Abstract
Individuals diagnosed with schizophrenia spectrum disorders (SSDs) often demonstrate alterations in the Theory of Mind Network (ToM-N). Here, in this proof-of-concept, single-arm pilot study, we investigate whether participants with an SSD (N = 7) were able to learn to volitionally control regions of the ToM-N (dorso/middle/ventromedial prefrontal cortex [D/M/VMPFC], left temporoparietal junction [LTPJ], precuneus [PC], right superior temporal sulcus [RSTS], and right temporoparietal junction [RTPJ]) using real-time fMRI neurofeedback (rtfMRI-NF). Region-of-interest analyses demonstrate that after neurofeedback training, participants were able to gain volitional control in the following ToM-N brain regions during the transfer task, where no active feedback was given: right temporoparietal junction, precuneus, and dorso/ventromedial prefrontal cortex (neurofeedback effect Fs > 6.17, ps < .05). These findings suggest that trained volitional control over the ToM-N is tentatively feasible with rtfMRI neurofeedback in SSD, although findings need to be replicated with more robust designs that include a control group and larger samples.
Keywords: Real-time fMRI, Neurofeedback, Theory of mind, Social cognition, Temporo-parietal junction
1. Introduction
Social dysfunction remains a prominent, impairing hallmark of schizophrenia spectrum disorders (SSDs) (Green et al., 2018). Importantly, many individuals with SSD note improvement in their social life as the most preferred outcome of treatment (Isvoranu et al., 2022). Recent research has demonstrated that these social difficulties are associated with alterations in Theory of Mind (ToM)–one's ability to understand others' minds–and its associated neural network (i.e., “ToM network” [ToM-N]; Couture et al., 2006; Fett et al., 2011; Kronbichler et al., 2017; Thibaudeau et al., 2021). Specifically, studies have found that individuals with an SSD demonstrate simultaneous over- and under-activation in ToM-N regions: under-activation has been observed in left, medial, and right prefrontal cortex (L/M/RPFC) (Brunet et al., 2003; Dodell-Feder et al., 2014; Kronbichler et al., 2017; Russell et al., 2000), superior temporal sulcus (STS) (Dodell-Feder et al., 2014) and posterior temporal parietal junction (TPJ) (Dodell-Feder et al., 2021; Kronbichler et al., 2017); over-activation has been observed in dorsal TPJ, and aberrant activation has been observed in MPFC and left dorsal TPJ (Kronbichler et al., 2017). Further, in several of these studies, altered neural activity in these regions were associated with performance on social cognitive tasks and/or real-world aspects of social behavior (Dodell-Feder et al., 2014, Dodell-Feder et al., 2021), highlighting the importance of the functioning of the ToM-N for real-world social behavior.
Taken together, these findings indicate that the ToM-N may be a promising neurobiological target for improving ToM, related aspects of social information processing, and their downstream consequences for social functioning. Towards directly targeting the ToM-N for intervention, previous studies have tested neuromodulatory techniques including transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS). This work has demonstrated a positive impact of these techniques on emotion regulation and social perception while targeting the left dorsolateral prefrontal cortex and right inferior parietal lobe, respectively, in participants with schizophrenia or depression (Yamada et al., 2022). However, findings across social cognitive outcomes are inconsistent, the clinical impact is unclear, and, like many pharmacological approaches, these methods are largely palliative in that they do not provide patients with skills or techniques to facilitate enduring neural and behavioral change. One emerging technique that may do so, by training volitional control over brain regions with excellent spatial resolution for the neural target, is real-time fMRI neurofeedback (rtfMRI-NF; Dudek and Dodell-Feder, 2021). RtfMRI-NF involves analyzing and presenting back to participants their brain activity in real-time through a neurofeedback signal. Using the signal, participants learn to modulate neural activity in the targeted brain regions and, ideally, demonstrate improvements in the processes supported by those regions. A recent meta-analysis demonstrated that rtfMRI-NF can be used to train volitional control of a wide variety brain regions involved in the pathophysiology of a wide variety of mental disorders (Dudek and Dodell-Feder, 2021). Our lab has also successfully used this method in a non-SSD sample to train volitional control of the ToM-N (Saxena et al., 2023). Specifically, volitional control was observed in most of the ToM-N regions during neurofeedback training and in transfer runs without active neurofeedback being given. Additionally, volitional control during up-regulation blocks was strongly associated with self-regulation strategies that involved social content. This prior study provides a good foundation for testing similar approaches with a patient sample that is characterized by ToM-N disruption.
Here, we present findings from a single arm, proof-of-concept, pilot study to investigate whether participants with SSDs can learn to volitionally control activity in the ToM-N with intermittent, activation-based rtfMRI-NF targeting key nodes of the ToM-N (temporoparietal junction; TPJ), delivered across three separate sessions. Additional aims included identifying successful strategies used to self-modulate neural activity, assessing for improvement in behavioral measures associated with the TPJ, and investigating brain-behavior associations.
2. Methods
2.1. Participants
This study was approved by the University of Rochester Research Subjects Review Board. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki and local guidelines. Participants were eligible for study enrollment if they were between the ages of 18–65 and fluent in English, had normal or corrected-to-normal vision and hearing, met SCID-5 criteria for an SSD (schizophrenia, schizophreniform, or schizoaffective disorder), and, for those taking medications, were on a stable medication regimen indicated by no medication changes in the past month. Participants were excluded if they did not meet criteria for ability to consent (i.e., IQ < 70 as assessed by the Wechsler Abbreviated Scale of Intelligence; Weschler, 2011), had a neurological disorder, and/or had an MRI contraindication (e.g., ferrous metal implants). Seven individuals with a SCID-5 diagnosis of an SSD were enrolled (Table 1).
Table 1.
Participant demographics.
| SSD diagnoses n (%) |
Schizophrenia Schizoaffective |
N = 7 5(71 %) 2(29 %) |
| Age (years) M (SD) |
41.1(11.5) | |
| Sex n (%) | Female | 3(43 %) |
| Race n (%) | White(European) Black(e.g., African, African Caribbean) Central/South American |
5(72 %) 1(14 %) 1(14 %) |
| IQ (WASI FSIQ) M (SD) |
107(11.1) | |
| PANSS total score M (SD) |
62.4(16.8) | |
| Medication status n (%) | Non-antipsychotic psychotropics Antipsychotics |
6(86 %) 5(71 %) |
Note. WASI FSIQ = Wechsler Abbreviated Scale of Intelligence Full Scale Intelligence Quotient, PANSS = Positive and Negative Syndrome Scale.
2.2. Design and procedure
The study was pre-registered on the Open Science Framework (https://osf.io/vxf9a) (see Supplementary Materials for deviations and additional results). We also provide the Consensus on the Reporting and Experimental Design of Clinical and Cognitive Behavioural Neurofeedback Studies Checklist (CRED-nf) (Ros et al., 2020) on Open Science Framework (https://osf.io/zs7wr). This study followed the same data collection and analysis procedures described in our study with non-SSD individuals, which we refer readers to for additional details (Saxena et al., 2023).
2.3. Study phases
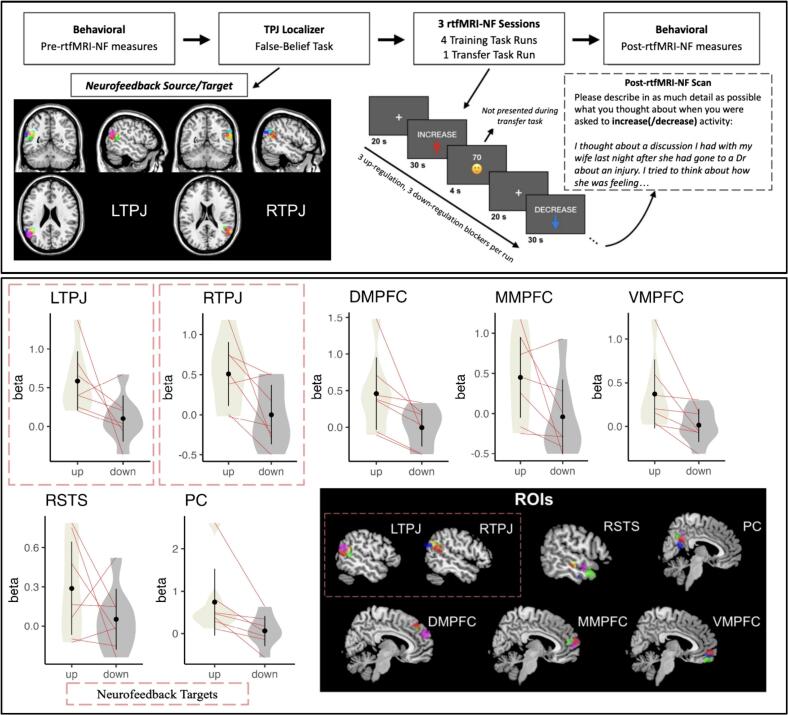
The experiment took place in 4 phases. Phase 1 involved an initial behavioral session where participants completed informed consent, eligibility assessments, and a battery of social and non-social behavioral tasks. Phase 2 involved an fMRI session to individually localize the left and right temporal parietal junction (L/RTPJ) with the False-Belief Task (Dodell-Feder et al., 2011; Saxe and Kanwisher, 2003), one of the most prominent tasks for assessing the functional neuroanatomy of ToM in the fMRI literature (Schurz et al., 2021). Phase 3 included three rtfMRI-NF sessions where participants completed 4 training runs of the NF task while receiving feedback from the TPJ, and, as a critical test of learning, 1 transfer run of the same task without neurofeedback. After each of these visits, participants rated enjoyment and difficulty of the procedure (0 = none, 100 = extremely), and provided descriptions of what mental strategies they used while attempting to up- and down-regulate the neurofeedback targets. Phase 4, the final phase, included a post-rtfMRI-NF behavioral session where participants completed the same battery of social and non-social behavioral tasks from Phase 1 (Fig. 1).
Fig. 1.
Task design & ROI analysis results.
Top A panel: study design and overlap of each participant's localized L/RTPJ neurofeedback target from the False-Belief Task. Bottom B panel: plots for extracted beta values representing up-regulation>baseline and down-regulation>baseline collapsed across the three sessions in each of the ROIs. Thin pink lines depict paired participant paired values. Bottom right: the overlap of individually-localized ROIs from the belief>physical representation contrast from the False-Belief Task. The colors shown depict individually localized ROIs and are subject specific; each color represents a different participant.
2.4. FMRI data collection, analysis, and real-time neurofeedback
MRI data were collected on a 3T Siemens Prisma scanner with a 64-channel headcoil at the University of Rochester Center for Advanced Brain Imaging & Neurophysiology. We collected a T1-weighted MPRAGE sequence (192 sagittal slices, voxel size = 1 × 1 × 1 mm3) and collected functional data using an echo-planar imaging (EPI) sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, FoV = 220 mm, 58 axial slices, voxel size = 2 × 2 × 2 mm3). FMRI data were preprocessed in SPM12 using standard steps (realignment to first functional image, co-registered to an anatomical image, normalized to the MNI template, smoothed using a 6 mm FWHM Gaussian kernel).
Neurofeedback targets (R/LTPJ) were identified in individual participants with the False-Belief Task (Saxe and Kanwisher, 2003) in which individuals read brief vignettes depicting (a) characters with false (i.e., outdated) beliefs, and (b) as the control condition, stories that describe false (i.e., outdated) physical representations of the world as depicted in photographs or maps. The task is designed to assess an individual's ability to understand that belief states are representational and that individuals behave in accordance with their beliefs (e.g., where they think an object is), and not necessarily the true state of the world (e.g., where an object actually is). The version of the task used here (Dodell-Feder et al., 2011) consisted of 10 belief stories and 10 physical representations stories. After each story, participants answered a corresponding true/false question. The stories were displayed for 12 s, the true/false question for 6 s, and finally a central fixation cross for 12 s.
We chose to use bilateral TPJ as the neurofeedback target because studies have shown that it demonstrates the most selective profile for mental state information (Molenberghs et al., 2016; Saxe and Powell, 2006) and may play a causal role in belief attribution (Filmer et al., 2019; Samson et al., 2004; Young et al., 2010). The TPJ was identified in individual subjects by contrasting neural activity for belief>photo using a voxel-wise family wise error rate (FWER)-corrected threshold of p < .05, k > 20. If we could not identify the TPJ at this threshold, we lowered the threshold to p < .0001 uncorrected and then p < .001 uncorrected. This was done to ensure that all participants had at least one individually-localized neurofeedback target.
Following other studies (Sukhodolsky et al., 2020) and current recommendations (Fede et al., 2020), prior to undergoing their first rtfMRI-NF scan, participants were given a brief and plain-language explanation of the function of the TPJ (i.e., that it activates in response to mental state information). They were then asked to generate their own mental strategies to the experimenter to ensure that as a starting point, they involved social content (e.g., thinking about a recent interaction with a friend). We emphasized that participants may use these strategies as a starting point but should alter their self-regulation strategy based on their feedback while in the scanner.
During the rtfMRI-NF scans, imaging data from the individually localized R/LTPJs were transmitted using Multivariate and Univariate Real-Time Functional Imaging (Hinds et al., 2011) scripts to a computer running OpenNFT (Koush et al., 2017). This software analyzed the data in real-time and presented the feedback back to the participant. In each of the three separate rtfMRI-NF sessions, participants completed two tasks: four runs of the training task, during which participants received intermittent, activation-based neurofeedback, and then one run of the transfer task, during participants performed the same task, but did not receive neurofeedback, which we used to assess learning/training generalization. During each of the 4 training runs, participants completed 6 blocks consisting of 20 s fixation (i.e., rest) on a central cross, 30 s of instruction to either up- or down-regulate (3 blocks of each, interleaved), followed by 4 s of feedback. Neurofeedback was calculated as median percent signal change in R/LTPJ during the prior regulation period related to the prior fixation period and converted to a number between 0 and 100. This value was accompanied by a smiley face that took on bigger smiles for higher/lower values (depending on whether it was an up- or down-regulation block). After completing the 4 training runs, participants completed the transfer run, which involved the same 6 block task without neurofeedback.
2.5. Moderators and behavioral outcome measures
As potential moderators, we reasoned that greater ability to vividly imagine and simulate social scenarios, and greater baseline propensity for perspective-taking and empathy, would be associated with greater volitional control. As such, participants were administered the
Vividness of Visual Imagery Questionnaire (Marks, 1973) and the Interpersonal Reactivity Index (Davis, 1983) to assess each potential moderator, respectively. We also explored the impact of IQ.
To evaluate behavioral effects of rtfMRI-NF, we administered the following social and non-social cognitive measures associated with neural activity in the neurofeedback targets: Hinting Task (Corcoran et al., 1995; Klein et al., 2020), which assesses the ability to understand intentions from indirect speech, Social Attribution Task-Multiple Choice (Bell et al., 2010), which assesses implicit social and mental state attribution, Multiracial Emotion Identification Task (Dodell-Feder et al., 2020), which assesses the ability to identify facial emotions from multiracial targets, Spontaneous Theory of Mind Protocol (Rice and Redcay, 2015), which assesses the propensity to spontaneously attend to and reason about mental states in film clips (using Linguistic Inquiry and Word Count [LIWC-22] software, Boyd et al., 2022; scores were calculated as the percentage of words falling within the following categories: affect, insight, positive emotion, and negative emotion), Mental State Fluency Task (Saxena et al., 2023), which assesses the fluency with which participants attribute mental states to social partners during a recent real-world social interaction, and Attentional Cueing Task (Krall et al., 2016; Vossel et al., 2009), which assesses the ability to rapidly identify validly- and invalidly-cued targets. See Saxena et al. (2023) for a detailed description of each task.
2.6. Data analysis
Data were analyzed in R Statistical Software (R Core Team, 2022) and R Studio (RStudio Team, 2020). Our primary question concerned whether participants successfully gained volitional control of the ToM-N during the critical transfer runs without active feedback being presented. To address this question, we conducted region-of-interest analysis in seven regions in the ToM-N that are consistently and robustly recruited during the False-Belief Task (Dodell-Feder et al., 2011; Dufour et al., 2013) and ToM tasks more generally (Molenberghs et al., 2016; Schurz et al., 2014): dorsomedial prefrontal cortex (DMPFC), left temporoparietal junction (LTPJ), middle medial prefrontal cortex (MMPFC), precuneus (PC), right superior temporal sulcus (RSTS), right temporoparietal junction (RTPJ), and ventromedial prefrontal cortex (VMPFC). These regions were individually-localized using the belief>photo contrast from the False-Belief Task using a threshold of p < .001, k > 10, uncorrected. We extracted beta values for up-regulation>baseline and down-regulation>baseline for all three runs of the transfer task from these seven regions and submitted these values to 2 condition (up-regulation, down-regulation) by 3 session repeated-measures ANOVA, separately for each region-of-interest. For those interested, we also analyzed data from the training task using a similar procedure (see Supplementary material).
To assess regulation strategies associated with volitional control, we submitted participant descriptions of their strategies to Linguistic Inquiry and Word Count software (LIWC-22) (Boyd et al., 2022), which calculated the proportion of words in 76 psychological categories (e.g., affect, social behavior, work, health, cognition, etc.). Using these data, we performed partial least squares regression, evaluating the association between components summarizing the LIWC dimensions and neural activity, separately for up-regulation and down-regulation (the number of components selected was based on the RMSE of prediction using leave-one-out, bias-corrected cross-validated predictions).
The association between hypothesized moderators and volitional control were tested with Pearson correlations using our measure of volitional control: the difference between up- and down-regulation. Changes in performance on the behavioral outcome measures were tested with paired-samples Welch's t-tests or repeated-measures ANOVAs. To assess for brain-behavior associations, we conducted Spearman rank correlations between pre-to-post behavioral changes and the difference between up- and down-regulation on the transfer task for each ROI. Given the novelty and exploratory nature of the study, our primary concern was Type II error. As such, no corrections for multiple testing were made. However, we note that the combination of our small sample size and large number of tests conducted increases the possibility of Type I error.
3. Results
Regarding the tolerability of rtfMRI-NF and the overall feasibility of our approach, participants rated the rtfMRI-NF procedure as moderately enjoyable (M = 50/100, SD = 19) and moderately difficult (M = 52/100, SD = 8), which is similar to ratings provided by non-SSD participants (Saxena et al., 2023).
3.1. FMRI
Using the false-belief task, we were able to localize both RTPJ and LTPJ in most participants. All seven participants had an identifiable RTPJ with an average voxel size of 215 (SD = 94). All participants except for one had an identifiable LTPJ with an average voxel size of 187 (SD = 94). Depictions of individually localized R/LTPJs are color coded and shown in Fig. 1.
Our primary aim was to evaluate whether SSD individuals demonstrated volitional control during the transfer task when no neurofeedback is provided, as evidenced by greater neural activity for up- versus down-regulation. Condition by session ANOVAs conducted separately for each of the 7 ROIs demonstrated a main effect of condition in DMPFC, PC, RTPJ, and VMPFC (Fs > 6.18, ps < .05) characterized by greater neural activity for up- versus down-regulation (Table 2). Collapsing across the three rtfMRI sessions, all ROIs demonstrated the expected difference between up- and down-regulation, with effects ranging from approximately medium (RSTS dz [95 % CI] =.47 [−.51, 1.36]) to large in magnitude (PC dz [95 % CI] =1.05 [.65, 1.50]). There was no effect of session nor a condition by session interaction for any ROI. Findings were generally similar for the training task, although condition differences were generally larger, ranging from moderate (MMPFC dz [95 % CI] = .67 [−.34, 5.23]) to very large in magnitude (VMPFC dz [95 % CI] = 2.43 [1.61, 4.18]); there was a main effect of condition in LTPJ, but not RTPJ; and we found condition by sessions interactions in several ROIs, in which volitional control (i.e., the difference between up- and down-regulation) was largest in the second session (Supplementary Materials).
Table 2.
Transfer task ROI analysis results.
| ROI | Term | F | p | η2G | dz [95 % CI] |
|---|---|---|---|---|---|
| DMPFC |
Condition Session Interaction |
6.51⁎ .43 2.05 |
.043 .663 .172 |
.158 .015 .075 |
.96 [.62, 1.51] |
| LTPJ | Condition Session Interaction |
3.88 .08 .43 |
.096 .926 .661 |
.144 .004 .026 |
.74 [−.60, 1.21] |
| MMPFC | Condition Session Interaction |
4.14 .52 2.05 |
.088 .610 .171 |
.135 .023 .038 |
.77 [−.01, 1.32] |
| PC |
Condition Session Interaction |
7.77⁎ .02 .58 |
.032 .984 .574 |
.172 .001 .010 |
1.05 [.64, 1.50] |
| RSTS | Condition Session Interaction |
1.55 1.46 1.43 |
.259 .271 .278 |
.066 .077 .044 |
.47 [−.51, 1.36] |
| RTPJ |
Condition Session Interaction |
6.67⁎ .77 .02 |
.042 .485 .982 |
.153 .048 .001 |
.98 [.20, 1.66] |
| VMPFC |
Condition Session Interaction |
6.18⁎ .33 .31 |
.047 .722 .741 |
.117 .023 .009 |
.94 [.54, 1.39] |
Results from repeated-measures ANOVAs testing the effect of regulation condition (up, down), session (1, 2, 3) and their interaction on neural activation in the ToM-N ROIs (betas) during the transfer task. Significant findings (p < .05) are in bold text. dz indicates the main effect of condition—up-regulation > down-regulation—collapsed across session and 95 % BCa CI generated from 10,000 bootstrap samples. DMPFC = dorsal medial prefrontal cortex, LTPJ = left temporoparietal junction, MMPFC = middle medial prefrontal cortex, PC = precuneus, RSTS = right superior temporal sulcus, RTPJ = right temporoparietal junction, VMPFC = ventromedial prefrontal cortex.
p < .05.
On moderators of volitional control in the transfer task, there was a significant, positive association between volitional control and visual imagery in in PC, RTPJ, and VMPFC (rs > .68, ps < .05), perspective taking in RSTS (rs = .74, ps < .05), and empathic concern in DMPFC, MMPFC, PC, RTPJ, and VMPFC (rs > .67, ps < .05) (Table 3). However, these correlations are certainly overestimates of true effects in the population and should be interpreted with caution. Correlations between volitional control during the transfer task in all ROIs and IQ were positive (range rs = .25[PC] - .57[RSTS]); however, none of these associations were statistically significant.
Table 3.
Potential moderators and volitional control.
| ROI | Vividness of visualizing imagery | Interpersonal Reactivity Index - perspective taking | Interpersonal Reactivity Index - empathic concern |
|---|---|---|---|
| DMPFC | −.04 [−1.00, .87] | .56 [−.78, .96] | .88 [−.13, 1.00] |
| LTPJ | .29 [−.75, 1.00] | .40 [−.65, .94] | .60 [−.67, 1.00] |
| MMPFC | .46 [−.62, 1.00] | .36 [−.89, .76] | .77 [.39, 1.00] |
| PC | .68 [−.70, 1.00] | .31 [−.94, .89] | .85 [−.07, 1.00] |
| RSTS | .57 [−.41, 1.00] | .74 [−.51, 1.00] | .50 [−.65, 1.00] |
| RTPJ | .75 [−.56, 1.00] | .56 [−.21, .96] | .67 [−.13, 1.00] |
| VMPFC | .71 [−.18, 1.00] | .56 [−.78, .96] | .88 [−.13, 1.00] |
Values represent Spearman's rank correlation coefficient and 95 % BCa CI generated from 10,000 bootstrap samples. Significant findings (p < .05) are in bold text.
3.2. Regulation strategies and volitional control
Concerning self-regulation strategies, we tested whether components summarizing the LIWC dimensions were able to explain variance in neural activity during up- and down-regulation, separately for each ROI. We found that across all ROIs except for LTPJ, we were able to explain roughly 58–98 % of variance in up-regulation with one LIWC component best summarized by social referents (e.g., “you, we, she”), affiliation (“our, us, help”), and drives (e.g., “success”, “bully”, “benefit”). See Fig. 2 for a depiction of these results in RTPJ. Across all ROIs except for PC, an intercept model best fit the data for down-regulation strategies, meaning that we were largely unable to explain neural activity for down-regulation with the LIWC dimensions. In the PC we were able to explain roughly 55 % of variance in down-regulation with one component that loaded onto perceptual process (e.g., “look,” “heard,” as in “I looked at spots in the scanner mirror”).
Fig. 2.
Up-regulation strategy and volitional control in RTPJ.
Data from the PLSR model using LIWC psychological components for up-regulation descriptions and volitional control of the RTPJ, where we were able to explain roughly 69 % of the variance. The magenta, positive loading components represent a positive correlation between the LIWC feature and volitional control. The green, negative loading components represent a negative correlation between the LIWC feature and volitional control.
3.3. Behavioral performance and brain-behavior associations
Approximately 28–71 % of participants showed increases in performance from pre-to-post rtfMRI on the behavioral tasks (Hinting Task, Social Attribution Task-Multiple Choice, Multiracial Emotion Identification Task, Spontaneous Theory of Mind Protocol, Mental State Fluency Task, and an Attentional Cueing Task) with effect sizes ranging from η2G = .01 (Mental State Fluency Task) to dz = .30 (Hinting Task). However, none of the pre-to-post-rtfMRI differences in behavioral performance were statistically significant. That said, pre-to-post performance changes on two tasks exceeded typical practice effects (Pinkham et al., 2018): the Hinting Task with an effect of dz = .30, exceeding a previously demonstrated practice effect of dz = .15 (Pinkham et al., 2018), and the Multiracial Emotion Identification Task with an effect of dz = .25, exceeding a previously demonstrated practice effect of a similar emotion identification task of dz = .12 (Pinkham et al., 2018) (Table 4).
Table 4.
Behavioral performance.
| Task/measure | Pre, M (SD) |
Post, M (SD) |
Statistical comparison | Effect size |
|---|---|---|---|---|
| Attentional Cueing Task Valid Invalid |
.62(.15) .64(.14) |
.59(.14) .61(.14) |
Time: F(1,6) = 5.05, p = .066; Trial Type: F (1,6) = 3.16, p = .126; Interaction: F(1,6) = .04, p = .847 |
Time: η2G = .12; Trial Type: ηG = .07; Interaction: η2G = .1. |
| Hinting Task | 15.1(3.1) | 15.7(3.1) | t(6) = .79, p = .228 | dz = .30 [−.73, 1.12] |
| Mental State Fluency Positive Negative |
595(182.9) 398.6(111.1) |
604.3(216.4) 623.7(171.8) |
Time: F(1,6) = 4.64, p = .079; Valence: F (1,6) = 2.36, p = .176; Interaction: F(1,6) = 2.93, p = .138 |
Time: η2G = .01; Valence: η G = .004; Interaction: η2G = .00006. |
| Multiracial Emotion Identification | .82(.15) | .86(.07) | t(6) = .67, p = .265 | dz = .25 [−.78, .83] |
| Spontaneous ToM Protocol | 1.9(1.2) | 2.3(1.2) | t(6) = .43, p = .342 | dz = .16 [−1.13, .98] |
| Social Attribution Task Multiple Choice |
14(3.8) | 12.9(6.1) | t(6) = −.53, p = .693 | dz = −.20 [−.91, .8] |
Pre-to-post changes in behavioral performance using paired-samples Welch's t-tests or repeated measures ANOVAs.
With respect to brain-behavior associations, correlations between volitional control during the transfer task in all ROIs and PANSS ratings were negative (range rs = −.21[PC]— -.82[LTPJ]). However, none of these associations were statistically significant. We found one statistically significant association between volitional control in RTPJ and fluency with reporting a social partner's mental states during a negatively-valenced interaction (i.e., Mental State Fluency Negative; rs = .71, p = .04) (Table 5). However, given the small sample, presumed modest reliabilities of each measure (which would produce a lower upper bound of the association between these measures; Schmidt and Hunter, 1996), and large number of tests conducted, this is almost certainly an overestimate of true effects in the population, and should be interpreted with caution.
Table 5.
Brain-behavior associations.
| ROI | Attentional Cueing Task | Hinting Task | Mental State Fluency Positive | Mental State Fluency Negative | Multi-Racial Emotion Identification | Spontaneous ToM Protocol | Social Attribution Task Multiple Choice | IQ | Positive and Negative Syndrome Scale |
|---|---|---|---|---|---|---|---|---|---|
| DMPFC | .11(−.96, .87) | .02(−.92, .96) | .00(−1.00, .88) | .64(−.18, 1.00) | −.56(−1.00, .92) | −.75(−1.00, .43) | −.11(−1.00, .89) | .50(−.96, 1.00) | −.53(−.89, .76) |
| LTPJ | .21(−.83, .92) | −.31(−.94, .84) | −.29(−1.00, .88) | .12(−.89, .96) | −.37(−.96, .97) | −.36(−1.00, .89) | −.21(−1.00, .89) | .54(−.69, 1.00) | −.82(−1.00, −.4) |
| MMPFC | .11(−.88, .88) | −.24(−.91, .94) | −.21(−1.00, .89) | .36(−.76, .89) | −.26(−.97, .94) | −.64(−1.00, 1.00) | −.39(−1.00, .89) | .46(−.70, 1.00) | −.54(−.96, .40) |
| PC | −.21(−1.00, .76) | .10(−.92, .88) | .00(−1.00, .96) | .64(−.75, 1.00) | −.56(−1.00, .24) | −.75(−1.00, .37) | −.11(−1.00, .78) | .25(−1.00, .96) | −.21(−.92, .87) |
| RSTS | .21(−.87, .89) | −.40(−.97, .92) | .11(−1.00, .96) | .46(−.35, 1.00) | −.37(−.97, .81) | −.43(−1.00, .68) | −.18(−1.00, .73) | .57(−.75, 1.00) | −.64(−1.00, .09) |
| RTPJ | −.14(−.89, .96) | −.02(−.97, .76) | .21(−.83, 1.00) | .71(−.18, 1.00) | −.63(−1.00, .14) | −.61(−1.00, .41) | .04(−1.00, .88) | .32(−.89, .96) | −.29(−1.00, .87) |
| VMPFC | .11(−.96, .89) | .02(−.92, .96) | .00(−1.00, .88) | .64(−.18, 1.00) | −.56(−1.00, .92) | −.74(−1.00, .43) | −.11(−1.00, .89) | .50(−.96, 1.00) | −.54(−.89, .76) |
Values represent Spearman's rho and 95 % BCa CI generated from 10,000 bootstrap samples. Significant findings (p < .05) are in bold text.
4. Discussion
This study presents pilot data from the first attempt at training volitional control of the ToM-N in SSD with rtfMRI-NF. We found that after training, during the transfer scan, when no neurofeedback was provided as a measure of learning, participants demonstrated volitional control of 1 of 2 neurofeedback targets (RTPJ) as well as many of the cortical midline structures of the ToM-N (DMPFC, PC, VMPFC), with effects ranging from medium to large in magnitude. There was no effect of session or a session by condition interaction, indicating that rtfMRI-NF-related learning may occur by the end of the first session, consistent with other rtfMRI-NF studies (Bauer et al., 2020; De Filippi et al., 2022). Although our ability to generalize results is extremely limited by the small sample and lack of control group, these data provide very preliminary evidence that rtfMRI-NF is tolerable in an SSD sample and that it may help to confer volitional control of a neural network critical for social information processing.
Although participants achieved volitional control in the majority of the ToM-N, volitional control was not observed in LTPJ, MMPFC, or RSTS. MMPFC and RSTS were not included in the neurofeedback signal, which may partially account for the absence of volitional control. However, LTPJ was a neurofeedback target and is believed to be a key component of the ToM-N, making its lack of volitional control more difficult to explain. That said, LTPJ was also the only ROI with variance not explained by LIWC components. Together, this may indicate that participants relied on specific self-regulation strategies that simply did not tap into specific processes mediated by LTPJ.
Regarding specific self-regulation strategies, the majority of variance in up-regulation was explained by a single psychological component, consisting of social, affiliation, and drive features. These findings are consistent with known functional properties of the ToM-N (Schurz et al., 2014). Further, these effects are being observed during the transfer task when no neurofeedback is given. This provides important, although tentative, evidence for learned volitional control that is maintained in the absence of active neurofeedback.
We observed no significant changes in pre-to-post rtfMRI behavior and only one significant brain-behavior correlation. That said, pre- to post-rtfMRI-NF performance exceeded known practice effects for two social cognitive tasks. It is possible that related behavioral changes are small in nature, and we were underpowered to detect them. Alternatively, it may be that volitional control of neural processes does not translate to these behavioral measures, that more training over a longer period is needed to observe meaningful changes, that detectable behavior change occurs well after the end of rtfMRI-NF once new self-regulation skills are practiced and honed (Rance et al., 2018), or that participants need assistance in translating what they learn with NF to behavior, in which case bridging/coaching groups may be helpful (Lejeune et al., 2021). More work is needed to investigate the clinical utility of rtfMRI-NF in social functioning interventions, with a focused need for follow-up assessments to capture temporal symptom change following neurofeedback (Rance et al., 2018).
Generally speaking, these findings are consistent with our similarly designed healthy control study (Saxena et al., 2023), indicating additional support for observed volitional control being driven by social thought processes. In both samples, we observed greater neural activity for up- versus down-regulation in the majority of the ROIs in the transfer task. Additionally, we observed no effect of session or session by condition interactions in healthy controls or SSDs. Regarding regulation strategies, in both samples, variance in up-regulation was significantly accounted for by social, affiliation, and drive features. In other words, SSD individuals are able to generate and implement the same effective strategies as individuals without social cognitive impairment, speaking to the potential utility of this method even for individuals who may have pre-existing difficulty with mental state attribution. Further, since our healthy volunteer sample was more than double the size (N = 16), a similar pattern of findings with our more modest SSD sample suggest that the findings observed here are not spurious. Lastly, lack of significant pre-post behavioral changes and brain-behavior associations, save for numerically improved pre- to post-rtfMRI-NF performance on the Hinting Task and Social Attribution Task in healthy controls, were consistent in both samples. This study contributes to the growing research demonstrating that rtfMRI-NF is feasible with, provides potential benefits for, and may be a worthwhile therapeutic intervention for additional research (Okano et al., 2020; Orlov et al., 2018; Ruiz et al., 2013). Additionally, our findings add to the literature demonstrating that rtfMRI-NF is useful for targeting social processes and the corresponding brain networks mediating these processes (Direito et al., 2021; Kanel et al., 2019).
These findings need to be interpreted in the context of the following critical limitations: small sample, the lack of a control group, and the lack of a pre-rtfMRI-NF transfer run, which would speak to the degree of volitional control prior to receiving any NF. Additionally, in an effort to avoid Type II error given the novelty of our approach, we did not implement multiple test correction. Results should therefore be interpreted critically and with caution. Although these findings lay a strong foundation for future work, additional research is needed to validate and extend upon these findings, including whether rtfMRI-NF outperforms placebo-control NF, and if so, the durability of rtfMRI-NF volitional control and long-term behavioral implications.
CRediT authorship contribution statement
Elizabeth A. Kruse: Writing – original draft, Investigation, Formal analysis, Data curation. Abhishek Saxena: Writing – review & editing, Investigation, Data curation. Bridget J. Shovestul: Writing – review & editing, Investigation, Data curation. Emily M. Dudek: Writing – review & editing, Project administration, Investigation, Data curation, Conceptualization. Stephanie Reda: Writing – review & editing, Investigation, Data curation. Jojo Dong: Writing – review & editing, Investigation, Data curation. Arun Venkataraman: Writing – review & editing, Resources, Methodology. J. Steven Lamberti: Writing – review & editing, Supervision, Resources. David Dodell-Feder: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
None.
Acknowledgments
This work was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation [grant #27282] and startup funds provided by the University of Rochester.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2024.100329.
Appendix A. Supplementary data
Supplementary material
Data availability
Data and analysis code available upon request from the corresponding author.
References
- Bauer C.C.C., Okano K., Ghosh S.S., Lee Y.J., Melero H., Angeles C. de los, Nestor P.G., del Re E.C., Northoff G., Niznikiewicz M.A., Whitfield-Gabrieli S. Real-time fMRI neurofeedback reduces auditory hallucinations and modulates resting state connectivity of involved brain regions: part 2: default mode network-preliminary evidence. Psychiatry Res. 2020;284 doi: 10.1016/j.psychres.2020.112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.D., Fiszdon J.M., Greig T.C., Wexler B.E. Social attribution test—multiple choice (SAT-MC) in schizophrenia: comparison with community sample and relationship to neurocognitive, social cognitive and symptom measures. Schizophr. Res. 2010;122(1):164–171. doi: 10.1016/j.schres.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R.L., Ashokkumar A., Seraj S., Pennebaker J.W. University of Texas at Austin; Austin, TX: 2022. The Development and Psychometric Properties of LIWC-22; p. 10. [Google Scholar]
- Brunet E., Sarfati Y., Hardy-Baylé M.-C., Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi: 10.1016/S0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Corcoran R., Mercer G., Frith C.D. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr. Res. 1995;17(1):5–13. doi: 10.1016/0920-9964(95)00024-G. [DOI] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. The functional significance of social cognition in schizophrenia: a review. Schizophr. Bull. 2006;32(suppl_1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44(1):113–126. doi: 10.1037/0022-3514.44.1.113. [DOI] [Google Scholar]
- De Filippi E., Marins T., Escrichs A., Gilson M., Moll J., Tovar-Moll F., Deco G. One session of fMRI-neurofeedback training on motor imagery modulates whole-brain effective connectivity and dynamical complexity. Cereb. Cortex Commun. 2022;3(3) doi: 10.1093/texcom/tgac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direito B., Mouga S., Sayal A., Simões M., Quental H., Bernardino I., Playle R., McNamara R., Linden D.E., Oliveira G., Castelo Branco M. Training the social brain: clinical and neural effects of an 8-week real-time functional magnetic resonance imaging neurofeedback Phase IIa Clinical Trial in Autism. Autism. 2021;25(6):1746–1760. doi: 10.1177/13623613211002052. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Koster-Hale J., Bedny M., Saxe R. fMRI item analysis in a theory of mind task. NeuroImage. 2011;55(2):705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Tully L.M., Lincoln S.H., Hooker C.I. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. NeuroImage Clin. 2014;4:154–163. doi: 10.1016/j.nicl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Ressler K.J., Germine L.T. Social cognition or social class and culture? On the interpretation of differences in social cognitive performance. Psychol. Med. 2020;50(1):133–145. doi: 10.1017/S003329171800404X. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Tully L.M., Dudek E., Hooker C.I. The representation of mental state information in schizophrenia and first-degree relatives: a multivariate pattern analysis of fMRI data. Soc. Cogn. Affect. Neurosci. 2021;16(6):608–620. doi: 10.1093/scan/nsab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek E., Dodell-Feder D. The efficacy of real-time functional magnetic resonance imaging neurofeedback for psychiatric illness: a meta-analysis of brain and behavioral outcomes. Neurosci. Biobehav. Rev. 2021;121:291–306. doi: 10.1016/j.neubiorev.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour N., Redcay E., Young L., Mavros P.L., Moran J.M., Triantafyllou C., Gabrieli J.D.E., Saxe R. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fede S.J., Dean S.F., Manuweera T., Momenan R. A guide to literature informed decisions in the design of real time fMRI neurofeedback studies: a systematic review. Front. Hum. Neurosci. 2020;14 doi: 10.3389/fnhum.2020.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A.-K.J., Viechtbauer W., Dominguez M.-G., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Filmer H.L., Fox A., Dux P.E. Causal evidence of right temporal parietal junction involvement in implicit theory of mind processing. NeuroImage. 2019;196:329–336. doi: 10.1016/j.neuroimage.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Green M.F., Horan W.P., Lee J., McCleery A., Reddy L.F., Wynn J.K. Social disconnection in schizophrenia and the general community. Schizophr. Bull. 2018;44(2):242–249. doi: 10.1093/schbul/sbx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O., Ghosh S., Thompson T.W., Yoo J.J., Whitfield-Gabrieli S., Triantafyllou C., Gabrieli J.D.E. Computing moment-to-moment BOLD activation for real-time neurofeedback. NeuroImage. 2011;54(1):361–368. doi: 10.1016/j.neuroimage.2010.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isvoranu A.-M., Ziermans T., Schirmbeck F., Borsboom D., Geurts H.M., de Haan L., GROUP Investigators Autistic symptoms and social functioning in psychosis: a network approach. Schizophr. Bull. 2022;48(1):273–282. doi: 10.1093/schbul/sbab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanel D., Al-Wasity S., Stefanov K., Pollick F.E. Empathy to emotional voices and the use of real-time fMRI to enhance activation of the anterior insula. NeuroImage. 2019;198:53–62. doi: 10.1016/j.neuroimage.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Klein H.S., Springfield C.R., Bass E., Ludwig K., Penn D.L., Harvey P.D., Pinkham A.E. Measuring mentalizing: a comparison of scoring methods for the hinting task. Int. J. Methods Psychiatr. Res. 2020;29(2) doi: 10.1002/mpr.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y., Ashburner J., Prilepin E., Sladky R., Zeidman P., Bibikov S., Scharnowski F., Nikonorov A., De Ville D.V. OpenNFT: an open-source Python/Matlab framework for real-time fMRI neurofeedback training based on activity, connectivity and multivariate pattern analysis. NeuroImage. 2017;156:489–503. doi: 10.1016/j.neuroimage.2017.06.039. [DOI] [PubMed] [Google Scholar]
- Krall S.C., Volz L.J., Oberwelland E., Grefkes C., Fink G.R., Konrad K. The right temporoparietal junction in attention and social interaction: a transcranial magnetic stimulation study. Hum. Brain Mapp. 2016;37(2):796–807. doi: 10.1002/hbm.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler L., Tschernegg M., Martin A.I., Schurz M., Kronbichler M. Abnormal brain activation during theory of mind tasks in schizophrenia: a meta-analysis. Schizophr. Bull. 2017;43(6):1240–1250. doi: 10.1093/schbul/sbx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune J.A., Northrop A., Kurtz M.M. A meta-analysis of cognitive remediation for schizophrenia: efficacy and the role of participant and treatment factors. Schizophr. Bull. 2021;47(4):997–1006. doi: 10.1093/schbul/sbab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D.F. Visual imagery differences in the recall of pictures. Br. J. Psychol. 1973;64(1):17–24. doi: 10.1111/j.2044-8295.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Molenberghs P., Johnson H., Henry J.D., Mattingley J.B. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2016;65:276–291. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Okano K., Bauer C.C.C., Ghosh S.S., Lee Y.J., Melero H., de los Angeles C., Nestor P.G., del Re E.C., Northoff G., Whitfield-Gabrieli S., Niznikiewicz M.A. Real-time fMRI feedback impacts brain activation, results in auditory hallucinations reduction: part 1: superior temporal gyrus-preliminary evidence. Psychiatry Res. 2020;286 doi: 10.1016/j.psychres.2020.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov N.D., Giampietro V., O’Daly O., Lam S.-L., Barker G.J., Rubia K., McGuire P., Shergill S.S., Allen P. Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl. Psychiatry. 2018;8(1) doi: 10.1038/s41398-017-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Harvey P.D., Penn D.L. Social cognition psychometric evaluation: results of the final validation study. Schizophr. Bull. 2018;44(4):737–748. doi: 10.1093/schbul/sbx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team R Foundation for Statistical Computing; 2022. R: a language and environment for statistical computing (4.2.0) [Computer software] https://www.R-project.org
- Rance M., Walsh C., Sukhodolsky D.G., Pittman B., Qiu M., Kichuk S.A., Wasylink S., Koller W.N., Bloch M., Gruner P., Scheinost D., Pittenger C., Hampson M. Time course of clinical change following neurofeedback. NeuroImage. 2018;181:807–813. doi: 10.1016/j.neuroimage.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K., Redcay E. Spontaneous mentalizing captures variability in the cortical thickness of social brain regions. Soc. Cogn. Affect. Neurosci. 2015;10(3):327–334. doi: 10.1093/scan/nsu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Enriquez-Geppert S., Zotev V., Young K.D., Wood G., Whitfield-Gabrieli S.…Thibault R.T. 2020. Consensus on the Reporting and Experimental Design of Clinical and Cognitive-Behavioural Neurofeedback Studies (CRED-nf Checklist) [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team RStudio: integrated development for R (1.3.1093) [Computer software] 2020. http://wwwrstudio.com
- Ruiz S., Lee S., Soekadar S.R., Caria A., Veit R., Kircher T., Birbaumer N., Sitaram R. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum. Brain Mapp. 2013;34(1):200–212. doi: 10.1002/hbm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell T.A., Rubia K., Bullmore E.T., Soni W., Suckling J., Brammer M.J., Simmons A., Williams S.C., Sharma T. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am. J. Psychiatry. 2000;157(12):2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Samson D., Apperly I.A., Chiavarino C., Humphreys G.W. Left temporoparietal junction is necessary for representing someone else’s belief. Nat. Neurosci. 2004;7(5):499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19(4):1835–1842. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 2006;17(8):692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxena A., Shovestul B.J., Dudek E.M., Reda S., Venkataraman A., Lamberti J.S., Dodell-Feder D. Training volitional control of the theory of mind network with real-time fMRI neurofeedback. NeuroImage. 2023;279 doi: 10.1016/j.neuroimage.2023.120334. [DOI] [PubMed] [Google Scholar]
- Schmidt F.L., Hunter J.E. Measurement error in psychological research: lessons from 26 research scenarios. Psychol. Methods. 1996;1(2):199–223. doi: 10.1037/1082-989X.1.2.199. [DOI] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Tholen M.G., Maliske L., Margulies D.S., Mars R.B., Sallet J., Kanske P. Toward a hierarchical model of social cognition: a neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol. Bull. 2021;147(3):293–327. doi: 10.1037/bul0000303. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky D.G., Walsh C., Koller W.N., Eilbott J., Rance M., Fulbright R.K., Zhao Z., Bloch M.H., King R., Leckman J.F., Scheinost D., Pittman B., Hampson M. Randomized, sham-controlled trial of real-time functional magnetic resonance imaging neurofeedback for tics in adolescents with Tourette syndrome. Biol. Psychiatry. 2020;87(12):1063–1070. doi: 10.1016/j.biopsych.2019.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaudeau É., Cellard C., Turcotte M., Achim A.M. Functional impairments and theory of mind deficits in schizophrenia: a meta-analysis of the associations. Schizophr. Bull. 2021;47(3):695–711. doi: 10.1093/schbul/sbaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S., Weidner R., Thiel C.M., Fink G.R. What is “odd” in Posner’s location-cueing paradigm? Neural responses to unexpected location and feature changes compared. J. Cogn. Neurosci. 2009;21(1):30–41. doi: 10.1162/jocn.2009.21003. [DOI] [PubMed] [Google Scholar]
- Weschler D. NCS Pearson; 2011. Wechsler Abbreviated Scale of Intelligence–Second Edition (WASI-II) [Computer software] [Google Scholar]
- Yamada Y., Inagawa T., Hirabayashi N., Sumiyoshi T. Emotion recognition deficits in psychiatric disorders as a target of non-invasive neuromodulation: a systematic review. Clin. EEG Neurosci. 2022;53(6):506–512. doi: 10.1177/1550059421991688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Camprodon J.A., Hauser M., Pascual-Leone A., Saxe R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc. Natl. Acad. Sci. 2010;107(15):6753–6758. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data and analysis code available upon request from the corresponding author.