Nutrition is an essential component of overall health and well-being and is identified as a major modifiable lifestyle factor. In recent years, the attention to the link between nutrition and persistent pain has increased. Yet, current pain management strategies do not address nutritional factors. This Comprehensive Pain Management Editorial Series seeks to advance the global application of pain science in clinical settings and to assist clinicians in offering personalized, multimodal lifestyle interventions with a focus on physical activity, stress, sleep, and nutrition for patients with persistent pain.1,2 Therefore, in this contribution to the Editorial Series, we will discuss the importance of nutritional factors in pain management. In particular, this paper will focus on nutritional habits of people with persistent pain, the link between pain-related mechanisms and nutrition, and evidence-based nutritional interventions in pain management.
Persistent pain and nutrition: the patient profile
The link between nutrition and pain is bidirectional. In a sample of more than 800 pain-free individuals, transitioning to a more pro-inflammatory diet (as measured by the dietary inflammatory index) increased the likelihood of pain incidence by more than 40 % over a three-year follow-up period.3 A pro-inflammatory diet is high in pro-inflammatory compounds that can increase circulating inflammatory markers and trigger the inflammatory cascade. Examples of the pro- and anti-inflammatory diet components can be found in Table 1.
Table 1.
Examples of pro- and anti-inflammatory diet components.
| Pro-inflammatory diet | Anti-inflammatory diet |
|---|---|
| Red and processed meat | Vegetables |
| Sugar sweetened beverages | Legumes |
| Refined carbohydrates | Fruits |
| Fried or ultra-processed foods | Nuts |
| Trans fats | Fatty fish |
On the other hand, persistent pain itself is reported to influence dietary behavior. For instance, compared to healthy controls, people with persistent pain showed neuroplastic changes in the central nervous system relating to the change in the hedonic value of fat which is also suggested to be one of the common mechanisms for obesity and persistent pain.4 Furthermore, observational studies also revealed that people with persistent pain tend to reduce their intake of fruits and vegetables and exhibit higher pro-inflammatory dietary behaviors and lower overall diet quality.5, 6, 7 Such unhealthy and pro-inflammatory dietary patterns can lead to deficiencies in essential nutrients that play crucial roles in pain mechanisms and nutrition, including antioxidants, omega-3 fatty acids, vitamin D, vitamins B6 and B12, magnesium, selenium, and zinc.8,9 Our own research also indicated a positive association between the pro-inflammatory characteristics of diets and pain sensitivity in patients with persistent low back pain; specifically, higher inflammatory dietary intake was correlated with increased pain sensitivity.10 Moreover, a recent study employing data from the United Kingdom Biobank and using a Mendelian randomization strategy revealed a causal relationship between dietary habits and pain scores.11 This study found a causal association between lower multisite persistent pain scores and higher consumption of fresh and dried fruits, cereals, and cheese. Additionally, the analysis indicated a link between higher levels of salt, alcohol, pork, and chicken consumption and increased multisite persistent pain scores.11
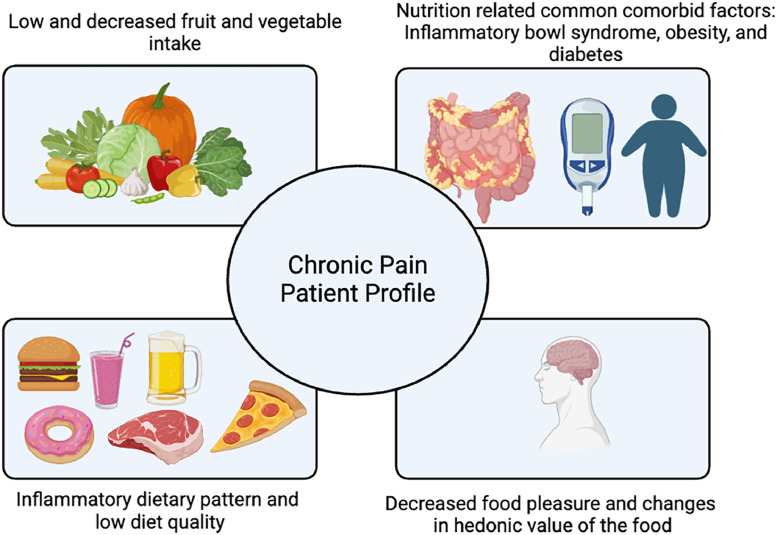
In the context of nutrition and pain, it is important to emphasize that common comorbid factors in people with persistent pain often include nutrition-related diseases and symptoms. For instance, the risk of being overweight/obese, developing type-2 diabetes, and inflammatory bowel syndrome is significantly higher in people with persistent pain compared to pain-free controls.12 Therefore, alongside the other components of the patient profile, as illustrated in Fig. 1, identification and management of these conditions is essential to comprehensive pain management.12
Fig. 1.
Nutritional profile of patients with persistent pain.
Persistent pain, nutrition, and the immune system: understanding the mechanisms
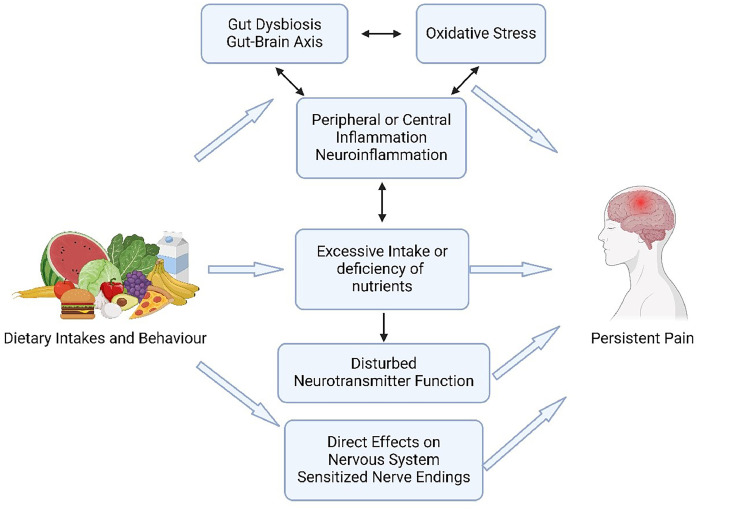
Immune activity and inflammation, as natural responses of the body, have been linked with various persistent health conditions and suggested as the dominant mechanism behind the link between nutrition and persistent pain as illustrated in Fig. 2.13 Cross-sectional analysis of data from over 50,000 participants recorded in the United Kingdom Biobank revealed a direct relationship between serum inflammatory biomarker levels, particularly C-reactive protein levels, and the presence of persistent pain compared to people reporting acute pain or no pain.14 Nutritional factors, including insufficient anti-inflammatory and antioxidative dietary intake, disrupt the body's detoxification and induce an increase in peripheral inflammatory biomarkers.15 When it persists, such peripheral inflammation triggers central immune responses.15 A prolonged pro-inflammatory state is linked to neuroinflammation which contributes to pain by directly enhancing the sensitivity of the peripheral and central nervous systems (i.e., sensitization) and contributes to the chronification of pain through aberrant glial cell and astrocyte activity and increased secretion of pro-inflammatory cytokines.16
Fig. 2.
Action mechanisms behind the link between nutrition and persistent pain.
Another factor contributing to central neuroimmune activity and pain sensitization is the dysregulation of the gut microbiome.15 Compared to healthy, pain-free individuals, data in people with persistent pain show evidence of gut dysbiosis, and an increased presence of certain microbes and viruses (e.g., Borrelia species, Mycobacterium leprae, and Human Immunodeficiency Virus).17 Nutritional factors but also physical (in)activity can directly impact the diversity and composition of microbes residing in the gastrointestinal tract. An unhealthy, pro-inflammatory dietary intake induces an inflammatory response in the gastrointestinal system via gut dysbiosis.9 An increased and sustained inflammatory response can trigger cascades of neuroinflammatory reactions (e.g., aberrant glial cell activity, inflammatory cytokine release as described above) in the central nervous system by activating vagal nerve afferents. Preclinical studies suggest that this nutrition and gut-brain axis can significantly modulate persistent pain via central sensitization,9 a key underlying mechanism of many patients with persistent pain.18
A last important aspect of the link between nutrition and pain can be found at the level of the nutrients as the nervous system requires certain nutrients to function properly. A deficiency or abundance of certain nutrients, either due to unhealthy dietary behavior or dysregulated nutrient metabolism, can directly affect the nervous system, impacting pain processing. For instance, an abundance of arachidonic acid, an omega-6 fatty acid, facilitates the secretion of prostaglandins, which can act as nociceptive factors and contribute to peripheral and central sensitization mechanisms.19 In contrast, sufficient omega-3 intake can have anti-inflammatory and antinociceptive effects similar to ibuprofen by decreasing cyclooxygenase activity and the secretion of prostaglandins.19 Furthermore, magnesium deficiency has been suggested to play a role in enhanced pain sensitivity, as magnesium acts as a blocker of N-methyl-d-aspartate (i.e., NMDA) receptors, which are essential for the sensation of pain.20 The hyperactivation of these receptors contributes to central sensitization and increased pain sensitivity by amplifying and modulating pain signals.20 Additionally, essential amino acids are required for the secretion of neurotransmitters with a significant impact on pain modulation, such as tryptophan for serotonin secretion.21 Thus, a dietary deficiency in certain precursors of neurotransmitters is suggested to play a role in the analgesic effect of dietary intakes.21 Moreover, besides increasing oxidative stress and leading to oxidative stress-induced inflammation, a prolonged hyperglycemic state and an abundance of serum glucose can directly interact with free nerve endings, decrease the pain threshold, and increase pain sensitivity.22
Evidence-based nutritional interventions for persistent pain problems
Targeting persistent pain with nutritional interventions not only holds potential for optimizing pain management but can also aid in the prevention of diseases and management of general health, constituting a sustainable intervention option. Identifying individual symptoms and needs, and addressing these needs based on the current best evidence, is the core of individualized nutritional interventions. From a dietary perspective, one can target a person's overall diet quality by targeting dietary patterns. Addressing overall diet quality using either predefined dietary indices or dietary patterns, ensures consideration of the complex interactions between the various foods and nutrients consumed together and is preferred over the focus on single nutrient intake. Overall, healthy plant-based dietary patterns such as vegan, vegetarian, and Mediterranean diets are classified as anti-inflammatory because they are rich in antioxidative and anti-inflammatory nutrients, contribute to gut health, and align with daily recommended nutrient intake levels.8,9,23,24 Additionally, the use of elimination diets such as low-FODMAP diets, aspartame/glutamate-free diets, or gluten- or lactose-free diets has been reported to have positive effects among the persistent pain population due to their consideration of individuals’ allergic reactions and intolerances to certain food components.8 Based on the available evidence, components of nutrition intervention in persistent pain and overall recommendations regarding these components are presented in Table 2.9,24, 25, 26, 27
Table 2.
Summary of evidence-based nutrition intervention in patients with persistent pain.
| Components | Recommendations |
|---|---|
| Dietary Intakes | |
| Carbohydrate Intake | Avoid ultra-processed foods and added-sugar, try low-glycemic indexed options over high-glycemic indexed ones. |
| Fat Intake | Reduce saturated fat intake, consider 1:1 ratio in omega-3/omega-6 poly unsaturated fat intake. Give preference to extra virgin olive oil. |
| Meat and Protein Intake | Avoid ultra processed meats, reduce red meat intake. Consume oily fish as it is high in omega 3. Consider legumes, nuts, and seeds for protein intake. |
| Dairy | Yogurt is rich in probiotics for gut health. Give preference to high quality dairy. |
| Fruits and Vegetables | Consume 4–5 servings a day. Give attention to high-FODMAP* fruits and vegetables in case of malabsorption. |
| Drinks | Reduce alcohol and caffeine intake and consume at least 1.5–2 L of water daily. |
| Dietary Patterns | |
| Plant-based Dietary Patterns (Vegan – Vegetarian) | Rich in antioxidants and pythonutrients and provides required daily nutrient intake, consistent with healthy eating guidelines. |
| Mediterranean Diet | Rich in fruits, vegetables, pre- and pro-biotics, and nutrients known for their antioxidative and anti-inflammatory properties. Thus, classified as anti-inflammatory and analgesic. |
| Elimination Diet | Allow the elimination of the nutrients and foods that patients are intolerant to or have association with their pain. Low-FODMAP diet, aspartame and glutamate eliminated diets, and gluten or lactose free diets are the common types of elimination diets. |
FODMAP – Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols are poorly absorbed short-chain carbohydrates.
In conclusion, integrating nutrition into the treatment of persistent pain should be considered as part of the pain management process. Emerging evidence suggests that dietary interventions can play a significant role in pain management, with potential benefits extending beyond pain relief to include offering sustainable solutions to long-term health and well-being, a preventive role against non-communicable diseases, and better management of comorbidities associated with persistent pain.
Declaration of competing interest
All authors were involved in the investigation of the link between persistent pain and nutritional factors for which their institutions received funding from various funding bodies, including the Research Foundation Flanders (FWO) and Bournemouth University Pump-Priming. This work was also supported by the Strategic Research Program SRP90 (‘Pain Never Sleeps: Unravelling the Sleep-Pain Interaction in Patients with Chronic Pain’) funded by the research council of the Vrije Universiteit Brussel, Brussels, Belgium. Jo Nijs and Vrije Universiteit Brussel received lecturing/teaching fees from various professional associations and educational organizations.
References
- 1.Nijs J., Lahousse A. Introducing the comprehensive pain management editorial series. Braz J Phys Ther. 2023;27(2) doi: 10.1016/j.bjpt.2023.100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nijs J., Lahousse A., Malfliet A. A paradigm shift from a tissue-and disease-based approach towards multimodal lifestyle interventions for chronic pain: 5 steps to guide clinical reasoning. Braz J Phys Ther. 2023;27(5) doi: 10.1016/j.bjpt.2023.100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carballo-Casla A., García-Esquinas E., Lopez-Garcia E., et al. The inflammatory potential of diet and pain incidence: a cohort study in older adults. J Gerontol: Series A. 2023;78(2):267–276. doi: 10.1093/gerona/glac103. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y., De Araujo I., Stanley G., Small D., Geha P. Chronic pain precedes disrupted eating behavior in low-back pain patients. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0263527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanDenKerkhof E.G., Macdonald H.M., Jones G.T., Power C., Macfarlane G.J. Diet, lifestyle and chronic widespread pain: results from the 1958 British Birth Cohort Study. Pain Res Manag. 2011;16(2):87–92. doi: 10.1155/2011/727094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zick S.M., Murphy S.L., Colacino J. Association of chronic spinal pain with diet quality. Pain Rep. 2020;5(5):e837. doi: 10.1097/PR9.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meleger A.L., Froude C.K., Walker I.II.J. Nutrition and eating behavior in patients with chronic pain receiving long-term opioid therapy. PM&R. 2014;6(1):7–12. doi: 10.1016/j.pmrj.2013.08.597. e11. [DOI] [PubMed] [Google Scholar]
- 8.Elma Ö., Yilmaz S.T., Deliens T., et al. Do nutritional factors interact with chronic musculoskeletal pain? A systematic review. J Clin Med. 2020;9(3):702. doi: 10.3390/jcm9030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elma Ö., Brain K., Dong H.-J. The importance of nutrition as a lifestyle factor in chronic pain management: a narrative review. J Clin Med. 2022;11(19):5950. doi: 10.3390/jcm11195950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elma Ö., Yılmaz S.T., Nijs J., et al. Proinflammatory dietary intake relates to pain sensitivity in chronic nonspecific low back pain: a case-control study. J Pain. 2024;25(2):350–361. doi: 10.1016/j.jpain.2023.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhou R., Zhang L., Sun Y., Yan J., Jiang H. Causal associations between dietary habits and chronic pain: a two-sample mendelian randomization study. Nutrients. 2023;15(17):3709. doi: 10.3390/nu15173709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert S.V., Green M.A., Mashaw S.A., et al. Assessing risk factors and comorbidities in the treatment of chronic pain: a narrative review. Curr Pain Headache Rep. 2024:1–10. doi: 10.1007/s11916-024-01249-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W.B.S., Meng J., Zhang J. Does low grade systemic inflammation have a role in chronic pain? Front Mol Neurosci. 2021;14 doi: 10.3389/fnmol.2021.785214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges S., Guler S., Sacca V., et al. Associations among acute and chronic musculoskeletal pain, sleep duration, and C-reactive protein (CRP): a cross-sectional study of the UK biobank dataset. Sleep Med. 2023;101:393–400. doi: 10.1016/j.sleep.2022.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minihane A.M., Vinoy S., Russell W.R., et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutrition. 2015;114(7):999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda M., Huh Y., Ji R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131–139. doi: 10.1007/s00540-018-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goudman L., Demuyser T., Pilitsis J.G., et al. Gut dysbiosis in patients with chronic pain: a systematic review and meta-analysis. Front Immunol. 2024;15 doi: 10.3389/fimmu.2024.1342833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nijs J., George S.Z., Clauw D.J., et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3(5):e383–e392. doi: 10.1016/S2665-9913(21)00032-1. [DOI] [PubMed] [Google Scholar]
- 19.DiNicolantonio J.J., O'Keefe J.H. Importance of maintaining a low omega–6/omega–3 ratio for reducing inflammation. Open Heart. 2018;5(2) doi: 10.1136/openhrt-2018-000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin H.-J., Na H.-S., Do S.-H. Magnesium and pain. Nutrients. 2020;12(8):2184. doi: 10.3390/nu12082184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shabbir F., Patel A., Mattison C., et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem Int. 2013;62(3):324–329. doi: 10.1016/j.neuint.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Nijs J., Elma Ö., Yilmaz S.T., et al. Nutritional neurobiology and central nervous system sensitisation: missing link in a comprehensive treatment for chronic pain? Br J Anaesth. 2019;123(5):539–543. doi: 10.1016/j.bja.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Barbaresko J., Koch M., Schulze M.B., Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–527. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- 24.Dragan S., Șerban M.-C., Damian G., Buleu F., Valcovici M., Christodorescu R. Dietary patterns and interventions to alleviate chronic pain. Nutrients. 2020;12(9):2510. doi: 10.3390/nu12092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rondanelli M., Faliva M.A., Miccono A., et al. Food pyramid for subjects with chronic pain: foods and dietary constituents as anti-inflammatory and antioxidant agents. Nutr Res Rev. 2018;31(1):131–151. doi: 10.1017/S0954422417000270. [DOI] [PubMed] [Google Scholar]
- 26.Field R., Pourkazemi F., Turton J., Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Medicine. 2021;22(3):694–714. doi: 10.1093/pm/pnaa378. [DOI] [PubMed] [Google Scholar]
- 27.Kaushik A.S., Strath L.J., Sorge R.E. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9:487–498. doi: 10.1007/s40122-020-00200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]