Abstract
Background
Sex-specific differences in left ventricular (LV) geometry might help in developing tailored strategies for hypertension management.
Objectives
The purpose of the study was to evaluate sex-related differences in LV geometry at baseline and over time in hypertension.
Methods
From a prospective registry, we included hypertensives without prevalent cardiovascular disease, incident myocardial infarction, chronic kidney disease > stage III, and with normal LV ejection fraction. LV mass index >115 g/m2 in males and >95 g/m2 in females, identified LV hypertrophy (LVH). Relative wall thickness ≥0.43 defined LV concentric geometry. LVH in presence of concentric geometry was defined as concentric LVH, whereas relative wall thickness <0.43 was categorized as eccentric. Concentric geometry, or LVH, identified LV remodeling.
Results
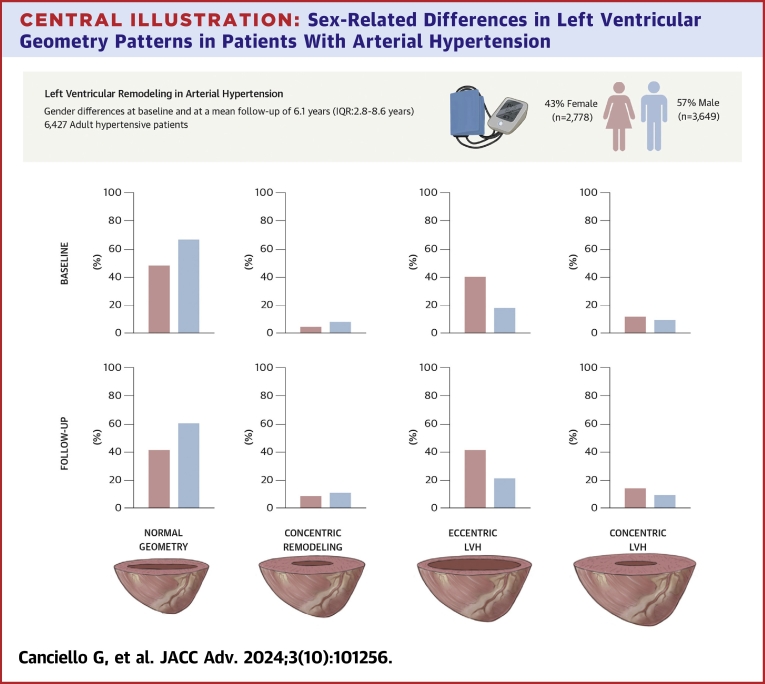
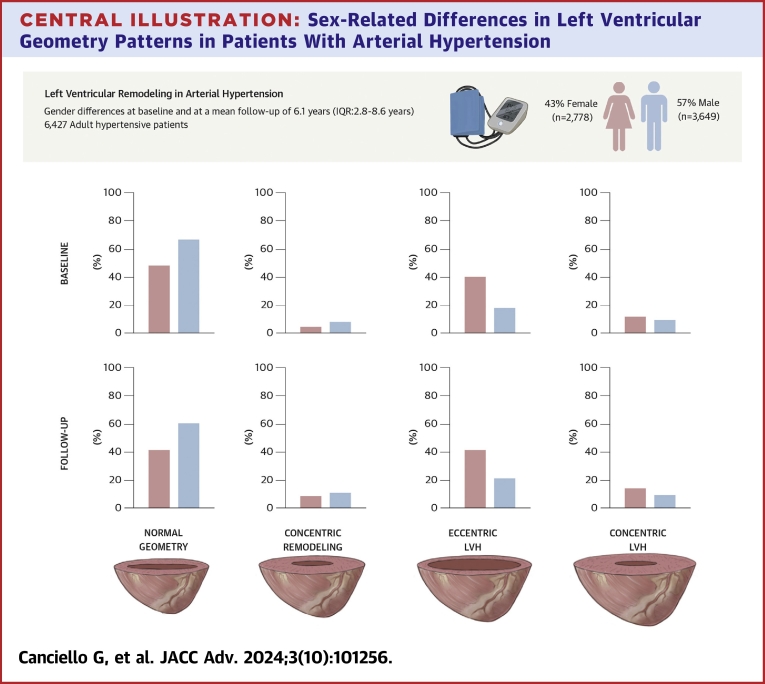
Six thousand four hundred twenty-seven patients (age 53 ± 11 years, 43% females) were included. At baseline, females showed lower prevalence of normal geometric pattern and higher prevalence of LVH than males (50% vs 72%, P < 0.001; 47% vs 23%, P < 0.001, respectively), with a higher prevalence of eccentric LVH (40% vs 18%, P < 0.001). Female sex was independently associated with LV remodeling (OR: 2.36; 95% CI: 2.12-2.62; P < 0.001). At long-term follow-up (mean 6.1 years, IQR: 2.8-8.6 years), prevalence of LV remodeling increased in both sexes, although a normal LV geometry remained less frequent in females than males (43% vs 67%, P < 0.001), with differences persisting in eccentric (41% vs 21%, P < 0.001) and concentric LVH (11% vs 5%, P < 0.001).
Conclusions
We found sex-related differences in LV geometry among hypertensives. Females have higher risk of LV remodeling at baseline compared with males, with differences persisting at long-term follow-up.
Key words: females, hypertension, left ventricular hypertrophy, sex-related differences
Central Illustration
Structural and functional characteristics of the left ventricle (LV) are highly correlated in the pathophysiology of arterial hypertension.1 The systemic hemodynamic profile often parallels the changes in LV geometry, with concentric remodeling and LV hypertrophy (LVH) associated with a higher peripheral resistance. Conversely, a supernormal cardiac index is often observed in patients exhibiting eccentric LV hypertrophy, with a low-to-normal range seen in cases of concentric LV remodeling.2 Despite extensive investigation on the relationship between arterial hypertension and LV remodeling,3 the impact of sex on this process remains underexplored.4 Data evaluating the role of sex on basal LV geometry, subsequent changes over time, and the mediating effects of treatment are scarce. Yet, emerging evidence highlights that sex-based differences significantly influence not only the prevalence and clinical presentation of hypertension but also impacts on morphofunctional cardiac adaptations in response to elevated blood pressure (BP).4 Therefore, identifying sex-specific differences in LV geometry is key for tailoring effective management strategies for arterial hypertension. Against this backdrop, we analyzed LV geometry patterns at baseline and their evolution during long-term follow-up within the Campania Salute Network registry, a large, prospective, observational study of patients with arterial hypertension.
Methods
The design and methodology of the Campania Salute Network registry (NCT02211365) have been described previously.5, 6, 7 Briefly, the Campania Salute Network is an open registry collecting information from general practitioners and community hospitals in the five districts of the Campania Region in Southern Italy, networked with the Hypertension Research Center of the Federico II University Hospital in Naples. All participants were referred to the center for baseline work-up screening and echocardiogram.5,8 The Campania Salute Network was approved by the institutional ethics committee, and a signed informed consent was obtained from all participants.
Definitions
Hypertension was diagnosed when office systolic BP readings were ≥140 mm Hg and/or diastolic BP readings were ≥90 mm Hg, or when antihypertensive therapy was prescribed.9 Systolic and diastolic BP was measured after 5 minutes resting in the sitting position by a trained physician or nurse, according to current guidelines and standard procedures of Campania Salute Network.10 Follow-up systolic and diastolic BP were also considered as the average office BP recorded during all control visits.11 Diabetes was classified following the 2007 American Diabetes Association criteria (fasting plasma glucose >125 mg/dL or antidiabetic treatment).12 Obesity was identified as a body mass index ≥30 kg/m2.12 The glomerular filtration rate was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula.11
Echocardiographic evaluation
Echocardiographic examinations were performed at our Hypertension Center, and quality-controlled validation was performed for each exam executed, as previously reported in detail.13 Echocardiograms recorded in our hypertension center using commercial machines and a standardized protocol were digitally mastered and read offline by one expert reader (ASE III level) under the supervision of a senior faculty member (ASE III level).13 All measurements were made following American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations.14,15
LV mass was estimated using a necropsy-validated formula, with the results indexed to body surface area (LV mass index).15 A LV mass index >115 g/m2 in males and >95 g/m2 in females identified LV hypertrophy (LVH).9,16 A relative wall thickness (RWT) ≥0.43 defined LV concentric geometry 9,17 in absence of LVH. LVH in presence of a concentric geometry was defined as concentric LVH, whereas a RWT <0.43 was categorized as eccentric. Concentric geometry, or LVH, either concentric or eccentric, identified LV remodeling. LV volumes were estimated from linear measures of LV diameters by the z-derived method and used to compute the LV ejection fraction.18 We considered two echocardiograms for each patient: that at baseline and the last available one, which was considered at follow-up.
Statistical analysis
Data are expressed as mean ± SD for continuous variables and as absolute frequency and percentage for categorical variables. The chi-square test was used to compare categorical variables with the Monte Carlo simulation to obtain exact P values. We used the 4-tier classification of LV geometry as suggested by recommendation: normal, concentric remodeling, eccentric, and concentric LVH.9,16,17
Firstly, associations between the covariates and the LV geometry pattern were assessed using crude and adjusted ordinal logistic regressions both at baseline (considering only baseline variable measures) and at the end of the follow-up period (considering only measures at follow-up). The results of the ordinal logistic regression models are reported as crude and adjusted ORs, which are to be interpreted as the estimated risk (by covariate) of changing the current LV geometry up by 1 tier. To investigate the determinants of the longitudinal changes in the LV geometry pattern, a multiple ordinal mixed-effect regression model with logit link was adopted, considering the patient’s ID as random-effect and follow-up time as a random-effects offset. Time considered as indexes for baseline and follow-up was also added as fixed effect. We have also run an additional analysis, dichotomizing the LV geometry pattern variable into normal and pathological pattern. The variables associated with a pathological LV pattern were evaluated using crude and adjusted logistic regression. As previously reported,13 the study accounts for antihypertensive therapy by calculating the total number of medications, including antirenin-angiotensin system (angiotensin converting enzyme inhibitors and AT1 receptor antagonists), calcium channel blockers, beta-blockers, and diuretics, at each visit. This total is reported based on the frequency of prescriptions during follow-up. If a medication was prescribed for more than 50% of control visits for a patient, it was included as a covariate in the follow-up multivariate analysis. To create a single variable representing overall antihypertensive therapy, the study adds up all the medications prescribed more than 50% of the time. This cumulative count is used as a continuous variable called “total therapy.” This variable quantifies the overall intensity of antihypertensive treatment each patient is receiving.
Patients with preserved normal geometry were the reference group in this analysis.
A two-tailed P value <0.05 was considered statistically significant in all analyses. Data were analyzed using SPSS (version 26.0, SPSS) and R Statistical Software (version 4.3.0).
Results
Between 1990 and 2014, a total of 14,161 hypertensive patients were included in the Campania Salute Network registry. We excluded patients under 18 years of age (N = 106), with prevalent cardiovascular disease (myocardial infarction, coronary revascularization, stroke, transient ischemic attack, valvular heart disease, N = 284), LV ejection fraction <50% (N = 1,588), chronic kidney disease stage >III (N = 2,198), incident myocardial infarction (N = 51), incomplete echocardiographic data at baseline and/or at follow-up (N = 452), and patients with less than 1-year follow-up (N = 3,055). Thus, the final population was of 6,427 patients, with a mean age of 53 ± 11 years, of which 2,778 (43%) were females. Mean follow-up time was not statistically different between the 2 sexes, with a mean of 6.2 ± 4.4 years for males and 5.8 ± 4.2 for females. Intraobserver and interobserver variability was assessed by the repeatability coefficient, determined as 1.96 × SD of the absolute value of the differences.19 The repeatability coefficient was assessed for the variables enabling the measurement of LVH and of RWT: interventricular LV septum, posterior wall, and end-diastolic diameter. The intraobserver and interobserver repeatability coefficients were 0.4 and 0.9 mm for interventricular septum, 0.7 and 1.0 mm for posterior wall, and 1.1 and 1.8 mm for end diastolic diameter, respectively. Table 1 shows the baseline characteristics of the study population stratified by sex. In comparison to males, female patients were older, had a longer history of hypertension, and had a higher prevalence of obesity. Additionally, females had a higher systolic and diastolic BP, heart rate, LV ejection fraction, and smaller left atrial and LV end-diastolic diameters. As shown in Table 1 and the Central Illustration, a normal geometric pattern was less common in female than in male patients (50% vs 72%, P < 0.001); concentric remodeling was present in few patients and was less common in females than in males (3% vs 5%, P < 0.001). LVH was more prevalent among females (47% vs 23%, P < 0.001), with females having a higher rate of eccentric LVH (40% vs 18%, P < 0.001) and a higher prevalence of concentric LV hypertrophy than males (7% vs 5%, P < 0.001) (Table 1, Central Illustration).
Table 1.
Baseline Clinical and Echocardiographic Characteristics of the Population Stratified by Sex
| Men (n = 3,649) | Female (n = 2,778) | Difference | 95% CI | |
|---|---|---|---|---|
| Age (y) | 52 ± 11 | 55 ± 11 | −0.24 | −0.29 to −0.19 |
| Years of hypertension | 5.7 ± 6.5 | 6.7 ± 7.3 | −0.15 | −0.20 to −0.10 |
| Obesity (%) | 864 (24%) | 724 (26%) | −2.4% | −4.6% to −0.22% |
| Diabetes (%) | 343 (9%) | 244 (9%) | 0.62% | −0.83% to 2.1% |
| Dyslipidemia | 2,933 (81%) | 2,381 (86%) | −5.3% | −7.1% to −3.4% |
| Systolic blood pressure (mm Hg) | 141 ± 17 | 144 ± 19 | −0.13 | −0.18 to −0.08 |
| Diastolic blood pressure (mm Hg) | 90 ± 11 | 88 ± 11 | 0.11 | 0.06-0.16 |
| Heart rate (bpm) | 74 ± 11 | 75 ± 12 | −0.12 | −0.17 to −0.07 |
| Left atrial diameter (cm) | 3.8 ± 0.3 | 3.6 ± 0.4 | 0.061 | 0.06-0.07 |
| Left ventricular end diastolic diameter (cm) | 5.1 ± 0.3 | 4.8 ± 0.3 | 1.1 | 1.0-1.3 |
| Left ventricular ejection fraction (%) | 66 ± 4 | 67 ± 4 | −0.20 | −0.25 to −0.15 |
| Relative wall thickness | 0.38 ± 0.04 | 0.38 ± 0.04 | −0.07 | −0.12 to −0.02 |
| Left ventricular mass (g/m2) | 105 ± 18 | 95 ± 15 | 0.55 | 0.49-0.60 |
| Left ventricular geometry patterns | ||||
| Normal left ventricular pattern | 72% | 50% | 22% | 20%-25% |
| Concentric left ventricular pattern | 5% | 3% | 1.8% | 0.85%-2.8% |
| Eccentric left ventricular hypertrophy | 18% | 40% | −22% | −24% to −19% |
| Concentric left ventricular hypertrophy | 5% | 7% | −2.5% | −3.8% to −1.3% |
Values are mean ± SD or n (%) unless indicated otherwise. Difference expressed as standardized mean difference for quantitative variables and as risk difference for proportions.
Central Illustration.
Sex-Related Differences in Left Ventricular Geometry Patterns in Patients With Arterial Hypertension
(Upper panel) Main methods of the study; (lower panels) main results of the study. LVH = left ventricular hypertrophy.
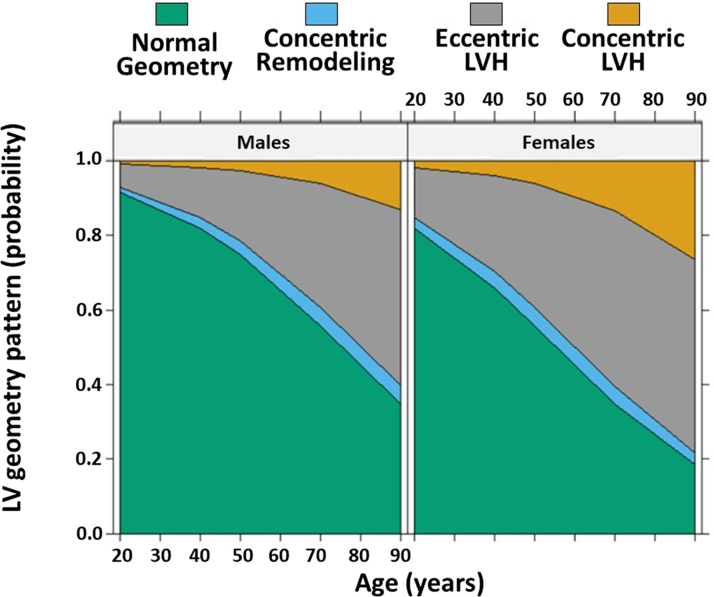
At multivariable analysis conducted at baseline (Table 2), female sex was independently associated with abnormal LV geometry (OR: 2.36; 95% CI: 2.12-2.62; P < 0.001). Other significant determinants included age, duration of hypertension, systolic BP, obesity, and diabetes. Figure 1 illustrates the age and sex-stratified adjusted estimated probability densities for each LV geometric pattern. The analysis showed that with each incremental year of age, the risk of an abnormal pattern increased by 4%, with females having a 2.36 times higher risk than males.
Table 2.
Determinants of Abnormal Left Ventricular Geometry at Baseline (Concentric Remodeling or Eccentric and Concentric Left Ventricular Hypertrophy): Univariable and Multivariable Logistic Regression Models
| OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Age (y) | 1.05 | 1.05-1.06 | <0.001 | 1.04 | 1.04-1.05 | <0.001 |
| Female | 2.59 | 2.35-2.87 | <0.001 | 2.36 | 2.12-2.62 | <0.001 |
| Duration of hypertension (y) | 1.05 | 1.05-1.06 | <0.001 | 1.02 | 1.01-1.03 | <0.001 |
| Systolic BP (mm Hg) | 1.02 | 1.02-1.03 | <0.001 | 1.02 | 1.01-1.02 | <0.001 |
| Diastolic BP (mm Hg) | 1.01 | 1.00-1.01 | <0.001 | 1.00 | 1.00-1.01 | 0.306 |
| Obesity | 1.45 | 1.29-1.62 | <0.001 | 1.35 | 1.20-1.52 | <0.001 |
| Diabetes | 1.81 | 1.53-2.12 | <0.001 | 1.23 | 1.04-1.46 | 0.018 |
Variables statistically significant at univariable were included in the multivariable logistic regression model.
BP = blood pressure.
Figure 1.
Effect Plot of the Adjusted Probability of Showing Each Left Ventricular Geometry Pattern by Sex and Age Estimated With Ordinal Logistic Regression
LV = left ventricle; LVH = left ventricular hypertrophy.
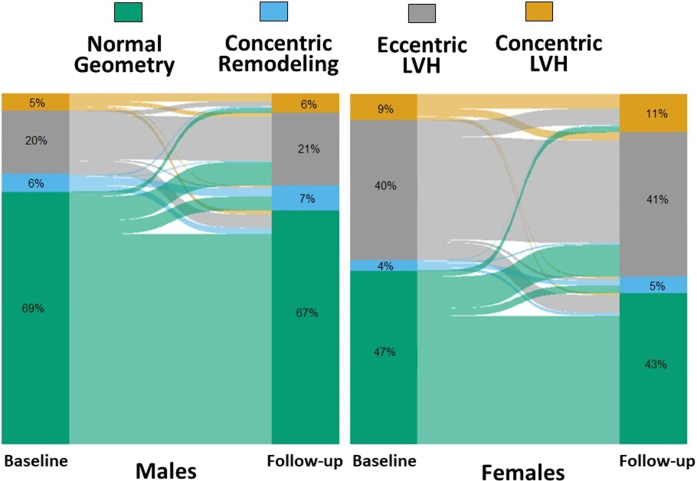
At long-term follow-up of 6.1 years (IQR: 2.8-8.6 years), we observed an increased proportion of patients with abnormal LV geometric patterns in both sexes (Table 3, Central Illustration). Specifically, concentric LVH increased mostly in females than in males (Table 3, Central Illustration). During follow-up, systolic and diastolic BP were different between males and females, with females having lower diastolic (83 ± 7 mm Hg vs 85 ± 7 mm Hg, respectively, P < 0.001) and higher systolic BP than men (138 ± 13 mm Hg vs 136 ± 11 mm Hg, respectively, P < 0.001). In addition, while the prescription of only 1 antihypertensive drug was similar among females than males (33% vs 34%, respectively, P = 0.195), the administration of more than two antihypertensive medications was more common in females than in males during follow-up (19% vs 16%, respectively, P = 0.003). Table 4 reports the results of the longitudinal model, which confirms a significant effect of age (24% higher risk per each increasing year of age) and a significant higher risk in females (250%) of progressing to a pathological LV geometry. Other significant covariates were duration of hypertension, abnormal BP control, and total therapy. Additionally, time was an independent predictor of progression. The full model outputs with R call and code for the models shown in Tables 3 and 4 and in the Supplemental Material. Table 5 outlines the variables associated with worsening patterns over time. Aging, obesity, diabetic status, and female sex together with a longer history of hypertension, poor BP control, and treatment with more antihypertensive drugs during follow-up were associated with worsening patterns at long-term follow-up. Alluvial plots of the individual changes in LV geometry pattern from baseline to follow-up in males and females are reported in Figure 2. Central Illustration reports the main methods and results of our study.
Table 3.
Univariable and Multivariable Ordinal Logistic Regression Model to Investigate the Determinants of Pathological LV Geometry at Follow-Up
| OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Age (y) | 1.06 | 1.05-1.06 | <0.001 | 1.04 | 1.04-1.05 | <0.001 |
| Female vs male | 2.69 | 2.44-2.96 | <0.001 | 2.50 | 2.25-2.77 | <0.001 |
| Duration of hypertension (y) | 1.05 | 1.05-1.06 | <0.001 | 1.01 | 1.00-1.02 | 0.003 |
| Normal vs abnormal BP control for more than 50% of visits during follow-up | 0.50 | 0.46-0.56 | <0.001 | 0.58 | 0.52-0.64 | <0.001 |
| Obesity at follow-up | ||||||
| Never obese | – | – | – | – | – | – |
| Ex obese | 1.77 | 1.44-2.18 | <0.001 | 1.69 | 1.35-2.10 | <0.001 |
| New obese | 1.12 | 0.91-1.38 | 0.266 | 1.19 | 0.96-1.48 | 0.112 |
| Ever obese | 1.56 | 1.38-1.76 | <0.001 | 1.41 | 1.24-1.61 | <0.001 |
| Diabetes at follow-up | ||||||
| Never diabetes | – | – | – | – | – | – |
| New diabetes | 1.44 | 1.21-1.71 | <0.001 | 1.16 | 0.97-1.39 | 0.099 |
| Ever diabetes | 2.16 | 1.83-2.55 | <0.001 | 1.40 | 1.17-1.67 | <0.001 |
| Follow-up years | 1.03 | 1.02-1.04 | <0.001 | 1.06 | 1.04-1.07 | <0.001 |
| Total therapy | ||||||
| No therapy | – | – | – | – | – | – |
| 1 drug | 1.29 | 1.09-1.52 | 0.002 | 1.18 | 0.99-1.41 | 0.061 |
| 2 drugs | 1.90 | 1.62-2.23 | <0.001 | 1.50 | 1.26-1.79 | <0.001 |
| 3 drugs | 2.73 | 2.27-3.29 | <0.001 | 1.79 | 1.46-2.19 | <0.001 |
| 4 drugs or more | 5.03 | 3.79-6.68 | <0.001 | 2.48 | 1.83-3.36 | <0.001 |
BP = blood pressure; LV = left ventricular.
Table 4.
Longitudinal Model of Progressing to a Pathological LV Remodeling
| Adjusted OR | 95% CI | P Value | |
|---|---|---|---|
| Age (y) | 1.24 | 1.21-1.26 | <0.001 |
| Female vs male | 2.50 | 2.25-2.77 | <0.001 |
| Duration of hypertension (y) | 1.04 | 1.00-1.07 | 0.026 |
| Normal vs abnormal BP control for more than 50% of visits during follow-up | 0.08 | 0.05- 0.12 | <0.001 |
| Obesity vs no obesity (longitudinal measure) | 1.10 | 0.83-1.45 | 0.508 |
| Diabetes vs no diabetes (longitudinal measure) | 1.23 | 0.88-1.72 | 0.229 |
| Total therapy | 1.80 | 1.45-2.23 | <0.001 |
| Time (follow-up vs baseline effect) | 2.09 | 1.86- 2.36 | <0.001 |
Abbreviations as in Table 3.
Table 5.
Univariable and Multivariable Binary Logistic Regression Model to Investigate the Determinants of Normal vs Pathological LV Pattern at Follow-Up
| OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Age (y) | 1.06 | 1.05-1.06 | <0.001 | 1.04 | 1.04-1.05 | <0.001 |
| Female vs male | 2.62 | 2.36-2.90 | <0.001 | 2.50 | 2.24-2.79 | <0.001 |
| Duration of hypertension (y) | 1.05 | 1.05-1.06 | <0.001 | 1.01 | 1.00-1.02 | 0.006 |
| Abnormal vs normal BP control for more than 50% of visits during follow-up | 0.53 | 0.47-0.58 | <0.001 | 0.58 | 0.52-0.65 | <0.001 |
| Obesity at follow-up | ||||||
| Never obesity | – | - | – | – | – | – |
| Ex obese | 1.76 | 1.41-2.21 | <0.001 | 1.74 | 1.36-2.22 | <0.001 |
| New obese | 1.10 | 0.89-1.35 | 0.393 | 1.14 | 0.91-1.44 | 0.256 |
| Ever obese | 1.56 | 1.38-1.77 | <0.001 | 1.43 | 1.25-1.65 | <0.001 |
| Diabetes at follow-up | ||||||
| Never diabetes | – | – | – | – | – | – |
| New diabetes | 1.40 | 1.17-1.67 | <0.001 | 1.14 | 0.93-1.38 | 0.202 |
| Ever diabetes | 2.27 | 1.89-2.73 | <0.001 | 1.51 | 1.23-1.85 | <0.001 |
| Follow-up years | 1.03 | 1.02-1.04 | <0.001 | 1.06 | 1.04-1.07 | <0.001 |
| Total therapy | ||||||
| No therapy | – | – | – | – | – | – |
| 1 drug | 1.29 | 1.10-1.53 | 0.002 | 1.10 | 0.92-1.33 | 0.061 |
| 2 drugs | 1.90 | 1.61-2.24 | <0.001 | 1.39 | 1.16-1.67 | <0.001 |
| 3 drugs | 2.70 | 2.23-3.27 | <0.001 | 1.66 | 1.34-2.06 | <0.001 |
| 4 drugs or more | 5.23 | 3.79-7.29 | <0.001 | 2.90 | 2.02-4.21 | <0.001 |
Abbreviations as in Table 3.
Figure 2.
Alluvial Plot of the Individual Changes in Left Ventricular Geometry Pattern From Baseline to Follow-Up
The height of each bar is proportional to the number of patients with the corresponding left ventricular geometry pattern, and the width of the ends of each flow line is proportional to the number of patients whose left ventricular geometry pattern changed at follow-up. LVH = left ventricular hypertrophy.
Discussion
Our study aimed to explore sex-related differences in LV geometry patterns in a large cohort of patients with arterial hypertension. We found that LV remodeling was more prevalent in females compared with males at the initial assessment, with a higher occurrence of LVH observed in females, including both eccentric and concentric LVH. The association between sex and LV remodeling persisted throughout the follow-up, indicating that these differences in LV geometry might even increase over time and therefore are not solely attributable to distinct clinical presentations. Prior studies from the Campania Salute Network registry had also noted a higher prevalence of LV hypertrophy in females.20,21 Our study builds on this by establishing a significant association between female sex and LV remodeling, irrespective of other cardiovascular risk factors like obesity, further emphasizing the role of sex in influencing LV remodeling. The persistent association between female sex and advanced LV remodeling patterns suggests sustained cardiovascular risk for females with arterial hypertension, highlighting the importance of long-term surveillance and tailored interventions to mitigate LV remodeling progression.
Several potential explanations may account for these sex-based differences. Complex social, economic, and structural disparities contribute to differing experiences between females and males.22 One plausible factor could be the historical oversight of early clinical signs in females, a phenomenon observed in other cardiac conditions such as coronary artery disease and heart failure.23 This oversight might be linked to factors such as symptom denial and a heightened emphasis on the health of other family members.23
Our findings suggest that females experience untreated hypertension for a longer duration than men before accessing health care services, potentially leading to a worse LV geometry pattern due to delayed treatment initiation. However, even after accounting for the duration of hypertension, female sex remained significantly associated with LV remodeling, along with age, diabetes, obesity, and baseline systolic BP. This aligns well with previous observations indicating that progressive BP elevation increases more rapidly in females than in males, starting as early as the third decade of life.24
A notable finding in our study is the higher prevalence of eccentric LVH pattern in females compared to males, both at baseline and during follow-up. Traditionally, female sex is linked to concentric remodeling in the general population, while male sex is linked to eccentric LV hypertrophy, likely due to the higher prevalence of coronary artery disease in males.25, 26, 27 By excluding patients with prevalent and incident myocardial infarction, we could partly eliminate this potential confounder.
Furthermore, the response to pathological conditions such as diabetes, obesity, and hypertension differs over time between males and females.28 Interestingly, while the expression of collagen in human hearts does not differ between sexes, regulators of collagen metabolism vary between sexes. Collagen types I and III are lower in young females than young males, but this ratio reverses with age, with females tending to express higher levels of both types compared to males.25,29 This difference in collagen composition might contribute to varying adaptation to cardiovascular risk factors between sexes.25,29
Additionally, we observed an increase in the prevalence of concentric hypertrophy mostly in females at follow-up. This may be attributed to visceral adiposity in older females, often associated with systemic inflammation.30 This inflammatory process can lead to cardiac fibrosis, impairing ventricular distensibility and causing diastolic filling abnormalities, resembling concentric LV hypertrophy.30
Study limitation
We recognize inherent limitations in our study due to the observational nature of the Campania Salute Network, which remains susceptible to biases despite extensive multivariable adjustment efforts, also considering that the results hereby reported were not corrected for multiple comparisons and thus the significance threshold of the analysis is to be interpreted with caution. Nonetheless, we have minimized selection and observational biases by enrolling all hypertensive patients and consistently applying a standardized protocol across participants. In addition, although patients were referred by general practitioners and community hospitals, we were unable to stratify the analyses according to the original referring site.
We acknowledge that classification and reclassification of LVH by echocardiography, based on a single assessment, might be challenging because of the intrinsic noise in the measurement. However, echocardiographic assessment of left ventricular mass has previously been demonstrated to maintain sufficient reliability to be used in clinical practice.21,31,32
Another limitation of our study was that we did not account for death as competing risk. However, we observed only 21 deaths during the follow-up time. Given that we have no information about the cause of deaths for these patients, we decided to exclude these patients from the analysis.
Observational studies like ours cannot establish causal relationships but may generate hypotheses for subsequent mechanistic investigations. Despite this limitation, they are adept at identifying predictors in real-world contexts, aligning with our investigation's primary objective.
Conclusions
The present study highlights significant sex-related differences in LV remodeling patterns among hypertensive patients, underscoring the need for sex-specific approaches in hypertension management and cardiovascular risk reduction strategies.
PERSPECTIVES.
COMPETENCY IN PRACTICE-BASED LEARNING: The study highlights the importance of sex-specific differences in LV geometry for tailoring effective management strategies in arterial hypertension. We identified female sex independently associated with a higher risk of LV remodeling at baseline and during follow-up compared to males.
TRANSLATIONAL OUTLOOK: Clinicians should incorporate sex-specific factors into risk stratification models for hypertensive patients, enabling more personalized treatment plans and monitoring strategies. Recognizing sex-specific risk factors allows for an increased understanding of cardiovascular risk, aiding clinicians in targeted interventions and follow-up protocols.
Funding support and author disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental material, please see the online version of this paper.
Supplementary data
References
- 1.Devereux R.B., Roman M.J. Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res. 1999;22:1–9. doi: 10.1291/hypres.22.1. [DOI] [PubMed] [Google Scholar]
- 2.Ganau A., Devereux R.B., Roman M.J., et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 3.Devereux R.B., de Simone G., Ganau A., Roman M.J. Left ventricular hypertrophy and geometric remodeling in hypertension: stimuli, functional consequences and prognostic implications. J Hypertens. 1994;12:S117–S127. [PubMed] [Google Scholar]
- 4.Tadic M., Cuspidi C., Grassi G. The influence of sex on left ventricular remodeling in arterial hypertension. Heart Fail Rev. 2019;24:905–914. doi: 10.1007/s10741-019-09803-3. [DOI] [PubMed] [Google Scholar]
- 5.D'Amato A., Mancusi C., Losi M.A., et al. Target organ damage and target systolic blood pressure in clinical practice: the Campania Salute Network. Am J Hypertens. 2018;31:658–664. doi: 10.1093/ajh/hpy007. [DOI] [PubMed] [Google Scholar]
- 6.Mancusi C., Gerdts E., Losi M.A., et al. Differential effect of obesity on prevalence of cardiac and carotid target organ damage in hypertension (the Campania Salute Network) Int J Cardiol. 2017;244:260–264. doi: 10.1016/j.ijcard.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Izzo R., de Simone G., Trimarco V., et al. Hypertensive target organ damage predicts incident diabetes mellitus. Eur Heart J. 2013;34:3419–3426. doi: 10.1093/eurheartj/eht281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losi M.A., Izzo R., Canciello G., et al. Atrial dilatation development in hypertensive treated patients: the campania-salute Network. Am J Hypertens. 2016;29:1077–1084. doi: 10.1093/ajh/hpw043. [DOI] [PubMed] [Google Scholar]
- 9.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 10.Mancusi C., Izzo R., Ferrara L.A., et al. Is increased uric acid a risk factor or a defensive response? The Campania Salute Network. Nutr Metabol Cardiovasc Dis. 2018;28:839–846. doi: 10.1016/j.numecd.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Mancusi C., Izzo R., de Simone G., et al. Determinants of decline of renal function in treated hypertensive patients: the Campania Salute Network. Nephrol Dial Transplant. 2018;33:435–440. doi: 10.1093/ndt/gfx062. [DOI] [PubMed] [Google Scholar]
- 12.de Simone G., Mancusi C., Izzo R., Losi M.A., Aldo Ferrara L. Obesity and hypertensive heart disease: focus on body composition and sex differences. Diabetol Metab Syndrome. 2016;8:79. doi: 10.1186/s13098-016-0193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losi M.A., Izzo R., De Marco M., et al. Cardiovascular ultrasound exploration contributes to predict incident atrial fibrillation in arterial hypertension: the Campania Salute Network. Int J Cardiol. 2015;199:290–295. doi: 10.1016/j.ijcard.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Marwick T.H., Gillebert T.C., Aurigemma G., et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European association of cardiovascular imaging (EACVI) and the American society of echocardiography (ASE) J Am Soc Echocardiogr. 2015;28:727–754. doi: 10.1016/j.echo.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Gaasch W.H., Zile M.R. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 16.de Simone G., Izzo R., Aurigemma G.P., et al. Cardiovascular risk in relation to a new classification of hypertensive left ventricular geometric abnormalities. J Hypertens. 2015;33:745–754. doi: 10.1097/HJH.0000000000000477. discussion 754. [DOI] [PubMed] [Google Scholar]
- 17.Mancusi C., Canciello G., Izzo R., et al. Left atrial dilatation: a target organ damage in young to middle-age hypertensive patients. The Campania Salute Network. Int J Cardiol. 2018;265:229–233. doi: 10.1016/j.ijcard.2018.03.120. [DOI] [PubMed] [Google Scholar]
- 18.de Simone G., Mancusi C., Esposito R., De Luca N., Galderisi M. Echocardiography in arterial hypertension. High Blood Pres Cardiovasc Prev. 2018;25:159–166. doi: 10.1007/s40292-018-0259-y. [DOI] [PubMed] [Google Scholar]
- 19.Losi M.A., Betocchi S., Barbati G., et al. Prognostic significance of left atrial volume dilatation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22:76–81. doi: 10.1016/j.echo.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Gerdts E., Izzo R., Mancusi C., et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network) Int J Cardiol. 2018;258:257–261. doi: 10.1016/j.ijcard.2017.12.086. [DOI] [PubMed] [Google Scholar]
- 21.Izzo R., Losi M.A., Stabile E., et al. Development of left ventricular hypertrophy in treated hypertensive outpatients: the Campania Salute Network. Hypertension. 2017;69:136–142. doi: 10.1161/HYPERTENSIONAHA.116.08158. [DOI] [PubMed] [Google Scholar]
- 22.Mosca L., Ferris A., Fabunmi R., Robertson R.M., American Heart A. Tracking women's awareness of heart disease: an American Heart Association national study. Circulation. 2004;109:573–579. doi: 10.1161/01.CIR.0000115222.69428.C9. [DOI] [PubMed] [Google Scholar]
- 23.Mosca L., Jones W.K., King K.B., Ouyang P., Redberg R.F., Hill M.N. Awareness, perception, and knowledge of heart disease risk and prevention among women in the United States. American heart association women's heart disease and stroke campaign task force. Arch Fam Med. 2000;9:506–515. doi: 10.1001/archfami.9.6.506. [DOI] [PubMed] [Google Scholar]
- 24.Ji H., Kim A., Ebinger J.E., et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5:19–26. doi: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusifov A., Woulfe K.C., Bruns D.R. Mechanisms and implications of sex differences in cardiac aging. J Cardiovasc Aging. 2022;2:20. doi: 10.20517/jca.2022.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunlay S.M., Roger V.L., Weston S.A., Jiang R., Redfield M.M. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitzman D.W., Gardin J.M., Gottdiener J.S., et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 28.Grilo G.A., Shaver P.R., Stoffel H.J., et al. Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes. J Mol Cell Cardiol. 2020;139:62–74. doi: 10.1016/j.yjmcc.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrov G., Regitz-Zagrosek V., Lehmkuhl E., et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122:S23–S28. doi: 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- 30.Packer M. The conundrum of patients with obesity, exercise intolerance, elevated ventricular filling pressures and a measured ejection fraction in the normal range. Eur J Heart Fail. 2019;21:156–162. doi: 10.1002/ejhf.1377. [DOI] [PubMed] [Google Scholar]
- 31.de Simone G., Muiesan M.L., Ganau A., et al. Reliability and limitations of echocardiographic measurement of left ventricular mass for risk stratification and follow-up in single patients: the RES trial. Working group on heart and hypertension of the italian society of hypertension. Reliability of M-mode echocardiographic studies. J Hypertens. 1999;17:1955–1963. doi: 10.1097/00004872-199917121-00027. [DOI] [PubMed] [Google Scholar]
- 32.Lonnebakken M.T., Izzo R., Mancusi C., et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network) J Am Heart Assoc. 2017;6:e004152. doi: 10.1161/JAHA.116.004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.