Abstract
IκB inhibitor proteins are the primary regulators of NF-κB. In contrast to the defined regulatory interplay between NF-κB and IκBα, much less is known regarding the regulation of IκBβ by NF-κB. Here, we describe in detail the regulation of IκBβ by RelA/p65. Using p65−/− fibroblasts, we show that IκBβ is profoundly reduced in these cells, but not in other NF-κB subunit knockouts. This regulation prevails during embryonic and postnatal development in a tissue-specific manner. Significantly, in both p65−/− cells and tissues, IκBα is also reduced, but not nearly to the same extent as IκBβ, thus highlighting the degree to which IκBβ is dependent on p65. This dependence is based on the ability of p65 to stabilize IκBβ protein from the 26S proteasome, a process mediated in large part through the p65 carboxyl terminus. Furthermore, IκBβ was found to exist in both a basally phosphorylated and a hyperphosphorylated form. While the hyperphosphorylated form is less abundant, it is also more stable and less dependent on p65 and its carboxyl domain. Finally, we show that in p65−/− fibroblasts, expression of a proteolysis-resistant form of IκBβ, but not IκBα, causes a severe growth defect associated with apoptosis. Based on these findings, we propose that tight control of IκBβ protein by p65 is necessary for the maintenance of cellular homeostasis.
The NF-κB transcription factor is a vital regulator of cellular processes involved in immune response, cellular proliferation, differentiation, and apoptosis (6, 38, 47, 53). Constitutive activation of NF-κB is also thought to contribute to multiple pathophysiological conditions such as rheumatoid arthritis (55), inflammatory bowel disease (60), and AIDS (22) and, with ever increasing evidence, cancer (1, 5, 33, 48). In mammals, the NF-κB family consists of RelA (from here on referred to as p65), c-Rel, and RelB, as well as p105 and p100 and their processed forms, p50 and p52, respectively (31). Each subunit contains a Rel homology domain (RHD) specifying DNA binding, protein dimerization, and nuclear localization. In addition, p65, c-Rel, and RelB contain transactivation domains (TAD) located at the carboxy terminus. Although in vitro most NF-κB subunits possess the ability to homo- or heterodimerize, in vivo, NF-κB primarily exists as a p50/p65 heterodimer.
Unlike the majority of transcription factors that reside in the nucleus, NF-κB is sequestered predominantly in the cytoplasm bound to IκB inhibitor proteins. IκBα is the prototypical IκB protein within a family that includes IκBβ, IκBɛ, p100, p105, Bcl-3, and newly described IκBζ (6, 7, 32, 71). With the exceptions of Bcl-3 and IκBζ, these proteins function as inhibitors through ankyrin repeats that interact with the RHD of NF-κB to mask the nuclear localization signal (NLS) and inhibit nuclear translocation. Classical activation of NF-κB proceeds by the degradation of IκB proteins, which is mediated by the activity of the IκB kinase complex (IKK) (31, 47). At the core of this large 700- to 900-kDa subunit complex are two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ or NEMO/IKKAP. In response to a multitude of factors that include inflammatory cytokines, bacterial products, viruses, double-stranded RNA, reactive oxygen species, and irradiation, IKK is activated, leading to the IKKβ-dependent phosphorylation of IκB on two N-terminal serine residues (74). This triggers polyubiquitination on neighboring lysines and subsequent IκB proteolysis via the 26S proteasome (11, 47). Upon IκB degradation, NF-κB translocates to the nucleus, where it binds to its cognate DNA sequence and interacts with basal transcription and chromatin remodeling factors to activate gene expression (4, 15, 61, 63, 75).
Over the past decade, there has been a concerted effort to understand the function by which IκB proteins regulate NF-κB activity. Earlier studies demonstrated that shortly following NF-κB activation, IκBα is resynthesized in an NF-κB-dependent manner (24, 44, 51). Following this resynthesis, IκBα enters the nucleus by a yet to be confirmed mechanism, where it then binds and removes NF-κB from the DNA and exports the complex back to the cytoplasm (2, 13, 20, 65). By this autoregulatory mechanism, NF-κB transcriptional activity remains transient, lasting between 1 and 4 h in most cells, with the exception in mature splenic B cells, where the p50/c-Rel complex is constitutively active (34, 54, 59). Disruption of this regulatory loop via the deletion of IκBα expression leads to persistent p65 activity and postnatal lethality (8, 49). More recently, structural analysis of IκBα in complex with the p50/p65 heterodimer revealed that IκBα ankyrin repeats 3 to 6 contribute the majority of p50/p65 binding while repeats 1 and 2 are more loosely associated with the NLS site of NF-κB (42, 45). The p50 NLS remains exposed when bound to IκBα (42, 45), which is thought to allow cytoplasmic to nuclear shuttling of the complex (56), while reverse shuttling is regulated by nuclear export signals located in the carboxyl- and amino-terminal halves of IκBα (3, 41, 46, 67), as well as in the TAD of p65 (37).
In contrast to this well-described regulatory interplay between NF-κB and IκBα, the regulation of NF-κB by IκBβ and a potential feedback mechanism has not been described. Unlike IκBα, the beta isoform is not rapidly degraded by classical NF-κB-inducing signals, and following NF-κB activation, IκBβ is also not resynthesized in an NF-κB-dependent manner. Depending on the cell type or stimulus, IκBβ may instead undergo persistent degradation, leading to constitutive NF-κB activity (12, 50, 69). Constitutive NF-κB activity is also regulated by a hypophosphorylated form of IκBβ that is capable of competing with IκBα for NF-κB binding but is incapable of dislodging NF-κB from the DNA (23, 66). Basal phosphorylation of IκBβ occurs in its carboxyl-terminal PEST domain that functions to inhibit NF-κB DNA binding and is thought to be primarily responsible for the formation of latent IκBβ/NF-κB complexes (58, 70). However, unlike IκBα/NF-κB, IκBβ/NF-κB complexes do not undergo nuclear-to-cytoplasmic shuttling (68) due to the addition of a linker region between ankyrin repeats 3 and 4 in IκBβ that binds κB-Ras to efficiently mask the second NLS in the NF-κB dimer complex (16, 17, 29, 57). Also, unlike IκBα (8, 49), mice lacking IκBβ were noted to have a mild phenotype (18), suggesting at first that these proteins are functionally distinct. However, knock-in expression of IκBβ under the control of the IκBα promoter rescued IκBα-associated lethality (18). This demonstrates that functional overlap between these proteins clearly exists, but given the overt phenotypic differences among respective IκB knockouts, it also points to the importance of spatiotemporal expression of IκB proteins in the regulation of NF-κB.
We have made the observation that IκBβ levels are dramatically reduced in mouse embryonic fibroblasts (MEFs) null for the p65 subunit of NF-κB. Although others have indirectly noted this phenomenon (9, 40), to date a detailed characterization of this regulation has not been performed. In this report, the specificity, mechanism, and physiological relevance of p65 regulation of IκBβ are described. Our results reveal a remarkable dependence of IκBβ for p65 in most, but not all, tissues. This dependence is mediated through the stabilization of IκBβ protein by p65 and its carboxyl terminus encompassing the TADs. Our results also show that IκBβ expression in p65-null MEFs has a severe impact on cell growth and viability. Interestingly, although p65 is considered constitutively expressed, studies have reported that p65 expression is in fact low or even undetectable in early development (21, 62) and in selective cell types (73). Based on such studies, as well as our own present findings, we suggest that the destabilization of IκBβ in cells lacking p65 is a regulatory process that might have emerged to ensure proper cell growth and viability.
MATERIALS AND METHODS
Materials.
Murine tumor necrosis factor alpha (TNF-α) was purchased from Roche Biochemicals (Indianapolis, IN). Antibodies to IκBα (C21), IκBβ (C20, N20), Bcl-3, IKKγ, and p100 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); myosin heavy chain and α-tubulin were from Sigma (St. Louis, MO); p65 was from Rockland Immunochemicals, Inc. (Gilbertsville, PA); and hemagglutinin (HA) was from Covance (Princeton, NJ). MG-132, ALLN, and lactacystin were purchased from Calbiochem (San Diego, CA); cycloheximide was from Sigma (St. Louis, MO); and λ-phosphatase was from New England Biolabs (Beverly, MA). [35S] Easy Tag protein labeling mix was purchased from NEN (Boston, MA), and methionine/cysteine-free Dulbecco's modified Eagle's medium (DMEM) was from Invitrogen (Carlsbad, CA). Materials required for immunohistochemical analysis were obtained from Vector Laboratories (Burlingame, CA).
Cell culture.
All fibroblast cells were cultured in high-glucose DMEM containing 10% fetal bovine serum and antibiotics. For p50 and Bcl-3 MEFs, mice null for these proteins (Jackson Laboratories, Bar Harbor, Maine) were crossed with their respective wild-type strains. Resulting heterozygotes were then bred, and MEFs were prepared from embryos at day 13.5 postcoitus. In a similar manner, p65 MEFs were generated from embryonic day 13.5 (E13.5) p65+/+, p65+/−, and p65−/− embryos. C2C12 myoblasts were cultured as previously described (35).
Plasmids.
Full-length p65 [p65(FL)] and carboxyl truncation mutants [p65(Δ534), p65(Δ521), and p65(Δ313)] were cloned into the pFLAG-CMV-2 expression plasmid as previously described (72). For the generation of p65(Δ431), Flag-tagged p65(FL) was used as a template and a DNA fragment corresponding to amino acids 1 to 431 was amplified with primers 5′-GATCAAGCTTGACGAACTGTTCCCCCTCATC and 3′-GATCGATATCTCAAGCCTGGGTGGGCTTGGGG. The DNA was subsequently cloned into the HindIII/EcoRV sites of a pFLAG-CMV-2 plasmid. Construct p65(Δ319) was generated in a similar manner using primers 5′-GATCAAGCTTGACGAACTGTTCCCCCTCATC and 3′-CGATATCTCAGCTGAAAGGACTCTTCTTCATG. Retroviral expression constructs for p65 and IκBβ were created by reverse transcription-PCR to amplify the respective cDNA from human fibroblasts and cloned into pBabepuro. The p65 construct was generated to produce the full-length protein. Wild-type IκBβ and IκBβ-SR (deleted in the signal response region, amino acids 1 to 54) were generated with an N-terminal HA epitope tag. pBabeIκBα-SR was generated by excising the cDNA of human IκBα-SR (mutated in Ser-32 and Ser-36 to alanines) from a pCMV4 expression plasmid (generously provided by D. Ballard, Vanderbilt University) with BglII/SmaI restriction enzymes and subcloned in BamH1/SnaB sites in pBabepuro. pCMVIκBβ(S19/23A) and pCMVIκBβ(S19/23E) were generated by site-directed mutagenesis from pCDNA3.1 and pHM6 expression plasmids containing IκBβ using the QuikChange site-directed mutagenesis kit (Stratagene) with primers S19/23A (5′-GAATGGTGCGACGCCGGCCTGGGCGCCCTGGGTCCG-3′) and S19/23E (5′-CAGATGAATGGTGCGACGAAGGCCTGGGCGAGCTGGGTCCGGAC-3′) (mutated residues are underlined). All clones were confirmed by DNA sequencing and Western blot analysis.
Immunoblotting, EMSA, and kinase assay.
Whole-cell extracts from cultured cells and immunoblotting were prepared as previously described (35). Extracts from mouse tissue were prepared by homogenization in lysis buffer (1% Triton X-100, 150 mM NaCl, 50 mM Tris [pH 7.5], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, standard protease inhibitors). Protein detection was obtained by enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA) and imaged either by using a Chemidoc gel documentation system (Bio-Rad Laboratories, Hercules, CA) or by exposing blots to film. All quantitation was performed using the ImageJ software (National Institutes of Health, Bethesda, MD). Electrophoretic mobility shift assays (EMSAs) and IKK kinase assays were performed as previously described (19, 25).
35S labeling and immunoprecipitations.
For labeling reactions, p65+/+ and p65−/− MEFs were cultured in DMEM lacking methionine or cysteine for 2 h and subsequently pulse-labeled with 35S-labeled methionine and cysteine for up to 1 h. Whole-cell extracts were prepared in a standard radioimmunoprecipitation assay buffer. For immunoprecipitation, 500 μg of protein was precleared with rabbit immunoglobulin G (IgG) for 2 h and nonspecific complexes were precipitated by centrifugation using 25 μl of A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Supernatants were then incubated overnight at 4°C with 1 μg of either IgG or an IκBβ antibody. The following day, complexes were precipitated by centrifugation using 30 μl of A/G agarose beads, washed three times in lysis buffer, resuspended in gel loading buffer, and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were dried and visualized on X-OMAT film.
Transfections and retrovirus infections.
Typically, 75% confluent MEFs were transfected in low-serum Opti-MEM medium using Lipofectamine reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Retrovirus production and infections were performed as previously described (35).
Immunohistochemistry.
E13.5 embryos either wild type or null for p65 were fixed in 10% formalin overnight at 4°C and then dehydrated and paraffin embedded. Longitudinal sections of the entire embryo and cross sections through selective tissues were prepared. Sections were deparaffinized by heating at 60°C for 1 h, followed by treatment with xylene and rehydration. Slides were treated with 3% hydrogen peroxide to block any endogenous peroxidase activity that could interfere with the detection reaction. Sections were steamed for 30 min in antigen retrieval solution and then incubated in avidin and biotin solutions. Following blocking for 1 h in 5% goat serum diluted in phosphate-buffered saline, sections were incubated with primary antibody against p65 (for 1 h; 1:5,000 dilution in 5% goat serum), IκBβ (for 1 h; 1:300 dilution in 5% goat serum), or myosin heavy chain (for 30 min; 1:2,000 dilution in mouse on mouse [MOM] diluent). Sections were subsequently incubated with a biotinylated secondary antibody. For p65 and IκBβ, the secondary antibody used was goat anti-rabbit (for 30 min; 1:250 in 5% goat serum) and for myosin heavy chain it was goat anti-mouse (for 10 min; 1:250 in MOM diluent). Sections were developed by incubation with avidin/biotin-complexed peroxidase to recognize secondary antibody (ABC Elite) and using 3,3′-diaminobenzidine as the enzyme substrate (DAB kit).
Real-time PCR.
RNA was prepared in TRIzol reagent (Invitrogen, Carlsbad, CA) as recommended by the manufacturer and further purified using RNeasy affinity columns (QIAGEN, Valencia, CA). cDNA was generated from 2 μg total RNA by reverse transcription with Superscript II (Invitrogen, Carlsbad, CA) according to the manufacturer's specifications. One microliter of cDNA was used as the template in a total reaction volume of 25 μl containing final concentrations of 1× iQ SYBR Green Super mix (Bio-Rad, Hercules, CA) and 0.5 μM each forward and reverse primers. The primer sequences used were as follows: IκBα, 5′-GGAGACTCGTTCCTGCACTTGG and 3′-AACAAGAGCGAAACCAGGTCAGG; IκBβ, 5′-ACACAGCCCTGCACTTGGCTG and 3′-GGTATCTGAGGCATCTCTTGGG; internal control GAPDH, 5′-GCAAATTCAACGGCACAGTCAAG and 3′-GTTCACACCCATCACAAACATGG. Data were read and collected on the Bio-Rad iCycler.
Mice and genotyping.
Animals were housed in the animal facility at the Ohio State University Heart and Lung Research Institute under supersterile conditions maintaining constant temperature and humidity and fed a standard diet. Treatment of mice was in accordance to the institutional guidelines of the Animal Care and Use Committee. p65−/− TNF-α−/− mice were generated as previously described (26). Briefly, p65+/− TNF-α+/+ mice were crossed to p65+/+ TNF-α−− mice (Jackson Laboratories, Bar Harbor, Maine). From this cross, resulting p65+/− TNF-α+/− mice were crossed to obtain p65+/+ TNF-α−/− and p65−/− TNF-α−/− mice in the expected Mendelian ratios. Genotypes of p65, TNF-α, NFKB1/p50, and Bcl-3 mice were confirmed by PCR analysis from prepared tail DNA.
Growth curves and flow cytometry.
p65−/− cells infected with pBabe vector, IκBβ-SR, or IκBα-SR were grown under puromycin selection. Cells (1 × 104) were plated in triplicate in 12-well cell culture plates and counted on indicated days. Apoptosis was evaluated using Annexin V-fluorescein isothiocyanate staining according to the manufacturer's specifications (Santa Cruz Biotechnology, Santa Cruz, CA). Briefly, 5 × 105 cells were washed with cold phosphate-buffered saline and suspended in 50 μl of annexin V-fluorescein isothiocyanate staining solution. After 15 min of incubation at room temperature, cells were fixed in 10% formaldehyde and subsequently analyzed on a FACScalibur flow cytometer (Becton-Dickinson, Mountain View, CA). Fluorescence data were analyzed using CellQuest Pro software.
RESULTS
Regulation of IκBβ is specific for the loss of p65.
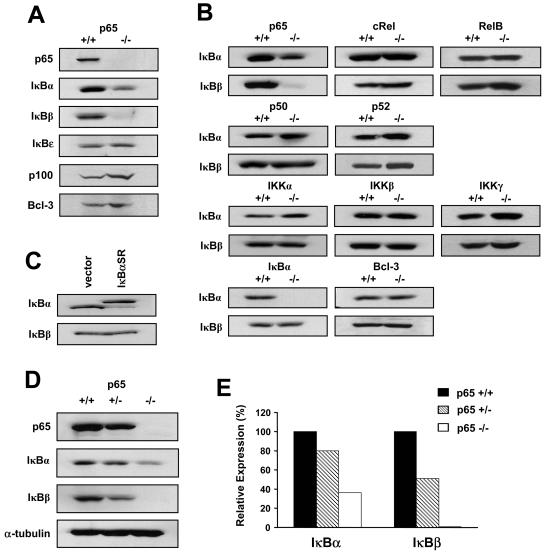
MEFs null for p65 were utilized in an attempt to gain insight into the mechanisms of NF-κB-dependent transcription. During the course of this analysis, we made the observation that IκBβ levels were strikingly lower in p65−/− fibroblasts compared to their wild-type counterparts (Fig. 1A). Although IκBα is a transcriptional target of p65, its level of regulation was noticeably less than that for IκBβ. In addition, no detectable changes in IκBɛ, p100, or Bcl-3 were seen between p65−/− and p65+/+ cells, demonstrating that p65 does not function as a general regulator of IκB proteins. To determine the specificity of this regulation, levels of IκBβ were compared in MEFs lacking other subunits in either the NF-κB family or the NF-κB signaling pathway. As opposed to the marked reduction of IκBβ in p65−/− MEFs, no changes in IκBβ were observed in fibroblasts lacking c-Rel, RelB, p50, p52, IKKα, IKKβ, IKKγ, IκBα, or Bcl-3 (Fig. 1B), implying that regulation of IκBβ is specific to p65.
FIG. 1.
IκBβ is specifically regulated by p65 in fibroblasts. (A) Whole-cell extracts (WCEs) were prepared from immortalized MEFs either wild type or null for the p65 subunit of NF-κB, and the levels of the indicated proteins were analyzed by Western blotting. (B) Western blot assays to probe for IκBα and IκBβ in MEFs either wild type or null for various components in the NF-κB signaling pathway. Genotypes for all cell lines were confirmed by PCR analysis. (C) WCEs were prepared from vector or IκBα-SR C2C12 myoblasts, and Western blot assays were performed to probe for IκB proteins. (D) Primary MEFs were isolated from E13.5 embryos and genotyped for p65 by PCR. WCEs were prepared from p65 wild-type, heterozygous, and null MEFs, and Western blot assays were performed to probe for p65, IκBα, and IκBβ. α-Tubulin was used as a loading control. (E) Quantitation of IκB proteins from two Western blot assays performed with WCEs obtained from two independent litters as described for panel D. Levels of IκB proteins were normalized to α-tubulin and compared to the expression of IκB proteins obtained from wild-type cells, which was set to a value of 100%.
To determine whether regulation of IκBβ was due to the physical absence of p65 or simply loss of its activity, we verified IκBβ protein levels in myoblast cells devoid of NF-κB transactivation function due to the stable expression of the degradation-resistant IκBα-SR mutant (36). In these cells, lack of NF-κB activity maintained IκBβ protein levels (Fig. 1C), indicating that reduction of IκBβ results from the physical loss of p65.
Since decreases in IκBβ were detected using established fibroblasts, it was important to determine whether this regulation was a consequence of the immortalization process due to the absence of p65. Mice heterozygous for p65 were therefore bred and E13.5 MEFs were prepared. In comparison to wild-type cells, p65+/− MEFs expressed approximately 50% less IκBβ and, strikingly, IκBβ was nearly undetectable in null cells (Fig. 1D and E). In contrast, IκBα expression was only slightly reduced in p65+/− MEFs while approximately 35% remained in cells lacking p65. These data demonstrate that in fibroblasts, p65 regulation of IκBβ is not a phenomenon of cellular immortalization and the degree to which p65 controls IκBβ expression is significantly higher than that for IκBα.
p65 regulation of IκBβ is maintained in embryonic and postnatal development.
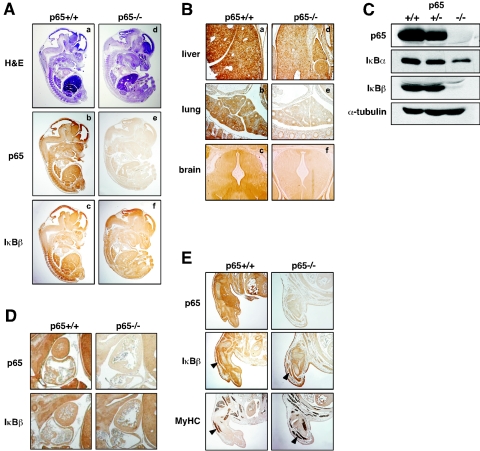
To determine if this regulation occurred in cells other than MEFs, immunohistochemical analysis of IκBβ was performed with p65+/+ and p65−/− embryos at day 13.5, a time that precedes liver apoptosis and lethality of p65-null mice (9). As expected, no overt morphological defects were observed at this developmental stage in embryos lacking p65 (Fig. 2A). Results revealed, however, that IκBβ expression was generally reduced in p65−/− embryos, with more apparent regulation occurring in the liver, lung, and brain (Fig. 2A and B). This implied that IκBβ regulation by p65 occurred in multiple cell types. To confirm that these findings were not due to staining artifacts, immunoblotting was performed from isolated fetal livers. In line with the immunohistochemistry data, IκBβ was found strongly repressed in p65−/− liver cells (Fig. 2C). Immunoblots also revealed that, similar to embryonic fibroblasts, the downregulation of IκBα in p65−/− livers was not nearly to the same extent as that for IκBβ, reaffirming the tight control of IκBβ expression by p65.
FIG. 2.
p65 regulation of IκBβ is not limited to fibroblasts. (A) Histological hematoxylin-and-eosin (H&E) and immunohistochemical staining of p65 and IκBβ from longitudinal sections of p65+/+ (a, b, c) and p65−/− (d, e, f) embryos (×1 magnification). (B) Immunohistochemical staining of IκBβ of liver (a, d), lung (b, e), and brain (c, f) tissues from p65+/+ and p65−/− embryos (×4 magnification). (C) Primary fetal liver cells were prepared from E13.5 embryos, and after genotypes were confirmed, Western blot assays were performed to probe for p65, IκBα, and IκBβ. α-Tubulin was used as a loading control. (D) p65 and IκBβ immunohistochemical staining of heart tissue from p65+/+ and p65−/− embryos (×4 magnification). (E) p65 and IκBβ immunohistochemical staining of forelimbs from p65+/+ and p65−/− embryos. To confirm skeletal muscle staining, serial sections of forelimbs were separately stained for myosin heavy chain (MyHC; arrowheads denote muscle tracks; ×4 magnification).
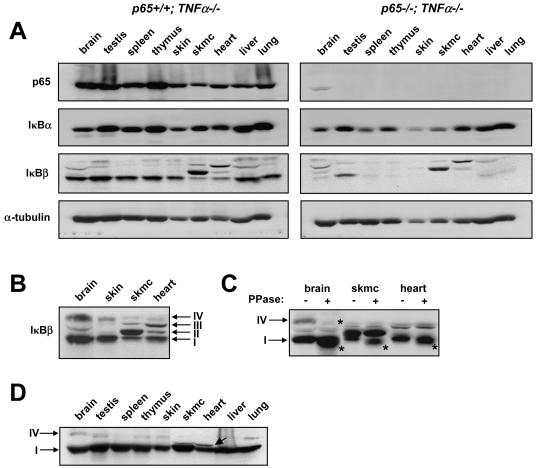
Next we asked whether p65 regulation of IκBβ could be maintained in adult mice. Although p65−/− mice die between E14.5 and E15.5 (9), liver apoptosis and embryonic lethality can be rescued with the additional deletion of TNF-α (27). Thus, p65−/− TNF-α−/− double knockouts were generated and at approximately 4 weeks of age, p65+/+ TNF-α−/− and p65−/− TNF-α−/− mice were sacrificed and tissues were processed for immunoblot analysis. Except for a p65-reactive band that reproducibly appeared in brain tissue, the complete absence of p65 staining in all remaining tissues confirmed the null phenotype of these mice (Fig. 3A). In agreement with immunohistochemical data, IκBβ was also found generally repressed in p65−/− tissues, suggesting that this regulation is maintained into adulthood (Fig. 3A). Of the p65−/− tissues examined, spleen, thymus, and skin samples contained the least IκBβ, with less but still significant reduction observable in the brain, liver, and lung compared to wild-type mice (Fig. 3A and B). Although IκBα was also downregulated in tissues lacking p65, similar to cultured MEFs and liver cells, the qualitative difference in expression compared to wild-type tissues was not as significant as that for IκBβ.
FIG. 3.
p65 regulates various forms of IκBβ in postnatal development in a tissue-specific manner. p65+/− TNF-α−/− mice were bred to generate p65+/+ TNF-α−/− and p65−/− TNF-α−/− progeny. (A) At 4 weeks of age, mice were sacrificed and tissue homogenates were prepared (skmc, skeletal muscle). Western blot assays were then performed to probe for p65, IκBα, IκBβ, and α-tubulin. Each Western blot assay is representative of a total of four different blot assays derived from two sets of littermates. (B) Western blot assay to probe for IκBβ in p65+/+ TNF-α−/− brain, skin, skeletal muscle, and heart tissues. Arrows denote IκBβ forms I through IV. (C) Similar extracts as in panel A were either left untreated or treated with phosphatase (PPase) enzyme, and Western blot assay was performed to probe for IκBβ. Arrows denote phosphorylated forms of IκBβ, and asterisks denote the shifted (dephosphorylated) forms of IκBβ. (D) The N-terminal IκBβ antibody (N20) was used in a Western analysis to verify IκBβ forms in p65+/+ TNF-α−/− tissues.
IκBβ is expressed in alternative forms that are differentially regulated by p65.
Interestingly, upon closer examination of immunohistochemical sections shown in Fig. 2, we observed that in heart and skeletal muscle, levels of IκBβ expression appeared almost comparable between p65+/+ and p65−/− embryos (Fig. 2D and E; note the colocalization of IκBβ and the skeletal muscle marker myosin heavy chain). This suggested that IκBβ regulation by p65 might also be tissue specific. Indeed, the immunoblot analysis in Fig. 3A showed significantly less reduction of IκBβ in skeletal muscle and heart compared to other tissues lacking p65. IκBβ levels in these mice were also largely retained in testis tissue, which is consistent with previous findings showing that IκBβ is particularly rich in this tissue (14). Of further interest was the identification that IκBβ produced from skeletal muscle and heart tissues migrated at a distinctly higher mobility compared to other tissues (Fig. 3A and B). In fact, as opposed to reports that murine cells produce only one form of IκBβ (39, 43), under our standard gel fractionation conditions, at least four forms of IκBβ were clearly discernible, which we refer to as forms I, II, III, and IV (Fig. 3B). IκBβ-I and IκBβ-IV represented the major and minor expressing forms in most tissues, respectively, and both were sensitive to p65 regulation, although IκBβ-I appeared more so then IκBβ-IV (Fig. 3A). However, in skeletal muscle and heart tissues, IκBβ-I and IV forms were less expressed, while IκBβ-II and IκBβ-III forms were readily detectable compared to other tissues. In addition, similar to what we had observed with IκBβ-I in testis tissue, the IκBβ-II and IκBβ-III forms also remained largely expressed in muscle tissues deficient in p65.
Since IκBβ is constitutively phosphorylated (58, 69, 70), tissue homogenates were phosphatase treated to further ascertain the characteristics of these various IκBβ-reactive polypeptides. This treatment caused a shift in IκBβ-I and -IV forms, promoting the appearance of a slightly faster-migrating IκBβ protein (Fig. 3C, denoted by asterisks). Based on these results, IκBβ-I is likely to represent the basally phosphorylated 45-kDa form of IκBβ that is most commonly described and whose stimulus-dependent hypophosphorylated state is associated with persistent activation of NF-κB (66, 69). Although IκBβ-IV is a minor component, the data also demonstrate that IκBβ can exist in a hyperphosphorylated state. In comparison, IκBβ-II and IκBβ-III were completely resistant to phosphatase treatment, which suggests that these proteins either represent unique forms of IκBβ devoid of phosphorylation or are IκBβ-like polypeptides that may have cross-reacted with this IκBβ carboxyl terminus-specific antibody used in immunoblot and immunohistochemical analyses. To make this distinction, immunoblots were repeated with a second antibody generated instead to the amino terminus of IκBβ (referred to as N20). Consistent with our previous results, the N20 antibody also reacted with IκBβ forms I and IV, thus validating the expression of these IκBβ forms in murine tissues (Fig. 3D). However, in contrast to the carboxyl-terminal antibody, the N20 antibody was clearly reactive with IκBβ-I, but less so with IκBβ-IV, in skeletal muscle and heart tissues (Fig. 3D). In addition, this IκBβ antibody again recognized altered IκBβ forms in skeletal muscle and heart tissues (Fig. 3D), but these forms did not migrate to the same mobility as forms II and III. Collectively, these data imply that skeletal muscle and heart tissues may be capable of synthesizing distinct IκBβ-like proteins. This same logic could apply to p65 expression in brain tissue, where a polypeptide migrating with an approximate mobility of 65 kDa, as described above, was reproducibly detected in p65-null mice (Fig. 3A). In any regard, it is clear that a more detailed investigation of IκBβ in muscle tissues will be required to determine whether such forms derive from posttranslational modifications or alternative splicing events.
Absence of p65 promotes IκBβ degradation by the 26S proteasome independent of IKK and phosphoacceptor serines 19 and 23.
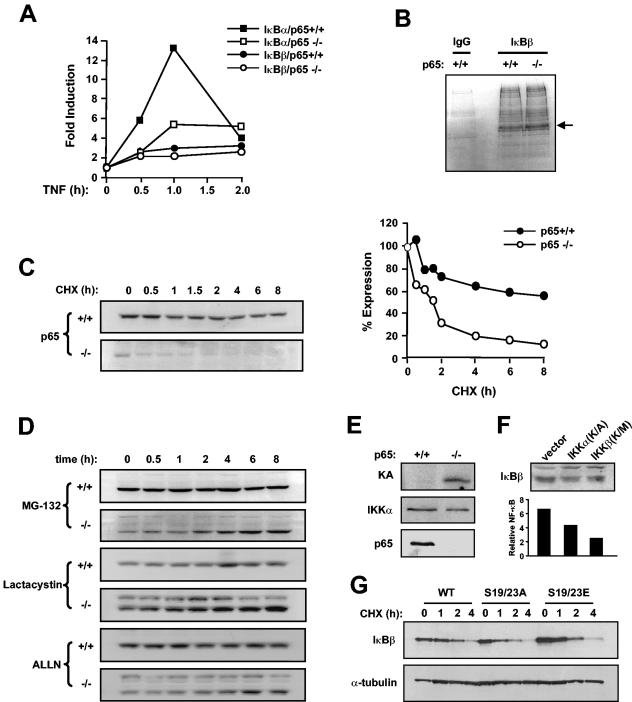
Next, we examined the mechanism by which p65 regulates IκBβ. Although a consensus NF-κB binding site is contained within the IκBβ promoter, overexpression of p65 has been shown to be incapable of stimulating IκBβ transcription (14), which argued, as others have before (69), that IκBβ is not a transcriptional target of p65. In line with these findings, we too could not detect any significant difference in steady-state levels of IκBβ mRNA between p65+/+ or p65−/− fibroblasts treated with TNF while, as expected, IκBα mRNA was readily induced by this cytokine in a p65-dependent manner (Fig. 4A).
FIG. 4.
IκBβ downregulation in p65−/− MEFs is regulated by the proteasome independent of classical IKK signaling. (A) MEFs wild type and null for p65 were treated with TNF-α, and at indicated time points a real-time PCR was used to measure IκBα and IκBβ RNAs. (B) p65+/+ and p65−/− MEFs were incubated for 2 h in methionine- and cysteine-free DMEM and subsequently labeled with a [35S]methionine/cysteine mix for an additional hour. Whole-cell extracts were prepared, and IκBβ was immunoprecipitated either with IgG (control) or with an IκBβ-specific antibody. Complexes were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by exposing dried gels to film for up to 3 days (arrow denotes IκBβ). (C) p65+/+ and p65−/− MEFs were treated with cycloheximide (CHX; 10 μg/ml), and at indicated times whole-cell extracts were prepared and Western blot assays performed to probe for IκBβ. Levels of IκBβ were quantitated from an average of three Western blot assays. (D) Similar conditions were used to treat p65+/+ and p65−/− MEFs with MG-132, ALLN, and lactacysteine (all at 10 μM). (E) Kinase assays (KA) were performed with IKKγ immunoprecipitates from p65+/+ and p65−/− MEFs incubated with glutathione S-transferase-IκBα as a substrate. Whole-cell extracts from p65+/+ and p65−/− MEFs used in kinase assay were analyzed by Western bloting to probe for IKKα and p65. (F) Western blot assays to probe for IκBβ and tubulin in p65−/− MEFs transfected with vector control or catalytically inactive IKKα or IKKβ proteins (top). Inhibitory activity of IKK proteins was verified by luciferase NF-κB reporter assays (bottom). (G) p65−/− MEFs were transfected with either HA-tagged wild-type (WT) IκBβ or HA-tagged IκBβ mutated at serines 19 and 23 to either alanine (S19/23A) or glutamic acid (S19/23E) residues. After 24 h, cells were treated with cycloheximide (10 μg/ml) for the indicated times. Whole-cell extracts were then prepared and Western blot assays performed to probe for HA and α-tubulin.
The above findings suggested that p65 regulation of IκBβ was not transcriptionally mediated and therefore is likely to occur at the protein level, affecting either the synthetic rate or stability of IκBβ. To address these possibilities, p65+/+ or p65−/− fibroblasts were metabolically labeled with 35S and IκBβ was subsequently analyzed by immunoprecipitation. Results showed similar expression levels of IκBβ following 10, 30, or 60 min of labeling (Fig. 4B and data not shown), indicating that loss of p65 does not affect the rate of IκBβ synthesis. To examine IκBβ stability, fibroblasts were treated with cycloheximide and IκBβ was analyzed over time. During an 8-h period, little destabilization of IκBβ was observed in p65+/+ cells, whereas in p65−/− cells, nearly a third of the protein was degraded after only 30 min of treatment (Fig. 4C). Further treatment of fibroblasts with MG-132 to inhibit proteasome activity demonstrated little increase in IκBβ stability in p65+/+ cells, while levels of IκBβ protein increased steadily over time in cells lacking p65 (Fig. 4D). Similar results were obtained with additional proteasome inhibitors, ALLN and lactacysteine (Fig. 4D), demonstrating that absence of p65 leads to IκBβ destabilization mediated by the 26S proteasome complex.
To further investigate the mechanism of IκBβ turnover, we asked whether this regulation by the proteasome was also dependent on IKK activity and Ser-19 and Ser-23 that are phosphorylated in response to a classical NF-κB-inducing signal. Interestingly, kinase assays revealed that p65−/− MEFs exhibited substantially higher basal IKK activity compared to wild-type cells (Fig. 4E). However, transient overexpression of catalytically inactive IKKα and IKKβ subunits (Fig. 4F) or treatment with IKK inhibitor compounds (Bay11-7085 and PS1145; data not shown) did not restore IκBβ levels in p65−/− MEFs. In addition, transient expression of HA-tagged IκBβ proteins mutated at Ser-19 and Ser-23 to alanine (S19/23A) or glutamic acid (S19/23E) residues had no significant effect on either the basal level or turnover rate of IκBβ in p65−/− cells (Fig. 4G). Therefore, despite elevated levels of IKK activity in p65−/− MEFs, IκBβ proteolysis in these cells does not appear to be regulated by the classical IKK signaling pathway.
The carboxyl terminus of p65 is required for IκBβ stability.
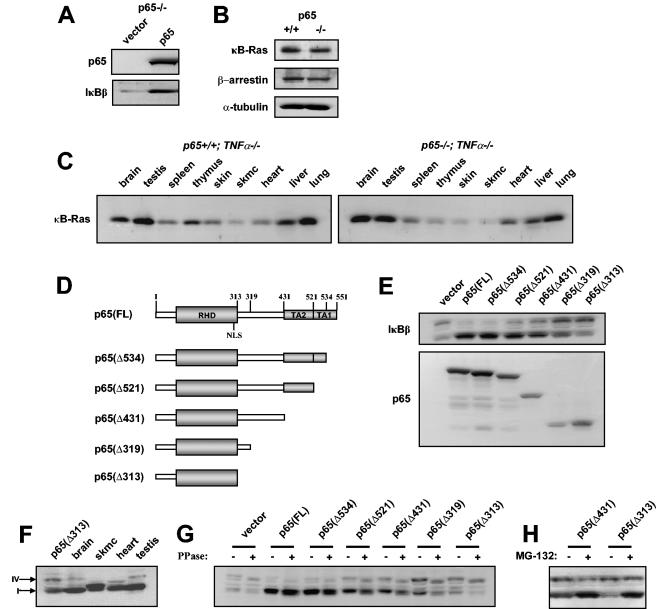
To address whether p65 is a direct regulator of IκBβ stability, p65 was reconstituted in null fibroblasts. Results showed that IκBβ expression was indeed restored in these cells (Fig. 5A). To further test the specificity of this regulation, we also examined the levels of κB-Ras, a Ras-like small GTPase recently shown to directly bind IκBβ and contribute to its stabilization in response to an NF-κB-inducing signal (16, 29). We considered the possibility that loss of IκBβ could be mediated by the preceding destabilization of κB-Ras resulting from the absence of p65. Our findings revealed, however, that κB-Ras expression was generally unaltered in either p65−/− MEFs or p65−/− tissues (Fig. 5B and C). Similar results were obtained with another recently identified IκBβ-stabilizing protein, β-arrestin (30) (Fig. 5B). Together, these findings support the notion that IκBβ stability is directly mediated by p65.
FIG. 5.
IκBβ stability is regulated by the carboxyl terminus of p65. (A) p65−/− MEFs were infected with either vector control retrovirus vector or virus expressing wild-type p65. Whole-cell extracts were prepared from a mixed population of drug selection-resistant cells, and Western blot assays were performed to probe for p65 and IκBβ. (B) Western blot assays to probe for κB-Ras and β-arrestin in p65+/+ and p65−/− MEFs. α-Tubulin was used as a loading control. (C) Western blot assays were performed with p65+/+ TNF-α−/− and p65−/− TNF-α−/− tissue homogenates to probe for κB-Ras. (D) Illustration of full-length and C-terminal truncation mutants generated in p65. The mutants are named according to the fragment of p65 expressed. For example, p65(Δ534) denotes p65 containing amino acids 1 to 534. (E) Western blot assays for IκBβ (top) and p65 (bottom) in p65−/− MEFs transfected with the indicated p65 truncation mutants. (F) Western blot assay for IκBβ in p65(Δ313)-expressing brain, skeletal muscle (skmc), heart, and testis cells from a p65+/+ TNF-α−/− mouse. Arrows denote forms IκBβ-I and IκBβ-IV. (G) Identical lysates as described in panel E were either treated or not treated with phosphatase (PPase), and Western blot assays were subsequently performed to probe for IκBβ. (H) p65−/− MEFs were transfected with p65(Δ431) or p65(Δ313) and subsequently treated with MG-132 or not treated. Western blot assay was then performed to probe for IκBβ.
Next, p65 deletion mutants were generated (Fig. 5D) and subsequently expressed in p65−/− MEFs in order to map the region in p65 responsible for IκBβ stability. Since IκBβ binding is known to occur through the RHD of NF-κB monomers, intuitively we did not consider the possibility that amino acids carboxyl to the NLS of p65 would contribute to this stability. Although deletion of the first 17 amino acids from the carboxyl terminus (Δ534) restored IκBβ to equivalent levels as that of wild-type p65(FL), to our astonishment, further deletion of the TA1 domain (Δ521) was sufficient to cause a minor but reproducible reduction of IκBβ (Fig. 5E). MG-132 treatment of p65(Δ521)-expressing cells restored IκBβ to wild-type levels, indicating that the reduction in IκBβ observed in p65(Δ521) cells was due to IκBβ destabilization (data not shown). Additional destabilization of IκBβ occurred when residues mapping to the second TA domain of p65 were removed (Δ431), and still further loss of IκBβ was observed upon deletion of residues lying just proximal to the RHD (Δ319). However, further deletion of residues to the NLS (Δ313) reproducibly had little to no further effect on IκBβ stability (Fig. 5E). Moreover, reconstitution of p65−/− MEFs with only carboxyl-terminal residues 313 to 551 of p65 was unable to restore IκBβ expression over that of vector control cells (data not shown), demonstrating that both the RHD and carboxyl residues 319 to 521 of p65 are critical to sustained IκBβ expression.
Also intriguing was the observation that destabilization of IκBβ due to carboxyl-terminal deletions of p65 led to the increased expression of a higher-molecular-weight form of IκBβ that appeared similar to the IκBβ-IV form that we had earlier identified in mouse tissues. Direct comparison of IκBβ forms from tissue and p65-reconstituted fibroblasts showed that the higher-molecular-weight form of IκBβ in fibroblasts expressing p65(Δ313) migrated to the same apparent molecular weight as IκBβ-IV from brain and testis tissues (Fig. 5F) and, like IκBβ-IV in tissues, was sensitive to phosphatase treatment (Fig. 5G). In addition, in contrast to IκBβ-I, MG-132 treatment was unable to further increase the expression of IκBβ-IV (Fig. 5H), suggesting that this form of IκBβ is resistant to 26S proteasome activity. This result is consistent with our previous observation that IκBβ-IV regulation appeared to be less dependent on p65 compared to IκBβ-I (Fig. 3A) and was unchanged in p65−/− MEFs treated with various proteasome inhibitors (Fig. 4D). Taken together, these results demonstrate that IκBβ dependence on p65 occurs due to the stabilization of IκBβ protein mediated largely through the p65 carboxyl terminus encompassing the TADs and that this regulation is specific to IκBβ-I.
Expression of IκBβ but not IκBα causes defects in cellular growth and survival.
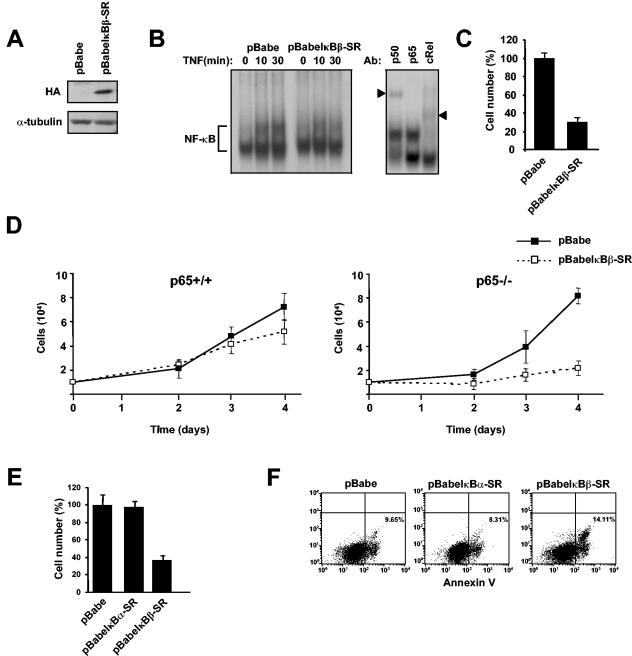
In the final analysis of this study, an attempt was made to understand the physiological relevance underlying the regulation of IκBβ by p65. Although it is widely believed that p65 is ubiquitously expressed, evidence suggests that p65 expression can be quite low or even undetectable during pregastrula development (21, 62) or in specific cell types in late embryogenesis (73). Based on these findings, as well as our present data, we asked if there was a reason why cells would need to degrade IκBβ under conditions where p65 expression is either low or absent. To address this question, an HA-tagged IκBβ retrovirus was generated in order to infect p65−/− MEFs. Attempts to stably express wild-type IκBβ or an S19/23A mutant by this system or by conventional cytomegalovirus expression plasmids proved unsuccessful. MG-132 treatment partially restored recombinant IκBβ expression, demonstrating that the inability to express IκBβ in p65−/− cells resulted from ongoing proteolysis (data not shown). We were, however, able to readily express a truncated form of IκBβ lacking the first 54 amino acids (Fig. 6A). This suggested that other determinants in the N terminus aside from Ser-19 and Ser-23 are required to mediate IκBβ proteasome-mediated degradation in p65−/− cells. We refer to this nondegradable mutant as an IκBβ superrepressor (pBabeIκBβ-SR).
FIG. 6.
MEFs stably expressing IκBβ exhibit a growth defect and increased apoptosis. p65−/− MEFs were infected with either a pBabe vector retrovirus or a virus expressing a degradation-resistant form of IκBβ tagged with an HA epitope (pBabeIκBβ-SR). (A) Extracts from p65−/−-expressing vector or HA-IκBβ-SR cells were prepared, and Western blot assays were performed to probe for HA and α-tubulin. (B) p65−/− cells expressing either the vector control or IκBβ-SR were treated with TNF-α, and at the indicated times nuclear extracts were prepared for EMSA. A supershift EMSA was performed with antibodies against p50, p65, or c-Rel to confirm the composition of NF-κB complexes (arrowheads denote supershifted subunits). (C) p65−/− MEFs were infected with a vector control or IκBβ-SR-expressing virus. Following 4 days of cell expansion under 1-μg puromycin selection, the cell number was determined in both cell lines. Cell number was normalized to vector control cells, which was set to a value of 100% growing cells. (D) Growth curves of p65+/+ or p65−/− cells expressing either a vector control or IκBβ-SR. (E) Growth curves identical to those performed in panel B from p65−/− cells infected with a vector control, IκBα-SR, or IκBβ-SR pBabe retrovirus. Cell number was normalized to the vector, which was set to a value of 100%. (F) Cells infected as in panel E were expanded under puromycin selection for 2 days and subsequently stained for Annexin V.
To address whether IκBβ-SR was functional, p65−/− vector or IκBβ-SR-expressing cells were treated with TNF-α and NF-κB activity was monitored by EMSA. Results showed that even in the absence of p65, an NF-κB complex was induced by TNF-α that by supershift analysis was found to contain p50 and c-Rel subunits (Fig. 6B). Expression of the IκBβ-SR transgene reduced this activation, which confirmed the inhibitory property of the IκBβ protein. But despite this function, IκBβ-SR expression was not found to increase the incidence of TNF-α-mediated killing over that of vector control cells, which suggested that IκBβ destabilization in p65−/− cells does not simply result to allow compensatory antiapoptotic function from c-Rel-containing complexes.
Clearly observable, however, was that p65−/− MEFs expressing IκBβ-SR, while under puromycin selection, grew at a considerably slower rate than vector control cells (Fig. 6C). Although not as evident, this growth defect was maintained even in the absence of antibiotic treatment (data not shown). To determine whether this effect was dependent on p65, IκBβ-SR retroviral infections were repeated in both p65+/+ and p65−/− MEFs. Over time, a growth reduction was also observed in p65+/+ MEFs, but not to the same extent as in p65−/− cells (Fig. 6D). To further examine the specificity of this phenotype, p65−/− MEFs were infected with viruses expressing nondegradable versions of either IκBα or IκBβ and growth rates were monitored compared to vector control cells. Results showed little growth difference between control and IκBα-SR-expressing cells, while again IκBβ-SR cells exhibited a clear growth defect (Fig. 6E). To address whether this defect was related to viability, cells were stained with Annexin V to monitor for apoptosis. Results showed that levels of apoptosis were nearly equivalent between vector control- and IκBα-SR-expressing cells. In contrast, apoptosis was increased approximately 65% in cells expressing IκBβ-SR (Fig. 6F). Collectively, these data demonstrate that IκBβ expression is linked to cellular growth defects, which provides at least one rationale to explain why IκBβ downregulation would be required in p65-deficient cells.
DISCUSSION
In contrast to IκBα, much less is known regarding the regulation of IκBβ by NF-κB. The present study was performed based on the observation that p65−/− MEFs contained dramatically lower levels of IκBβ. Although our group is not the first to note such a phenomenon (9, 40), a comprehensive study as to how p65 regulates IκBβ had yet to be undertaken. We have now performed such an analysis, which we believe provides fresh insight into the specificity, mechanism, and biological significance of this regulation.
One question we sought to address was the specificity of IκBβ regulation by p65. By using fibroblasts null for various components of NF-κB and its signaling pathway, we determined that regulation of IκBβ only occurred in cells lacking p65. By this genetic criterion, the data strongly support the idea that IκBβ regulation is specific to this NF-κB subunit. In addition to p65, IκBβ can also associate with c-Rel. It thus remains possible that the inability to detect IκBβ regulation in c-Rel−/− MEFs may be due to underrepresentation of c-Rel in mouse fibroblasts. Nonetheless, to the best of our knowledge, regulation of IκBβ in tissues from c-Rel−/− mice has not been reported, which further supports the specificity of p65 in this regulation.
Our findings also revealed the differences in the degree to which IκBα and IκBβ expression is dependent on p65. IκBα is the prototypical IκB protein whose basal and stimulated expression is controlled by p50/p65 DNA binding sites within its promoter (24, 44, 51). From this perspective, it stands to reason why IκBα has long been considered to be the most highly regulated IκB protein by NF-κB, specifically by the p65 subunit. Our present data, however, challenge this thinking. We found that IκBα levels were indeed downregulated in p65−/− primary MEFs and fetal liver cells, but only to about 60% of that of wild-type cells (Fig. 1). Such data support the role of p65 in regulating IκBα transcription and/or protein stability (64), but they also highlight the requirement for other factors in this regulation. In comparison, IκBβ expression was almost completely absent in these same cells, accentuating this protein's dependency on p65. These data provide compelling evidence that of these two IκB proteins, IκBβ is the one most tightly regulated by the p65 subunit of NF-κB.
Analysis into the mechanism of IκBβ downregulation revealed the regulation by the proteasome complex. Unlike stimulus-induced activation of NF-κB that requires IKK-dependent phosphorylation of serine residues and subsequent polyubiquitination in the N terminus, we found that proteasome-mediated degradation of IκBβ was independent of both IKK and serines 19 and 23. However, deletion of the first 54 amino acids stabilized IκBβ, suggesting that other determinants within the signal response element are required for IκBβ turnover in cells lacking p65. This regulation is highly reminiscent of the mechanism controlling IκBα degradation in response to UV treatment, which also requires the N terminus but not IKK activity or phosphorylation of N-terminal serines (10, 52). Similar to UV-induced degradation of IκBα (10), it remains to be seen whether polyubiquitination in the signal response element of IκBβ is critical for its decay in p65−/− cells.
The facts that the expression of p65 could completely restore IκBβ in p65−/− MEFs and that κB-Ras and β-arrestin levels were unaffected in these cells suggested that p65 was a direct regulator of IκBβ stabilization (Fig. 5). Data derived from the IκBβ/p65 crystal structure have shown that much of the binding from the IκBβ inhibitor ankyrin repeats occurs in the RHD of p65, between residues 191 and 319 (57). Because these structures lacked the TADs of p65, it has not been possible to formally conclude whether residues in the carboxyl half of the RHD participate in IκBβ binding. To our surprise, however, it is precisely this region of p65, between residues 319 and 534, that was found to be required for maximal IκBβ stability. Based on these findings, we predict that IκBβ stabilization is directly regulated by contributions from both the RHD and the carboxyl-terminal half of p65. This carboxyl terminus is likely to adopt a particular conformation when p65 is activated and involved in interactions with the basal transcription machinery and transcriptional coactivators in the nucleus. As an inactive cytoplasmic complex, we foresee that the carboxyl half takes on a different structure as a result of direct or indirect contacts with IκBβ. Interestingly, S. Ghosh and colleagues had previously proposed that p65 bound to IκBβ in the cytoplasm exists as an intramolecular structure that, when phosphorylated on serine 276, undergoes a conformational change that is necessary for nuclear CBP/p300 interaction and p65 TAD function (76). In an analogous fashion, we reason that the carboxyl-terminal half of p65 within this intramolecular structure plays a critical role in mediating IκBβ stability.
Our results also revealed that destabilization of IκBβ due to removal of the p65 carboxyl half gave way to increased expression of an IκBβ variant which co-migrated with IκBβ-IV in tissues and existed in a hyperphosphorylated state (Fig. 5). Intriguingly, unlike IκBβ-I, which was stabilized by proteasome inhibition in p65−/− MEFs, IκBβ-IV levels did not increase under these same conditions, nor were levels stabilized by proteasome inhibition in p65−/− cells expressing carboxyl deletion mutants of p65. This implies that hyperphosphorylation of IκBβ-IV may be a contributing factor in the stability of this IκBβ form. While phosphorylation is generally considered to target proteins for degradation by the 26S proteasome, studies have also demonstrated the requirement of phosphorylation for protection from the proteasome (28). So perhaps deletion of the p65 carboxyl terminus can lead to IκBβ hyperphosphorylation, resulting in a protein now refractory to 26S proteasome activity. It will be interesting to ascertain in future studies whether stability associated with IκBβ-IV is a result of phosphorylation and whether this minor form of IκBβ that exists in tissues possesses a functional activity distinct from the predominantly expressed IκBβ-I form.
In the final aspect of our study, we attempted to address the physiological significance underlying the tight control of IκBβ by p65. Our data revealed that p65−/− MEFs stably expressing a nondegradable form of IκBβ exhibited an extreme growth defect that could at least partially be attributed to apoptosis (Fig. 6). A similar but less severe phenotype was seen in wild-type cells, which argues that this defect is p65 dependent but also reveals the general susceptibility of cells to IκBβ expression. Importantly, our data demonstrated that this effect on growth and survival was specific to IκBβ since a comparable phenotype was not observed in cells expressing a nondegradable form of IκBα. Taken together, these results provide a rationale for why IκBβ may undergo such strong proteolysis in cells lacking p65 and the more pronounced regulation of IκBβ compared to IκBα. Our results indicate that even in p65-containing cells, proper regulation of IκBβ turnover is likely to be required to circumvent growth defects associated with stable expression of this protein. Although p65 is widely considered to be a constitutively expressed transcription factor, studies have shown that certain development stages early in embryogenesis (21, 62), or specific cell compartments of the thymus (73), do in fact possess low levels of p65. Such regulation of p65 has also been observed in selective neurons throughout development and adulthood (K. Pahan, personal communication). Based on our findings, we predict that it is precisely under such conditions that IκBβ proteolysis would be required in order to allow the proper development and maintenance of tissue homeostasis.
Acknowledgments
We thank U. Siebenlist and F. Weih for MEF knockout cell lines, A. Beg for RelA/p65 mice, S. Ghosh for κB-Ras antibody, W. Miller for β-arrestin antibody, M. Karin for cell lines and expression plasmids, and A. Baldwin for plasmids and helpful discussion. We also thank S. Acharyya and M. Carathers for technical assistance and the rest of the Guttridge laboratory for support and insight throughout the course of this study.
This work was supported by NIH grant CA97953 and startup funds to D.C.G. and the OSU Up on the Roof Fellowship to J.W.
REFERENCES
- 1.Amit, S., and Y. Ben-Neriah. 2003. NF-κB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin. Cancer Biol. 13:15-28. [DOI] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos, F., J. Thompson, M. S. Rodriguez, F. Bachelerie, D. Thomas, and R. T. Hay. 1995. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol. Cell. Biol. 15:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos, F., P. Turpin, M. Rodriguez, D. Thomas, R. T. Hay, J. L. Virelizier, and C. Dargemont. 1997. Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J. Cell Sci. 110:369-378. [DOI] [PubMed] [Google Scholar]
- 4.Ashburner, B. P., S. D. Westerheide, and A. S. Baldwin, Jr. 2001. The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 21:7065-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin, A. S. 2001. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J. Clin. Investig. 107:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin, A. S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 7.Bates, P. W., and S. Miyamoto. 2004. Expanded Nuclear Roles for IκB s. Sci. STKE 254:pe48. [DOI] [PubMed] [Google Scholar]
- 8.Beg, A. A., W. C. Sha, R. T. Bronson, and D. Baltimore. 1995. Constitutive NF-κB activation, enhanced granulopoiesis, and neonatal lethality in IκBα-deficient mice. Genes Dev. 9:2736-2746. [DOI] [PubMed] [Google Scholar]
- 9.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 10.Bender, K., M. Gottlicher, S. Whiteside, H. J. Rahmsdorf, and P. Herrlich. 1998. Sequential DNA damage-independent and -dependent activation of NF-κB by UV. EMBO J. 17:5170-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Neriah, Y. 2002. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 3:20-26.11753406 [Google Scholar]
- 12.Bourke, E., E. J. Kennedy, and P. N. Moynagh. 2000. Loss of IκB-β is associated with prolonged NF-κB activity in human glial cells. J. Biol. Chem. 275:39996-40002. [DOI] [PubMed] [Google Scholar]
- 13.Brown, K., S. Park, T. Kanno, G. Franzoso, and U. Siebenlist. 1993. Mutual regulation of the transcriptional activator NF-κB and its inhibitor, IκB-α. Proc. Natl. Acad. Sci. USA 90:2532-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budde, L. M., C. Wu, C. Tilman, I. Douglas, and S. Ghosh. 2002. Regulation of IκBβ expression in testis. Mol. Biol. Cell 13:4179-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, L., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653-1657. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., S. Vallee, J. Wu, D. Vu, J. Sondek, and G. Ghosh. 2004. Inhibition of NF-κB activity by IκBβ in association with κB-Ras. Mol. Cell. Biol. 24:3048-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, Y., J. Wu, and G. Ghosh. 2003. κB-Ras binds to the unique insert within the ankyrin repeat domain of IκBβ and regulates cytoplasmic retention of IκBβ × NF-κB complexes. J. Biol. Chem. 278:23101-23106. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, J. D., R. P. Ryseck, R. M. Attar, D. Dambach, and R. Bravo. 1998. Functional redundancy of the nuclear factor κB inhibitors IκBα and IκBβ. J. Exp. Med. 188:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheshire, J. L., and A. S. Baldwin, Jr. 1997. Synergistic activation of NF-κB by tumor necrosis factor alpha and gamma interferon via enhanced IκBα degradation and de novo IκBβ degradation. Mol. Cell. Biol. 17:6746-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiao, P. J., S. Miyamoto, and I. M. Verma. 1994. Autoregulation of IκBα activity. Proc. Natl. Acad. Sci. USA 91:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa, R. G., V. Tergaonkar, J. K. Ng, I. Dubova, J. C. Izpisua-Belmonte, and I. M. Verma. 2004. Characterization of NF-κB/IκB proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 24:5257-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLuca, C., H. Kwon, R. Lin, M. Wainberg, and J. Hiscott. 1999. NF-κB activation and HIV-1 induced apoptosis. Cytokine Growth Factor Rev. 10:235-253. [DOI] [PubMed] [Google Scholar]
- 23.DeLuca, C., L. Petropoulos, D. Zmeureanu, and J. Hiscott. 1999. Nuclear IκBβ maintains persistent NF-κB activation in HIV-1-infected myeloid cells. J. Biol. Chem. 274:13010-13016. [DOI] [PubMed] [Google Scholar]
- 24.de Martin, R., B. Vanhove, Q. Cheng, E. Hofer, V. Csizmadia, H. Winkler, and F. H. Bach. 1993. Cytokine-inducible expression in endothelial cells of an IκBα-like gene is regulated by NF-κB. EMBO J. 12:2773-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiDonato, J. A. 2000. Assaying for IκB kinase activity. Methods Enzymol. 322:393-400. [DOI] [PubMed] [Google Scholar]
- 26.Doi, T. S., M. W. Marino, T. Takahashi, T. Yoshida, T. Sakakura, L. J. Old, and Y. Obata. 1999. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl. Acad. Sci. USA 96:2994-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doi, T. S., T. Takahashi, O. Taguchi, T. Azuma, and Y. Obata. 1997. NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J. Exp. Med. 185:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng, J., R. Tamaskovic, Z. Yang, D. P. Brazil, A. Merlo, D. Hess, and B. A. Hemmings. 2004. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 279:35510-35517. [DOI] [PubMed] [Google Scholar]
- 29.Fenwick, C., S. Y. Na, R. E. Voll, H. Zhong, S. Y. Im, J. W. Lee, and S. Ghosh. 2000. A subclass of Ras proteins that regulate the degradation of IκB. Science 287:869-873. [DOI] [PubMed] [Google Scholar]
- 30.Gao, H., Y. Sun, Y. Wu, B. Luan, Y. Wang, B. Qu, and G. Pei. 2004. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol. Cell 14:303-317. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:81-96. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 33.Gilmore, T. D. 2003. The Re1/NF-κB/IκB signal transduction pathway and cancer. Cancer Treat. Res. 115:241-265. [PubMed] [Google Scholar]
- 34.Grumont, R. J., and S. Gerondakis. 1994. The subunit composition of NF-κB complexes changes during B-cell development. Cell Growth Differ. 5:1321-1331. [PubMed] [Google Scholar]
- 35.Guttridge, D. C., C. Albanese, J. Y. Reuther, R. G. Pestell, and A. S. Baldwin, Jr. 1999. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19:5785-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttridge, D. C., M. W. Mayo, L. V. Madrid, C.-Y. Wang, and A. S. Baldwin, Jr. 2000. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289:2363-2366. [DOI] [PubMed] [Google Scholar]
- 37.Harhaj, E. W., and S. C. Sun. 1999. Regulation of RelA subcellular localization by a putative nuclear export signal and p50. Mol. Cell. Biol. 19:7088-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-κB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 39.Hirano, F., M. Chung, H. Tanaka, N. Maruyama, I. Makino, D. D. Moore, and C. Scheidereit. 1998. Alternative splicing variants of IκBβ establish differential NF-κB signal responsiveness in human cells. Mol. Cell. Biol. 18:2596-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann, A., T. H. Leung, and D. Baltimore. 2003. Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J. 22:5530-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang, T. T., N. Kudo, M. Yoshida, and S. Miyamoto. 2000. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NF-κB/IκBα complexes. Proc. Natl. Acad. Sci. USA 97:1014-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huxford, T., D. B. Huang, S. Malek, and G. Ghosh. 1998. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell 95:759-770. [DOI] [PubMed] [Google Scholar]
- 43.Inan, M. S., R. Place, V. Tolmacheva, Q. S. Wang, A. K. Hubbard, D. W. Rosenberg, and C. Giardina. 2000. IκBβ-related proteins in normal and transformed colonic epithelial cells. Mol. Carcinogen. 29:25-36. [DOI] [PubMed] [Google Scholar]
- 44.Ito, C. Y., A. G. Kazantsev, and A. S. Baldwin, Jr. 1994. Three NF-κB sites in the IκB-α promoter are required for induction of gene expression by TNF-α. Nucleic Acids Res. 22:3787-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs, M. D., and S. C. Harrison. 1998. Structure of an IκBα/NF-κB complex. Cell 95:749-758. [DOI] [PubMed] [Google Scholar]
- 46.Johnson, C., D. Van Antwerp, and T. J. Hope. 1999. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα. EMBO J. 18:6682-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 48.Karin, M., Y. Cao, F. R. Greten, and Z. W. Li. 2002. NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2:301-310. [DOI] [PubMed] [Google Scholar]
- 49.Klement, J. F., N. R. Rice, B. D. Car, S. J. Abbondanzo, G. D. Powers, P. H. Bhatt, C. H. Chen, C. A. Rosen, and C. L. Stewart. 1996. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol. Cell. Biol. 16:2341-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladner, K. J., M. A. Caligiuri, and D. C. Guttridge. 2003. Tumor necrosis factor-regulated biphasic activation of NF-κB is required for cytokine-induced loss of skeletal muscle gene products. J. Biol. Chem. 278:2294-2303. [DOI] [PubMed] [Google Scholar]
- 51.Le Bail, O., R. Schmidt-Ullrich, and A. Israel. 1993. Promoter analysis of the gene encoding the IκB-α/MAD3 inhibitor of NF-κB: positive regulation by members of the rel/NF-κB family. EMBO J. 12:5043-5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li, N., and M. Karin. 1998. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc. Natl. Acad. Sci. USA 95:13012-13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 54.Liou, H. C., W. C. Sha, M. L. Scott, and D. Baltimore. 1994. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol. Cell. Biol. 14:5349-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makarov, S. S. 2001. NF-κB in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 3:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malek, S., Y. Chen, T. Huxford, and G. Ghosh. 2001. IκBβ, but not IκBα, functions as a classical cytoplasmic inhibitor of NF-κB dimers by masking both NF-κB nuclear localization sequences in resting cells. J. Biol. Chem. 276:45225-45235. [DOI] [PubMed] [Google Scholar]
- 57.Malek, S., D. B. Huang, T. Huxford, S. Ghosh, and G. Ghosh. 2003. X-ray crystal structure of an IκBβ × NF-κB p65 homodimer complex. J. Biol. Chem. 278:23094-23100. [DOI] [PubMed] [Google Scholar]
- 58.McKinsey, T. A., Z. L. Chu, and D. W. Ballard. 1997. Phosphorylation of the PEST domain of IκBβ regulates the function of NF-κB/IκBβ complexes. J. Biol. Chem. 272:22377-22380. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto, S., M. J. Schmitt, and I. M. Verma. 1994. Qualitative changes in the subunit composition of κB-binding complexes during murine B-cell differentiation. Pro. Natl. Acad. Sci. USA 91:5056-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neurath, M. F., C. Becker, and K. Barbulescu. 1998. Role of NF-κB in immune and inflammatory responses in the gut. Gut 43:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perkins, N. D., L. K. Felzien, J. C. Betts, K. Leung, D. H. Beach, and G. J. Nabel. 1997. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523-527. [DOI] [PubMed] [Google Scholar]
- 62.Richardson, J. C., A. M. Garcia Estrabot, and H. R. Woodland. 1994. XrelA, a Xenopus maternal and zygotic homologue of the p65 subunit of NF-κB. Characterisation of transcriptional properties in the developing embryo and identification of a negative interference mutant. Mech. Dev. 45:173-189. [DOI] [PubMed] [Google Scholar]
- 63.Sheppard, K. A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun, S. C., J. Elwood, C. Beraud, and W. C. Greene. 1994. Human T-cell leukemia virus type I Tax activation of NF-κκB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Mol. Cell. Biol. 14:7377-7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun, S. C., P. A. Ganchi, D. W. Ballard, and W. C. Greene. 1993. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science 259:1912-1915. [DOI] [PubMed] [Google Scholar]
- 66.Suyang, H., R. Phillips, I. Douglas, and S. Ghosh. 1996. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol. Cell. Biol. 16:5444-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tam, W. F., L. H. Lee, L. Davis, and R. Sen. 2000. Cytoplasmic sequestration of rel proteins by IκBα requires CRM1-dependent nuclear export. Mol. Cell. Biol. 20:2269-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tam, W. F., and R. Sen. 2001. IκB family members function by different mechanisms. J. Biol. Chem. 276:7701-7714. [DOI] [PubMed] [Google Scholar]
- 69.Thompson, J. E., R. J. Phillips, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1995. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell 80:573-582. [DOI] [PubMed] [Google Scholar]
- 70.Tran, K., M. Merika, and D. Thanos. 1997. Distinct functional properties of IκBα and IκBβ. Mol. Cell. Biol. 17:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verma, I. M., J. K. Stevenson, E. M. Schwartz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-κB /IκB family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 72.Wang, D., and A. S. Baldwin, Jr. 1998. Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 273:29411-29416. [DOI] [PubMed] [Google Scholar]
- 73.Weih, F., D. Carrasco, and R. Bravo. 1994. Constitutive and inducible Rel/NF-κB activities in mouse thymus and spleen. Oncogene 9:3289-3297. [PubMed] [Google Scholar]
- 74.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 75.Zhong, H., M. J. May, E. Jimi, and S. Ghosh. 2002. The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol. Cell 9:625-636. [DOI] [PubMed] [Google Scholar]
- 76.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]