, Montefiori D C, Robinson H L. Studies of the neutralizing activity and avidity of antihuman immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72:9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmond J F L, Mustafa F, Lu S, Santoro J C, Weng J, O'Connell M, Fenyo E M, Hurwitz J L, Montefiori D C, Robinson H L. Screening of HIV-1 Env glycoproteins for the ability to raise neutralizing antibody using DNA immunization and recombinant vaccinia virus boosting. Virology. 1997;230:265–274. doi: 10.1006/viro.1997.8478. [DOI] [PubMed] [Google Scholar]
- 14.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 16.Shiver J W, Davies M-E, Perry H C, Freed D C, Liu M A. Humoral and cellular immunities elicited by HIV-1 DNA vaccination. J Pharm Sci. 1996;85:1317–1324. doi: 10.1021/js9600991. [DOI] [PubMed] [Google Scholar]
- 17.Shiver J W, Perry H C, Davies M-E, Freed D C, Liu M A. Cytotoxic T lymphocyte and helper T cell responses following HIV polynucleotide vaccination. DNA Vaccines. 1995;772:198–208. doi: 10.1111/j.1749-6632.1995.tb44745.x. [DOI] [PubMed] [Google Scholar]
- 18.Shiver J W, Ulmer J B, Donnely J J, Liu M A. Humoral and cellular immunities elicited by DNA vaccines: application to the human immunodeficiency virus and influenza. Adv Drug Del Rev. 1996;21:19–31. [Google Scholar]
- 19.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization-resistant, clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72:7840–7845. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatatos L, Lim M, Cheng-Mayer C. Generation and structural analysis of soluble oligomeric envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV-1 isolates. AIDS Res Hum Retrovir. 2000;16:981–994. doi: 10.1089/08892220050058407. [DOI] [PubMed] [Google Scholar]
- 22.zur Megede J, Chen M-C, Doe B, Schaefer M, Greer C E, Selby M, Otten G R, Barnett S W. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J Virol. 2000;74:2628–2635. doi: 10.1128/jvi.74.6.2628-2635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]