Abstract
Siamois is the transcriptional mediator of the dorsal Wnt signaling pathway and is necessary for formation of the Spemann organizer and dorsoanterior development in Xenopus. We have determined that XIC, a Xenopus I-mfa domain protein that regulates Tcf3 binding, is required for dorsoaxial development and specifically for Siamois activity in establishing the dorsal organizer. In loss-of-function studies, we found that embryos injected with a morpholino to XIC mRNA (XIC morphpolino) are missing head structures, neural tube, notochord, and paraxial mesoderm as well as NCAM and XMyoD expression. Although Siamois, Twin, and Xnr3 expression is normal in morpholino-injected embryos, levels of downstream organizer factors, including goosecoid, Xnot, Cerberus, and chordin, are severely reduced. Ectopic axis formation induced by Siamois is repressed by injection of the XIC morpholino and further repressed by coinjection of β-catenin or a constitutively active Tcf3/HMG/G4A fusion. Activation of reporters driven by the Siamois-responsive proximal element of the goosecoid promoter is inhibited in the presence of the morpholino and can be rescued by murine I-mfa and by a dominant-negative Tcf3. The data indicate a role for XIC in limiting Tcf3-dependent repression of Siamois activities that are required for goosecoid transcription and for dorsal organizer formation.
An essential component of amphibian axis formation is the Spemann-Mangold organizer, a dorsal territory that induces the expression of a set of transcription factors and secreted products that orchestrate differentiation and morphogenesis (17). The organizer is specified by maternal components of the canonical Wnt signaling pathway that are localized during cortical rotation (34, 45), resulting in accumulation of β-catenin and its association with Tcf3 to activate dorsal-specific transcription. In early blastula embryos, direct targets of the maternal β-catenin/Tcf3 complex that contribute to organizer formation are Siamois (4), Twin (29), and Xnr3 (32). After the midblastula transition, components of the canonical Wnt signaling pathway, including Tcf3, lose the ability to induce a dorsal axis and specific gene targets such as Siamois (10). Instead, during late blastula and gastrula stages, β-catenin-dependent signaling restricts formation of dorsal structures, promotes ventrolateral mesoderm development, and patterns the neuraxis (8, 16, 31). Although a temporal restriction on dorsal induction has been recognized for at least 30 years, little is known about the molecular mechanisms responsible for the shift from maternal dorsal-promoting to zygotic ventrolateral-promoting responses to the canonical Wnt signaling pathway.
The homeodomain transcription activator Siamois mediates the dorsalizing role of the Wnt pathway and is necessary for Spemann organizer formation (14, 26). Siamois is one of the earliest zygotic transcription factors expressed in response to the Wnt signaling pathway and is unique in its ability to induce a complete secondary axis (5, 30). When expressed in marginal zone blastomeres, Siamois reportedly cooperates with transforming growth factor β (TGF-β) signals to promote formation of a complete organizer (13). Cooperation with growth factor signals expressed in the mesoderm is reflected in spatial regulation of Siamois along the animal-vegetal axis (11). Analysis of the promoters of two direct targets of Siamois, goosecoid and Cerberus, reveals binding and cooperative activation by Siamois and activin-induced proteins and thus supports synergistic regulation by Wnt and TGF-β pathways in formation of dorsal mesoderm (48, 50). Siamois activity is also regulated temporally: when expressed under the control of a hormone-inducible promoter on the ventral side of embryos, it loses the ability to activate goosecoid transcription during late blastula (28). The molecular details for regulation of the functions of Siamois activity in dorsoanterior development are largely unknown.
XIC, an I-mfa domain protein expressed in early Xenopus development, can inhibit DNA binding by Tcf3, block the canonical Wnt signaling pathway, and repress dorsoanterior development (44). XIC therefore has the potential to participate in early axis formation. I-mfa domain proteins are characterized by a cysteine-rich carboxy-terminal domain (“a”-specific domain) that binds Tcf3 as well as basic helix-loop-helix transcription factors and inhibits their activity (6, 44). In Xenopus embryos, overexpression of XIC inhibits the β-catenin/Tcf3 activation of Siamois transcription and prevents dorsal axis specification. We sought to reveal an endogenous role for XIC in dorsoanterior development through loss-of-function experiments. The results revealed that XIC is required downstream of Siamois for expression of many organizer factors and for subsequent axial development. Coinjection of a morpholino to XIC mRNA (XIC morpholino, or XIC Mo) with Siamois RNA suppresses ectopic axis formation and activation of Siamois-responsive goosecoid reporter constructs. The data reveal that XIC is necessary for Siamois-mediated formation of the dorsal organizer and provide evidence for a molecular model in which XIC limits Tcf3-dependent negative regulation of Siamois function consistent with a role for XIC in modulating transitions in the dorsalizing activity of canonical Wnt signaling.
MATERIALS AND METHODS
DNA constructs.
The vector CS2Gal4BD contains nucleotides 1 to 147 of Gal4A as well as the polylinker transferred from pSG424 (39) positioned between the BamHI and XbaI sites of CS2. Siamois coding sequences were ligated to this vector using SmaI and XbaI sites of the polylinker to generate CS2Gal4BD-Siam.
Embryo manipulations and microinjection.
Embryos were obtained and cultured as described previously (44) and staged according to Nieukoop and Faber (36). Synthetic capped RNA was prepared using an Ambion MEGAscript transcription kit and linearized DNA template according to the manufacturer's directions. The red substrate for the β-galactosidase tracer was Red Gal (Research Organics). Embryos were injected at the four-cell stage, equatorially at the midline of dorsal or ventral blastomeres as determined by pigmentation. For certain goosecoid luciferase reporter assays, injections were performed subequatorially at the eight-cell stage. Duplicate axes were generated by microinjection of 20 pg of Siamois RNA into two ventral blastomeres at the four-cell stage, and embryos were allowed to develop to Niewkoop Faber (NF) stage 30 to 32 for morphological assessment of the extent of axis duplication. Coinjection of 4 or 2 ng of mouse I-mfa, 300 ng of pt β-catenin (51a), or 50 pg of Tcf3/HMG/G4A was performed with Siamois in some experiments.
XIC Mo.
The XIC Mo was designed against the 5′ untranslated region of XIC mRNA, overlapping the AUG start codon. The sequence of the antisense oligonucleotide is 5′ GGGACATCTGCCAGGCCTCTCAGCA 3′ (Gene Tools). Control oligonucleotides of random sequence or the sense strand of the antisense morpholino were also obtained from Gene Tools. The injected dose of morpholino used in these experiments was the lowest that consistently gave a specific phenotype with high penetrance.
RT-PCR.
Reverse transcription-PCR (RT-PCR) experiments were performed as previously described (44). Additional primer pairs for RT-PCR included VegT (53) and Xwnt8 (9). Cycle numbers were as follows: XIC, 29; siamois, 28; and VegT, Xwnt8, gscd, and Xnr3, 25.
Luciferase reporter assays.
Embryos were injected with 100 ng of goosecoid luciferase reporter DNA (48) or CS2Gal4 luciferase reporter DNA, alone or in combination with 40 ng XIC morpholino or control morpholino. The following RNA was included as indicated: 200 pg Siamois (30), 0.1 to 4 ng murine I-mfa (6), 0.5 ng dominant-negative Tcf3 (dnTcf3) (33), 2.4 ng “a”-specific domain of I-mfa, 200 pg VP16/Sia (26), or 200 pg Gal4BD-Siam. Pools of five embryos were collected at NF 10.5 in triplicate and lysed in 100 μl 1% Triton X-100, 25 mM glycyl-glycine, pH 7.8, 15 mM MgCl2, 4 mM EGTA, and 1 mM dithiothreitol. Twenty microliters was assayed; results were averaged and expressed as actual luciferase activity.
In situ hybridization, immunohistochemistry, and histology.
In situ hybridization was performed using digoxigenin-labeled antisense RNA probes essentially as described previously (41). For detection of XIC transcripts, hybridizations were performed for 36 h and incubation with chromogenic reagents for 24 h in 10% polyvinyl alcohol. DNA templates for probe synthesis were prepared from the following plasmids: XIC (44), NCAM (27), XMyoD (38), goosecoid (8), chordin (40), Xnot (47), En2 (19), Wnt8 (7), Krox20 (3), Xnr3 (43), Vent1 (15), Cerberus (2), and Xanf (52). Plasmids for synthesis of Xbra and Otx (pXOT 30.1) probes were obtained from R. T. Moon. Pigmented embryos were bleached with hydrogen peroxide following staining and postfixation with Bouin's fixative. Immunohistochemistry for detection of epidermal cells was performed as described previously (41) using undiluted EpA supernatant (24) and goat anti-mouse alkaline phosphatase-conjugated secondary antibody (Roche). For histological examination, NF stage 32 embryos were fixed in 4% paraformaldehyde and dehydrated in ethanol followed by toluene and Paraplast infiltration for three times for 20 min each at 60°C. Ten-micrometer sections were mounted on Superfrost Plus slides (Fisher Scientific) for hematoxylin-and-eosin staining (25).
RESULTS
Developmental expression pattern of endogenous XIC.
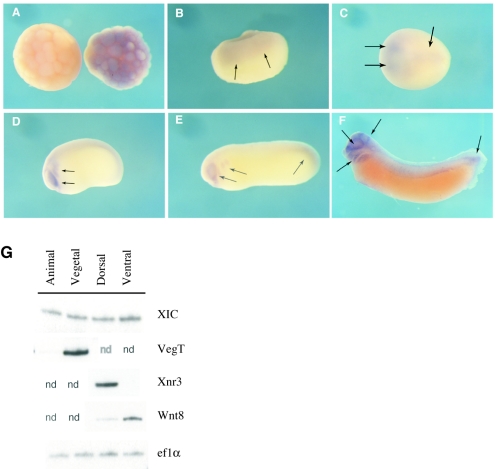
Our previous examination by RT-PCR of the temporal expression of XIC RNA indicated that XIC RNA expression is both maternal and zygotic and continues to at least tadpole stages (44). To determine the spatial distribution of XIC transcripts in Xenopus embryos, we performed in situ hybridization using a full-length antisense cDNA probe. Experimental conditions required for all expression analyses indicate that XIC is a relatively low-abundance mRNA. Consistent with RT-PCR results, XIC RNA is detected throughout the animal hemisphere in early-blastula-stage embryos prior to midblastula transition (MBT). There are no apparent dorsal-ventral differences in expression levels (Fig. 1A). During early gastrula stages, transcripts for XIC continue to be localized uniformly throughout the animal region (Fig. 1B) and are undetected in the vegetal region by in situ hybridization. RNA begins to accumulate in the dorsoanterior region of the embryo during neural plate stage (Fig. 1C), and as neurulation progresses, XIC RNA accumulates in the eye, developing branchial arches, and tailbud (Fig. 1D and E). In tailbud stage embryos, XIC RNA expression increases in discrete anterior regions, including the eye, central nervous system (CNS), cranial mesenchyme, and prospective branchial arches as well as in the tailbud (Fig. 1F).
FIG. 1.
Developmental expression pattern of XIC RNA. Panels A to F show results of whole-mount in situ hybridization, and arrows indicate regions of endogenous XIC expression. (A) XIC RNA expression at blastula stages is uniform in the animal region; antisense probe was hybridized to the embryo on the right and sense probe to the embryo on the left. (B) In early-gastrula-stage embryos, XIC is uniformly expressed in the animal region. (C) At neural plate stages (NF13), XIC accumulates in dorsal and anterior regions of the embryo. Dorsal view, with anterior to the left. (D, E) XIC levels increase around the eye and in the developing branchial arches and in the tailbud as neurulation continues through NF22 and NF27 (F) In tadpole stages, XIC RNA is strongly expressed in the branchial arches, around the eye, in specific regions of the CNS, and in the tailbud. Panels B, D, E, and F are lateral views; panel C is a dorsal view; and panel A is an animal view. (G) In RT-PCR analysis, XIC RNA is expressed uniformly in animal, vegetal, dorsal, and ventral halves of early-gastrula-stage embryos. Expression of Xwnt8, VegT, and Xnr3 was monitored for identity of ventral, vegetal, and dorsal halves, respectively and ef1α was an internal control for RNA quantitation. Samples not tested are marked “nd” to distinguish them from samples that gave no detectable signal with the specific primers indicated.
To support the in situ results, we further investigated spatial distribution of XIC transcripts during early gastrula stages by RT-PCR analysis. RNA was isolated from embryos dissected into animal and vegetal or dorsal and ventral halves. Levels of expression of Xnr3, Wnt8, and VegT RNA were used as a control for dorsal, ventral, and vegetal identities, respectively, of the halves. XIC RNA was expressed at similar levels in all four samples (Fig. 1G). We had not detected XIC mRNA in the vegetal region by in situ hybridization, perhaps due to a combination of low expression level and interference by the yolk content of vegetal blastomeres. These results and the in situ hybridization analysis demonstrate a broad expression pattern of XIC RNA in the early embryo.
XIC is required for the development of dorsoanterior structures.
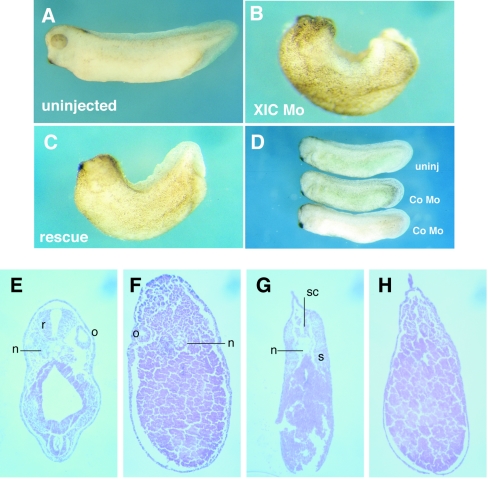
Morpholinos, modified antisense oligonucleotides, have been used to interfere with RNA translation and to phenocopy null mutations. To assess the role of XIC in developing embryos, we generated a morpholino to XIC mRNA that specifically inhibited the in vitro translation of XIC mRNA but not an unrelated mRNA, that of myogenin (data not shown). We injected the XIC morpholino into the marginal zone of dorsal or ventral blastomeres of four-cell embryos and evaluated the requirement for XIC in dorsoventral axis formation. Figure 2A shows an uninjected tailbud embryo. In contrast, embryos collected at a comparable developmental stage but injected at the midline of the dorsal marginal zone with the XIC morpholino failed to develop normal dorsoanterior structures (Fig. 2B). These embryos exhibit loss of neural folds during neurula stages (not shown) and subsequently have missing or reduced head structure, eyes, and cement gland. In addition, the dorsal fin is deformed and the tail reduced. Importantly, the XIC morpholino phenotype was mostly rescued by coinjection of murine I-mfa RNA (Fig. 2C) (6), indicating specificity of the knock-down effect. The “a”-specific domains of XIC and I-mfa have been functionally equivalent in all assays performed for Xenopus axis studies. This morphological rescue was strongly supported by rescue of a transcriptional target of XIC regulation, the goosecoid promoter, by coinjection of I-mfa RNA with the XIC morpholino (see Fig. 6A). Rescue of the morphological phenotype was complicated by the fact that overexpression of I-mfa also leads to embryos exhibiting disturbed dorsoanterior development (44); thus, careful titration of the rescue molecule was required and complete reversal of the morpholino phenotype in whole embryos was difficult to achieve. In a separate experiment, the random sequence control morpholino had no significant effects on formation of dorsoanterior structures at any stage of development (Fig. 2D) or on other measured outcomes in this and all later experiments in our studies. The sense-strand control morpholino gave a similar result (not shown). Injection of the XIC morpholino into ventral blastomeres had only a minimal effect of shortening of the anteroposterior axis (not shown).
FIG. 2.
XIC morpholino leads to loss of dorsoanterior structures in developing embryos. (A to D) Morphology of control and morpholino-injected embryos. Embryos were injected at the four-cell stage into the marginal zone of two dorsal blastomeres and examined at NF 35 (A, B, and C) or NF 28 (D). (A) An uninjected control embryo. (B) A representative embryo injected with 40 ng of XIC morpholino (Mo) loses head, eyes, cement gland, tail, and axial structures. (C) Coinjection of 40 ng of XIC morpholino with 0.3 ng of mouse I-mfa RNA mostly rescues dorsoaxial structures, including the cement gland, tail, dorsal fin, and head. (D) A control (Co) morpholino does not affect dorsoanterior development as compared to uninjected (uninj) embryos of the same age. (E to H) Hematoxylin-and-eosin-stained transverse sections of stage 32 embryos. (E) An uninjected embryo and (F) an XIC morpholino-injected embryo sectioned at the level of the otic vesicle. This particular morpholino-injected embryo retains some notochord. (G) A control embryo and (H) an XIC morpholino-injected embryo with sections taken at comparable levels posterior to the otic vesicle. Abbreviations: r, rhombencephalon (hindbrain); o, otic vesicle; n, notochord; sc, spinal chord; s, somite.
FIG. 6.
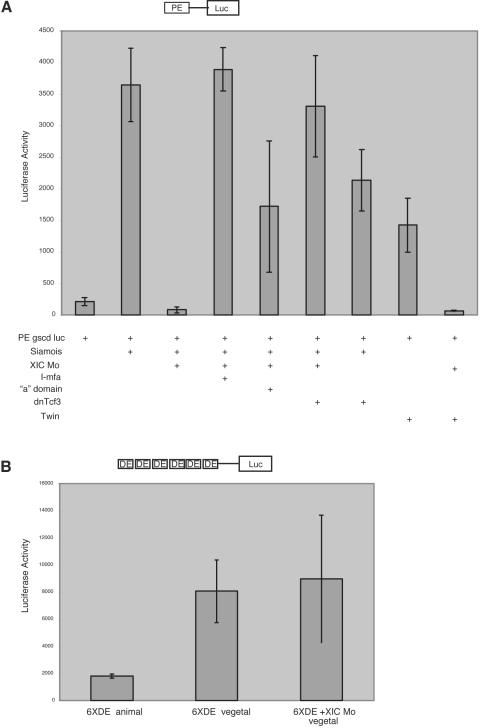
XIC is required for Siamois activation of the goosecoid PE. (A) Four-cell embryos were injected on the ventral side with 100 pg −155 gscd/Luc reporter and 200 pg siamois or Twin RNA, 40 ng morpholino, 2.4 ng I-mf “a”-specific domain, or 0.5 ng dnTcf3 RNA as indicated. (B) To test whether XIC is required for activation of the DE of the goosecoid promoter, embryos were injected with 200 pg DE(6X) −104 gscd/Luc reporter in the animal or vegetal hemisphere at the two-cell stage. Additional embryos were injected in vegetal blastomeres with the DE(6X) reporter and 40 ng of XIC morpholino. Embryos were collected at NF 10.5 in pools of five and assayed in triplicate for luciferase activity. Schematic diagrams of the reporter constructs are shown at the top of each plot.
The morphological loss of anterior and axial structures was supported by analysis of stained transverse sections of tadpole-stage embryos taken at the level of the otic vesicle. In XIC morpholino-injected embryos, the hindbrain is missing and the notochord is substantially reduced or absent (compare a control embryo in Fig. 2E with a morpholino-injected embryo in Fig. 2F). Sections taken at a more posterior position show that defects persist throughout the trunk: XIC morpholino-injected embryos do not develop a spinal chord, and reduction of notochord is apparent (compare an uninjected control embryo in Fig. 2G and an XIC morpholino-injected embryo in Fig. 2H). In addition, loss of paraxial mesoderm is indicated by the absence of somites from the XIC morpholino-injected embryos (Fig. 2H).
XIC is required for the expression of dorsal markers and organizer factors.
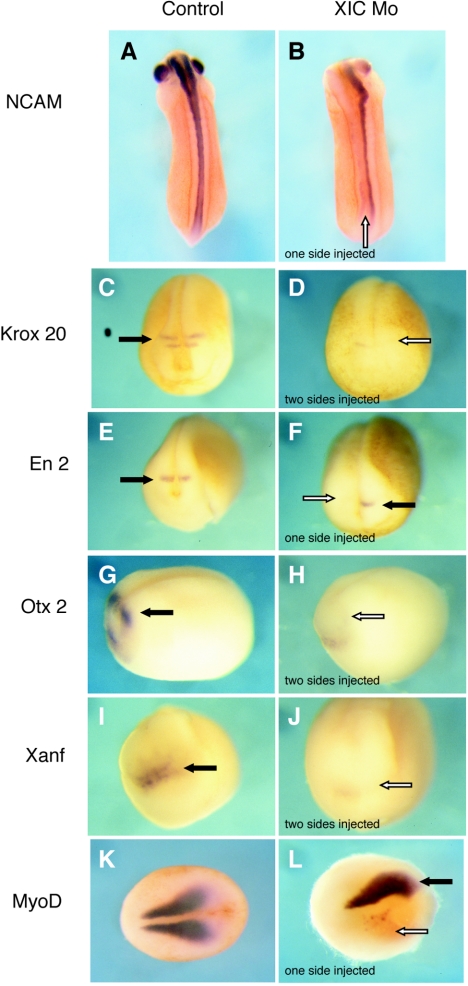
Molecular analysis by in situ hybridization confirmed a significant developmental perturbation in the XIC morpholino-injected embryos. When the XIC morpholino was introduced into one dorsal blastomere of four-cell embryos, we found that NCAM RNA, a pan neural marker (27), was absent or reduced from the morpholino-injected side of embryos (Fig. 3B). This is most apparent in the eye and anterior portion of the embryo but persists beyond the posterior boundary of the CNS and is consistent with the loss of neural tube observed during morphological analyses. NCAM is not expressed in morpholino-injected embryos at any developmental stage that we have examined between NF 13 and NF 35 (data not shown). Anteroposterior markers of neural patterning, Krox-20 (3), En-2 (19), Otx2 (1), and Xanf (52), which are expressed after neural induction, were all significantly reduced or eliminated by XIC morpholino injection (Fig. 3D, F, H, and J). Examination at earlier and later time points indicated that these results are not due to temporal changes in expression of the selected markers (data not shown). The loss or reduction of all of these markers indicates a requirement for XIC expression for an early event in neural development prior to patterning of the neuraxis.
FIG. 3.
XIC morpholino inhibits expression of neural and paraxial mesoderm markers. Embryos shown are either controls (A, C, E, G, I, and K) or were injected with 40 ng of XIC morpholino into one or two dorsal blastomeres at the four-cell stage, as indicated (B, D, F, H, J, and L). Filled arrows indicate control expression patterns, and white arrows indicate the expected location of marker expression in the embryos that received morpholino. Marker gene expression was detected by whole-mount in situ hybridization using antisense probes for NCAM, Krox 20, En2, Otx2, Xanf, and MyoD. Injection of the XIC morpholino eliminated or severely reduced expression of all tested neural and mesoderm markers. Panels A, B, K, and L are dorsal views; panels C to F, I, and J are anterior views; and panels G and H are lateral views.
MyoD is a key component of the myogenic pathway and a marker for somitic mesoderm (21). Consistent with the absence of somites observed in histological analysis, in situ hybridization shows that expression of mRNA for MyoD is severely reduced on the XIC morpholino-injected side in late-gastrula-stage embryos (Fig. 3L).
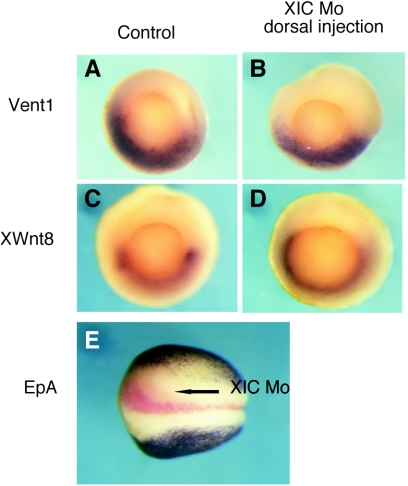
We attempted to determine the alternate fates of dorsal mesoderm and neurectoderm when XIC expression is reduced and focused on the possibility that ventral markers expand into the dorsal territory. The expression domains of dorsal and ventral genes are determined in part by ventral signaling inhibitors produced in the dorsal organizer of the embryo, a region that is deficient (discussed below) in XIC morpholino-injected embryos. As shown in Fig. 4A to D, both Xwnt8 (8) and Vent-1 (15) exhibit normal expression patterns and do not expand into the dorsal region in gastrula-stage embryos that were injected with XIC morpholino on the future dorsal side. Similarly, we asked whether the ectoderm of XIC-injected embryos adopts the ventral, epidermal fate in the absence of neural marker expression. Figure 4E shows a typical neurula-stage embryo that was coinjected with XIC morpholino and β-galactosidase tracer into one dorsal cell at the four-cell stage. Following disclosure of the tracer using a red substrate, embryos were immunostained for an epidermal marker, EpA (24). In no case did we observe expansion of epidermis toward the dorsal midline on the injected side. As noted above and verified by staining of additional embryos from this experiment, the injected side also fails to express the neural marker NCAM (data not shown).
FIG. 4.
Ventral markers do not expand into dorsal regions of XIC morpholino-injected embryos. (A to D) Control embryos and embryos injected with XIC morpholino into two dorsal blastomeres at the four-cell stage were examined by in situ hybridization for expression of ventral markers Xwnt8 and Vent1 at NF 11.5. Views are of the blastopore with dorsal at the top of each image. (E) Immunostaining was used to detect expression of the epidermal marker EpA in embryos injected with XIC morpholino in one dorsal blastomere at the four-cell stage. The injected side of neurula-stage embryos was identified with the lineage tracer β-galactosidase, shown in red.
Finally, we tested whether absence of dorsal marker expression indicates that XIC is necessary for cell survival by performing terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assays (20) on embryos injected with the XIC morpholino. There was no detectable increase in cell death in XIC-depleted embryos (data not shown). Therefore, although embryos require XIC for normal dorsoanterior development, the alternate fates of the XIC-depleted dorsal mesoderm and neurectoderm have not yet been determined.
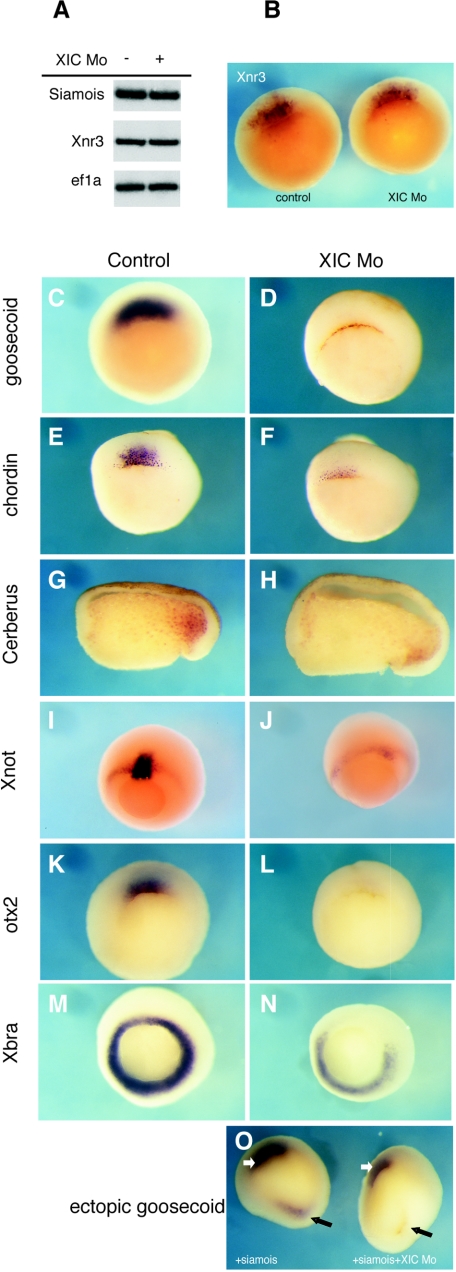
In light of morphological and molecular data indicating that XIC is required for dorsal structures to form and our previous characterization of XIC as a potential modulator of Tcf3 activity, we examined the expression of direct targets of Tcf3/β-catenin that are indicative of dorsal axis specification: Siamois and Xnr3. Late-blastula- and early-gastrula-stage embryos injected with the XIC morpholino in both dorsal blastomeres at the four-cell stage had comparable levels of RNA for these factors, as determined by RT-PCR and in situ hybridization analyses (Fig. 5A and B). Therefore, XIC is not required for expression of early targets of the maternal Wnt signaling pathway.
FIG. 5.
XIC morpholino injection reduces expression of dorsal organizer markers but not direct targets of maternal Tcf3/β-catenin. (A) RT-PCR analysis shows RNA levels for Xnr3 and Siamois in control and XIC morpholino-injected embryos at NF stage 10.25. Ef1α RNA was examined as an internal control. (B) In situ hybridization with a probe for Xnr3 in early gastrula embryos confirms the RT-PCR results. (C to N) Embryos shown are controls (C, E, G, I, K, and M) or were injected in two dorsal blastomeres of four-cell-stage embryos with 40 ng of XIC morpholino (D, F, H, J, L, and N) and analyzed by whole-mount in situ hybridization for organizer markers at stages 10.25 to 11.5. Probes used are indicated along the left of the figure. Embryos probed for Cerberus expression were bisected through the dorsal lip prior to processing. Staining intensity of the embryo shown in panel N does not indicate reduced ventral expression of Xbra RNA. Expression of ectopic goosecoid is inhibited by coinjection of XIC morpholino with Siamois into ventral blastomeres. White and black arrows indicate endogenous and ectopic goosecoid, respectively (O).
We next examined several components of the Spemann organizer that are downstream of Siamois and that characterize its function in dorsal axis formation. goosecoid is directly activated by Siamois and the closely related protein Twin as well as by factors that mediate the maternal activin signaling pathway (29, 48). Compared to uninjected controls, levels of goosecoid RNA were reproducibly reduced by injection of XIC morpholino (Fig. 5D). Chordin is a secreted inhibitor of ventral bone morphogenetic protein (BMP) signaling that has recently been reported to be a target of Siamois (12, 49). chordin RNA was reduced by the XIC morpholino (Fig. 5F) as was expression of mRNA for Cerberus, another secreted factor that inhibits Wnt, BMP, and nodal signaling (37) (Fig. 5H). Similarly, Xnot RNA is severely reduced (Fig. 5J). Xnot is a homeodomain organizer protein expressed in the most dorsal portion of the marginal zone during early gastrulation and in the notochord during neurulation (47). Dorsal expression of additional organizer transcription factors, Xotx2 (1) and Xbra (42), was also reduced when XIC was depleted in the early gastrula dorsal marginal zone by morpholino injection (Fig. 5L and N). All of these factors, with the exception of Xotx2, have been shown to be dependent upon Siamois activity for their expression in the Spemann organizer (14, 26). Consistent with analysis of these endogenous expression patterns, the XIC morpholino also inhibited induction of ectopic goosecoid mRNA by ventral injection of Siamois (Fig. 5O).
XIC morpholino blocks activation of the goosecoid promoter.
In order to understand the potential requirement for XIC in organizer formation by Siamois, we investigated the role of XIC in expression of a specific Siamois target. Goosecoid expression is regulated by Siamois through a proximal element (PE) in the goosecoid promoter, whereas a distal element (DE) mediates activation by activin/BVg-1 signaling (48). To evaluate whether XIC is necessary for Siamois to activate the goosecoid promoter, we assayed the activity of reporter constructs driven by goosecoid promoter fragments. Initially, we injected a reporter containing both the DE and PE elements (−226 gscd/Luc) with and without the XIC morpholino into the dorsovegetal region of eight-cell embryos where they are subject to endogenous Siamois, as well as TGF-β family signals (48). The XIC morpholino, but not a control morpholino, diminished the activity of reporter constructs containing the PE, indicating that XIC is necessary for Siamois to activate the goosecoid promoter during development (data not shown). Little activation and no morpholino effect were detected on a −104 gscd/Luc reporter that contains no PE.
To further characterize the requirement for XIC in activation of the goosecoid promoter, we examined the activation of the PE goosecoid reporter construct (−155 gscd/Luc) on the ventral side of the embryo, where its activity is dependent on coinjection of Siamois RNA. The XIC morpholino blocked Siamois-mediated activation of the reporter, and the block could be rescued by coinjection of full-length murine I-mfa RNA (Fig. 6A). Coinjection of the C-terminal “a”-specific domain that interacts with Tcf3 also rescued morpholino inhibition of reporter activity, although less efficiently than full-length I-mfa. Based on our previous characterization of XIC as an inhibitor of Tcf3 binding, we tested whether a form of Tcf3 that is a dominant negative in the canonical Wnt signaling pathway modulates the effects of XIC depletion in the reporter assay. Injection of Siamois RNA and the XIC morpholino with RNA encoding dnTcf3 (33) that cannot be activated by β-catenin rescued the morpholino block in Siamois-dependent −155 gscd/Luc reporter activity. In the absence of the morpholino, injection of RNA encoding dnTcf3 did not significantly affect Siamois-induced reporter activity. The XIC morpholino similarly repressed reporter activity mediated by Twin, a homeodomain protein that shares functional and DNA binding activities with Siamois.
To evaluate the possibility of a requirement for XIC in transcription activity at the distal element of the goosecoid promoter, we also tested the morpholino effect on activation of DE(6×) −104 gscd/Luc, a reporter driven by the multimerized activin-responsive distal element (48). Figure 6B confirms previously reported evidence that DE(6×) luciferase reporter activity is increased in vegetal blastomeres relative to animal blastomeres and shows that coinjection of XIC morpholino does not inhibit transcription driven by the activin-dependent element. These results demonstrate that XIC is required for activation of transcription by the Siamois-responsive proximal element of the goosecoid promoter.
XIC morpholino blocks axis formation downstream of Siamois.
We next tested the ability of the XIC morpholino to inhibit a secondary axis induced by ectopic expression of Siamois so as to order these components within a pathway. Siamois induced complete secondary axes when expressed on the ventral side of Xenopus embryos, whereas coinjection of Siamois RNA with the XIC morpholino diminished the formation of secondary axes (Table 1), similar to its inhibition of the endogenous dorsal axis. A control morpholino had no effect on secondary axis formation (data not shown). The specificity of the XIC morpholino is demonstrated by the dose-dependent rescue of secondary axis formation when murine I-mfa RNA is included with Siamois and the XIC morpholino. Furthermore, neither β-catenin nor a Tcf3/HMG/G4A fusion (44) comprising the Tcf3 DNA binding domain and Gal4 activation domain rescued the formation of secondary axes by Siamois in the presence of the XIC morpholino. Instead, β-catenin and the constitutively active Tcf3 construct significantly enhanced the morpholino-dependent repression of ectopic axes. Ventral injection of β-catenin and Siamois without the XIC morpholino produces hyperdorsalized embryos (data not shown). These results are consistent with XIC being required for Siamois function because Siamois mediates β-catenin/Tcf3 activity in axis induction. Furthermore, they indicate that depletion of XIC exposes a negative role for β-catenin and Tcf3 in Siamois-induced axis formation, perhaps in inducing expression of a protein that blocks Siamois activity.
TABLE 1.
Effects of XIC morpholino on Siamois secondary axis formation
| Injected RNAa | n | Complete secondary axis (%) | Partial secondary axis (%)b
|
Single axis (%) | ||
|---|---|---|---|---|---|---|
| +++ | ++ | + | ||||
| Siamois | 81 | 91 | 9 | |||
| Siamois + XIC Mo | 93 | 4 | 21 | 10 | 65 | |
| Siamois + XIC Mo + I-mfa (4 ng) | 65 | 54 | 25 | 18 | 3 | |
| Siamois + XIC Mo + I-mfa (2 ng) | 26 | 30 | 37 | 26 | 7 | |
| Siamois + XIC Mo + pt β-catenin | 30 | 100 | ||||
| Siamois + XIC Mo + Tef3/HMG/G4A | 41 | 15 | 2 | 83 | ||
Two ventral blastomeres of four-cell-stage embryos were injected with the indicated RNA and XIC morpholino combinations.
Embryos with partial secondary axes were grouped according to the approximate percentage of secondary axis that formed; +++ represents 75%, missing eyes and cement gland; ++ and + represent shorter axes with less anterior development.
XIC is required for Siamois to interact with the goosecoid promoter.
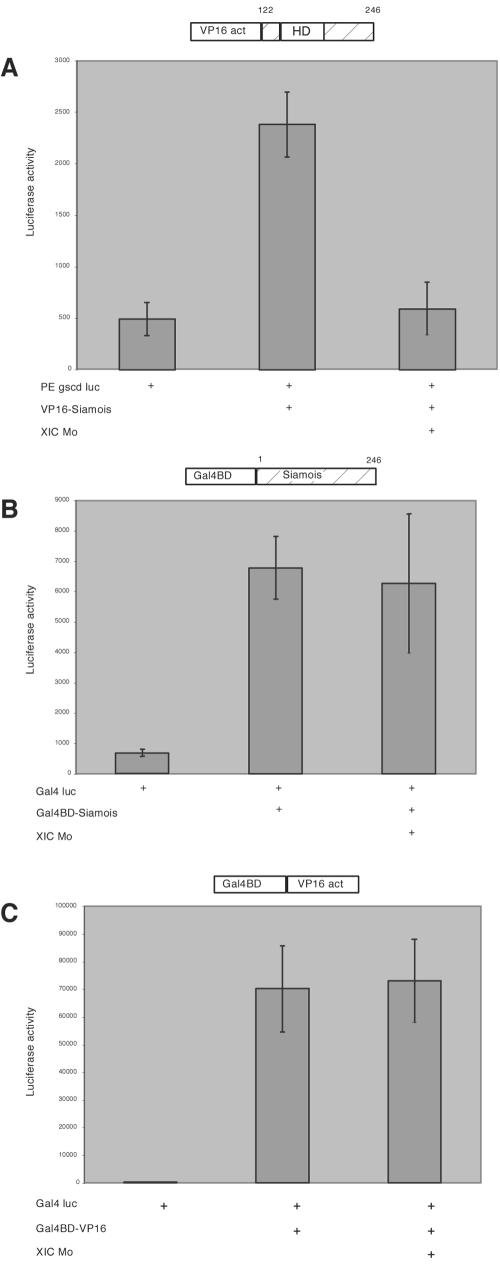
To determine whether XIC was required for the activity of the Siamois DNA binding domain or the transactivation domains, we used two fusion constructs: VP16-Siam, a C-terminal fragment containing the Siamois homeobox DNA binding domain fused to the transactivation domain of VP16 (26), making transcriptional activation dependent on the interaction of Siamois with the PE sequence in the −155 gscd/Luc reporter; and Gal4DBD-Siam, a fusion of the Gal4 DNA binding domain to the full-length Siamois, which utilizes Siamois elements for transcriptional activation and the Gal4DBD for binding to a Gal4-luciferase (Gal4-Luc) reporter. When VP16-Siam RNA is injected into the ventral marginal zone of four-cell embryos, it activates the −155 gscd/Luc reporter and coinjection of the XIC morpholino blocks this activation (Fig. 7A). In contrast, the activation of the Gal4-Luc reporter by Gal4DBD-Siam is not inhibited by the XIC morpholino (Fig. 7B). In addition, the XIC morpholino does not inhibit a GAL4DBD-VP16 construct (Fig. 7C). Therefore, XIC is not necessary for the transactivation function of Siamois or VP16 but is necessary for Siamois to effectively target these elements to the goosecoid promoter.
FIG. 7.
XIC is required for Siamois to target transactivation domains to the goosecoid promoter. (A) Ventral blastomeres of four-cell embryos were injected with −155 gscd/Luc reporter DNA, VP16-Sia RNA encoding a fusion activator protein, and the XIC morpholino as indicated. (B) Activation of a Gal4 luciferase reporter by a Gal4BD-Siam fusion containing full-length Siamois protein was not repressed by the XIC morpholino injection. (C) The XIC morpholino has no effect on activation of the Gal4 luciferase reporter activated by a Gal4BD-VP16 fusion protein. Embryos were injected with 100 pg of reporter DNA and 200 pg of activator RNA. Embryos were collected in triplicate samples for luciferase assays at NF 10.5. Schematic diagrams of the activator constructs are shown at the top of each plot.
DISCUSSION
Understanding the formation and function of the dorsal organizer is one of the most active areas of investigation in developmental biology. Although many organizer-specific factors have been identified, little is known about the regulation and coordination of their activities. In this study, we demonstrate a biological role for XIC in the regulation of dorsoanterior development in Xenopus embryos. The novel finding of our study is that XIC is required for Siamois function, but not its expression, and therefore provides a unique basis for investigations of very early zygotic mechanisms that regulate dorsal axis development. Specifically, XIC appears necessary for Siamois to activate downstream targets in formation of a functional dorsal organizer and subsequent development of dorsoanterior structures. Reducing endogenous levels of XIC by injection of an antisense morpholino blocked expression of Siamois-responsive endogenous targets, inhibited formation of ectopic dorsal axes by Siamois, and repressed the activity of reporter constructs containing Siamois response elements from the goosecoid promoter. Inhibition of duplicate axes and goosecoid reporter activity by the XIC morpholino was sensitive to constitutively active and dominant-negative forms of Tcf3. These molecular data affirm that XIC has a necessary biological function in execution of the dorsal organizer program and indicate that it regulates a previously unrecognized role for zygotic Tcf3.
XIC is required for normal dorsal development.
Our current understanding of the molecular requirements for dorsoanterior development revolves around formation of the organizer and its function in dorsalization of mesoderm and neuralization of ectoderm. The organizer forms as a result of combined activities of the maternal TGF-β and Wnt signaling pathways leading to expression of gene-specific transcription factors as well as secreted inhibitors of growth factor signaling to establish the entire body plan of the organism (17). Execution of region-specific developmental programs such as head and trunk induction is reflected in physical and functional regionalization of the organizer itself (35). Siamois, as the zygotically expressed transcriptional mediator of the maternal Wnt signaling pathway, is necessary for organizer formation and unique in its ability to induce a complete secondary axis when expressed in marginal zone blastomeres.
XIC morpholino-injected embryos, while phenotypically similar to those produced when an organizer is removed as a result of surgical ablation (46), depletion of β-catenin, or introduction of a dominant-negative Tcf3 (18, 33), are significantly different in that they maintain expression of Siamois RNA. Our characterizations of XIC activity based on this observation support a role for XIC in Siamois induction of the dorsal organizer.
The XIC morpholino phenotype is consistent with that of embryos injected with a Siamois-engrailed repressor domain fusion (Eng-Sia) in which dominant interference with Siamois function results in loss of dorsoaxial structures as well as loss of expression of many organizer factors (14, 26). Both phenotypes are characterized by absence of specific neural and mesodermal molecular markers and structures as well as by reduction of many head and trunk organizer factors, including goosecoid, Cerberus, chordin, and Xnot. Repressors of signaling by BMP, Wnt, and nodal that are required for head and trunk induction are reduced in XIC morpholino-injected and Eng-Sia-injected embryos. An important common feature of the XIC morpholino and Eng-Sia phenotypes is that neither can be rescued by coexpression of effectors of the maternal Wnt signaling pathway such as β-catenin or a Tcf3/HMG/G4A fusion molecule that mimics constitutive Tcf3 activity. These upstream inducers of Siamois expression and organizer formation are not effective when Siamois function is blocked. As previously reported, this confirms the key role of Siamois as a mediator of maternal signaling and also supports a requirement for XIC in Siamois function.
There are substantial similarities between the XIC morpholino and the Eng-Sia fusion RNA-injected embryos; however, the two loss-of-function phenotypes are not identical. XIC morpholino-injected embryos are not severely ventralized, as judged by morphological features, as are those injected with Eng-Sia. The XIC-deficient embryos are rarely radially symmetric, form a dorsal lip but not neural folds, retain a recognizable anterior-posterior axis, and are sometimes more severely affected in development of anterior than trunk regions. We did not observe the reduction of Xnr3 or enhancement of XWnt8 mRNA expression that was described for the Eng-Sia embryos. Similarly, XIC morpholino and Eng-Sia repression of ectopic axis formation by Siamois are not identical. Whereas the Siamois-engrailed fusion eliminates the secondary axis induced by ventral injection of Siamois (26), coinjection of the morpholino with Siamois often results in development of partial secondary axes. Significantly, the partial axes can be completely repressed by coinjection of β-catenin or activated Tcf3 with the XIC morpholino and Siamois. We conclude that XIC provides a spatial or temporal limit on negative regulation of Siamois function by activators of the Wnt signaling pathway. To what extent the differences between the XIC morpholino and Eng-Sia phenotypes reflect inhibition at a partially overlapping set of targets remains to be investigated. Alternatively, reduction of XIC levels may be a less efficient mechanism for repression of Siamois function than is the introduction of a fusion of Siamois with the strong repressor domain of Drosophila engrailed.
We have previously demonstrated that XIC overexpression represses Tcf3 binding, leading to reduction of Siamois RNA expression (44). Therefore, a significant aspect of the XIC loss-of-function phenotype is that Siamois mRNA levels are unchanged by morpholino injection. Levels of RNA from two other direct targets of Tcf3, Xnr3 and Twin, are also unaffected by the morpholino. This suggests that XIC may not be a required component of the maternal Wnt pathway. However, the design of our experimental system does not allow us to conclusively eliminate a role of XIC in maternal Wnt signaling through Tcf3. XIC mRNA can be detected as early as the eight-cell stage of development. Therefore, depending on its stability, XIC protein synthesized prior to inhibition of translation by the morpholino may be available in the embryo to perform pre-MBT functions. Attempts to examine XIC protein in embryos using a XIC-specific antibody have been unsuccessful, possibly due to very low expression levels. Elimination of XIC protein from oocytes would be necessary to definitively establish its earliest role.
XIC regulates effective recruitment of Siamois activity to the goosecoid promoter.
Siamois is a paired-type homeodomain transcription activator necessary for dorsoventral axis development (14, 26) through induction of Spemann organizer factors, including goosecoid. The PE of the goosecoid promoter has been characterized as a direct target of Siamois (26) and therefore provides a model for determining the molecular basis for XIC regulation of Siamois activity. Using reporter constructs driven by fragments of the goosecoid promoter, we determined that XIC is required for Siamois activation of transcription through a fragment containing the PE, but not for activin-mediated transcription through a distal element, DE. In light of the currently limited characterization of the goosecoid promoter and the proximal element, we used an indirect approach to gain insight into the role of XIC in Siamois function on this promoter.
Fusion proteins were designed to begin to distinguish between a requirement for XIC in Siamois binding and in Siamois transcriptional activation. Reporter assays demonstrate that XIC is necessary for the DNA binding domain of Siamois to effectively target the VP16 activation domain to the PE. However, XIC is not required for full-length Siamois to activate transcription when bound to reporter DNA through a heterologous DNA binding element. Together, these results suggest that XIC regulates the binding of Siamois to the PE. Because we have not been able to demonstrate a direct interaction between XIC and Siamois (L. Snider, unpublished) and cannot disrupt the interaction between Siamois and the proximal element with XIC in a gel mobility shift assay using in vitro-translated proteins (Snider, unpublished), the most straightforward model based on our data is that XIC regulates expression of another factor that interferes with Siamois binding to its target promoter.
A role for Tcf3 in XIC regulation of Siamois activity.
A predicted consequence of XIC loss of function is liberation of Tcf3 that could act as a transcriptional activator or repressor depending on availability of the coactivator β-catenin (22, 23). Results of two of our experiments are consistent with Tcf3 functioning in an activator capacity that leads to suppression of Siamois activity and axial development when XIC levels are reduced. Note that while this proposed function for Tcf3 is independent of its maternal role in inducing Siamois expression, it is consistent with Tcf3 being a positive regulator on the dorsal side of embryos when β-catenin accumulates. First, when β-catenin or Tcf3/HMG/Gal4A is coinjected with Siamois and the XIC morpholino in an axis duplication assay (Table 1), the loss of the ectopic axis is significantly enhanced. If liberated Tcf3 were a repressor of transcription in this experiment, the addition of β-catenin or the constitutively active form of Tcf3 would be expected to reverse its effects. Instead, on the ventral side of the embryo where nuclear β-catenin is limited and where Tcf3 functions as a negative regulator (4), the supplemental β-catenin may enable activation of Tcf3 and full suppression of the ectopic axis. The ability of Tcf3/HMG/G4A, a fusion of the Tcf3 DNA binding domain and the Gal4 activation domain that functions independently of β-catenin, to perform the same role indicates that Tcf3 or a closely related family member is a critical component of this response. Second, injection of a dnTcf3 molecule that cannot interact with β-catenin rescues morpholino-mediated repression of Siamois responsive reporter activity. Therefore, an interpretation that incorporates all of our data is that in the absence of XIC an activated form of Tcf3 mediates repression of Siamois activity, perhaps through synthesis of a molecule that interferes with Siamois binding. Experiments to confirm this model and to identify Tcf targets that regulate Siamois activity are ongoing in our laboratory.
An alternative mechanism by which liberated Tcf3 could influence Siamois activity is direct interaction with Siamois. We have not formally tested this possibility; however, it is more difficult to include a role for β-catenin or another coactivator in such a model. Moreover, results obtained using the Tcf3/HMG/Gal4A fusion indicate a requirement for Tcf3 binding to DNA in axis suppression, and there are no consensus Tcf3 binding elements in the PE of goosecoid (29).
A temporal shift in the ability of the canonical Wnt pathway and activated Tcf3 to promote dorsal development has been widely reported and recently attributed to mechanisms that function at the level of specific target promoters (10, 51), including the Siamois promoter. Similarly, there is a tight window of competence during which Siamois is active such that delayed expression of Siamois cannot activate transcription of the goosecoid gene (28). Results from our XIC morpholino experiments indicate that the negative regulation of Siamois activity results from relief of Tcf3 repression. Investigation of the target-specific mechanisms by which XIC modulates Tcf3 and regulates Siamois activity will broaden our understanding of the dynamic role of the canonical Wnt signaling pathway in dorsal development.
Acknowledgments
We thank R. T. Moon, K. Cho, and D. Kimelman for plasmids and members of the Tapscott laboratory for support and discussions.
This work was supported by NIAMS AR45113 to S.J.T.
REFERENCES
- 1.Blitz, I. L., and K. W.-Y. Cho. 1995. Anterior neurectoderm is progressively induced during gastrulation: the role of the Xenopus homeobox gene orthodenticle. Development 121:993-1004. [DOI] [PubMed] [Google Scholar]
- 2.Bouwmeester, T., S.-H. Kim, Y. Sasai, B. Lu, and E. M. DeRobertis. 1996. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature 382:595-601. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, L. C., A. Snape, S. Bhatt, and D. G. Wilkinson. 1993. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech. Dev. 40:73-84. [DOI] [PubMed] [Google Scholar]
- 4.Brannon, M., M. Gomperts, L. Sumoy, R. T. Moon, and D. Kimelman. 1997. A B-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 11:2359-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnac, G., L. Kodjabachian, J. B. Gurdon, and P. Lemaire. 1996. The homeobox gene Siamois is a target of the Wnt doralization pathway and triggers organiser activity in the absence of mesoderm. Development 122:3055-3065. [DOI] [PubMed] [Google Scholar]
- 6.Chen, A. C.-M., N. Kraut, M. Groudine, and H. Weintraub. 1996. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell 86:731-741. [DOI] [PubMed] [Google Scholar]
- 7.Christian, J. L., B. J. Gavin, A. P. McMahon, and R. T. Moon. 1991. Isolation of cDNAs partially encoding four Xenopus wnt-1/Int-1 related proteins and characterization of their transient expression during embryonic development. Dev. Biol. 143:230-234. [DOI] [PubMed] [Google Scholar]
- 8.Christian, J. L., and R. T. Moon. 1993. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 7:13-28. [DOI] [PubMed] [Google Scholar]
- 9.Cui, Y., J. D. Brown, R. T. Moon, and J. L. Christian. 1995. Xwnt-8b: a maternally expressed Xenopus Wnt gene with a potential role in establishing the dorsoventral axis. Development 121:2177-2186. [DOI] [PubMed] [Google Scholar]
- 10.Darken, R. S., and P. A. Wilson. 2001. Axis induction by Wnt signaling: target promoter responsiveness regulates competence. Dev. Biol. 234:42-54. [DOI] [PubMed] [Google Scholar]
- 11.Darras, S., Y. Marikawa, R. P. Elinson, and P. Lemaire. 1997. Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinants: implications for the patterning of the organiser. Development 124:4275-4286. [DOI] [PubMed] [Google Scholar]
- 12.DeRobertis, E. M., J. Larrain, M. Oelgeschlager, and O. Wessely. 2000. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 1:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engleka, M. J., and D. S. Kessler. 2001. Siamois cooperates with TGFΒ signals to induce the complete function of the Spemann-Mangold organizer. Int. J. Dev. Biol. 45:241-250. [PubMed] [Google Scholar]
- 14.Fan, M. J., and S. Y. Sokol. 1997. A role for Siamois in Spemann organizer formation. Development 124:2581-2589. [DOI] [PubMed] [Google Scholar]
- 15.Gawantka, V., H. Delius, K. Hirschfield, C. Blumenstock, and C. Niehrs. 1995. Antagonizing the Spemann organizer: role of the homeobox gene, Xvent-1. EMBO J. 14:6268-6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton, F. S., G. N. Wheeler, and S. Hoppler. 2001. Difference in XTcf3 dependency accounts for change in response to B-catenin-mediated Wnt signalling in Xenopus blastula. Development 128:2063-2073. [DOI] [PubMed] [Google Scholar]
- 17.Harland, R., and J. Gerhart. 1997. Formation and function of Spemann's organizer. Annu. Rev. Cell Dev. Biol. 13:611-667. [DOI] [PubMed] [Google Scholar]
- 18.Heasman, J., M. Kofron, and C. Wylie. 2000. Β-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 222:124-134. [DOI] [PubMed] [Google Scholar]
- 19.Hemmati-Brivanlou, A., J. R. de la Torre, C. Holt, and R. Harland. 1991. Cephalic expression and molecular characterization of. Xenopus En-2. Development 111:715-724. [DOI] [PubMed] [Google Scholar]
- 20.Hensey, C., and J. Gautier. 1998. Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev. Biol. 203:36-48. [DOI] [PubMed] [Google Scholar]
- 21.Hopwood, N. D., A. Pluck, and J. B. Gurdon. 1989. Myod expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 8:3409-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houston, D. W., M. Kofron, E. Resnik, R. Langland, O. Destree, C. Wylie, and J. Heasman. 2002. Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development 129:4015-4025. [DOI] [PubMed] [Google Scholar]
- 23.Hurlstone, A., and H. Clevers. 2002. T-cell factors: turn-ons and turn-offs. EMBO J. 21:2303-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, E. A., and H. R. Woodland. 1986. Development of the ectoderm in Xenopus: tissue specification and the role of cell association and division. Cell 44:345-355. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, G. M., D. W. Eib, and R. T. Moon. 1991. Histological preparation of Xenopus laevis oocytes and embryos. Methods Cell Biol. 36:389-417. [DOI] [PubMed] [Google Scholar]
- 26.Kessler, D. S. 1997. Siamois is required for formation of Spemann's organizer. Proc. Natl. Acad. Sci. USA 94:13017-13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kintner, C. R., and D. A. Melton. 1999. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development 125:311-325. [DOI] [PubMed] [Google Scholar]
- 28.Kodjabachian, L., and P. Lemaire. 2001. Siamois functions in the early blastula to induce Spemann's organiser. Mech. Dev. 108:71-79. [DOI] [PubMed] [Google Scholar]
- 29.Laurent, M. N., I. L. Blitz, C. Hashimoto, U. Rothbacher, and K. W.-Y. Cho. 1997. The Xenopus homeobox gene Twin mediates Wnt induction of Goosecoid in establishment of Spemann's organizer. Development 124:4905-4916. [DOI] [PubMed] [Google Scholar]
- 30.Lemaire, P., N. Garrett, and J. B. Gurdon. 1995. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81:85-94. [DOI] [PubMed] [Google Scholar]
- 31.McGrew, L. L., K.-I. Takemaru, R. Bates, and R. T. Moon. 1999. Direct regulation of the Xenopus engrailed-2 promoter by the Wnt signaling pathway, and a molecular screen for Wnt-responsive genes, confirm a role for wnt signaling during neural patterning in Xenopus. Mech. Dev. 87:21-32. [DOI] [PubMed] [Google Scholar]
- 32.McKendry, R., S.-C. Hsu, R. M. Harland, and R. Grosschedl. 1997. LEF-1/TCF proteins mediate Wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev. Biol. 192:420-431. [DOI] [PubMed] [Google Scholar]
- 33.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates Β-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 34.Moon, R. T., and D. Kimelman. 1998. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. BioEssays 20:536-545. [DOI] [PubMed] [Google Scholar]
- 35.Niehrs, C. 2004. Regionally specific induction by the Spemann-Mangold organizer. Nat. Rev. Genet. 5:425-434. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwkoop, P. D., and J. Faber. 1967. Normal table of Xenopus laevis (Daudin). North Holland, Amsterdam, The Netherlands.
- 37.Piccolo, S., E. Agius, L. Leyns, S. Bhattacharyya, H. Grunz, T. Bouwmeester, and E. M. De Robertis. 1999. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397:707-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rupp, R. A. W., L. Snider, and H. Weintraub. 1994. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 8:1311-1323. [DOI] [PubMed] [Google Scholar]
- 39.Sadowski, I., and M. Ptashne. 1989. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 17:7539-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasai, Y., B. Lu, H. Steinbeisser, D. Geissert, L. K. Gont, and E. M. De Robertis. 1994. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sive, H., R. M. Grainger, and R. R. Harland. 2000. Early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Smith, J. C., B. M. J. Price, J. B. A. Green, D. Weigel, and B. G. Herrman. 1991. Expression of a Xenopus homologue of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67:79-87. [DOI] [PubMed] [Google Scholar]
- 43.Smith, W. C., R. Mckendry, S. Ribisi, and R. Harland. 1995. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell 82:37-46. [DOI] [PubMed] [Google Scholar]
- 44.Snider, L., H. Thirlwell, J. R. Miller, R. T. Moon, M. Groudine, and S. J. Tapscott. 2001. Inhibition of Tcf3 binding by I-mfa domain proteins. Mol. Cell. Biol. 21:1866-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokol, S. Y. 1999. Wnt signaling and dorsoventral axis specification in vertebrates. Curr. Opin. Genet. Dev. 9:405-410. [DOI] [PubMed] [Google Scholar]
- 46.Stewart, R. M., and J. Gerhart. 1990. The anterior extent of dorsal development of the Xenopus embryonic axis depends on the quantity of organizer in the late blastula. Development 122:363-372. [DOI] [PubMed] [Google Scholar]
- 47.von Dassow, G., J. E. Schmidt, and D. Kimelman. 1993. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeobox gene. Genes Dev. 7:355-366. [DOI] [PubMed] [Google Scholar]
- 48.Watabe, T., S. Kim, A. Candia, U. Rothbacher, C. Hashimoto, K. Inoue, and K. W.-Y. Cho. 1995. Molecular mechanisms of Spemann's organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 9:3038-3050. [DOI] [PubMed] [Google Scholar]
- 49.Wessely, O., J. I. Kim, D. Geissert, U. Tran, and E. M. De Robertis. 2004. Analysis of Spemann organizer formation in Xenopus embryos by cDNA macroarrays. Dev. Biol. 269:552-566. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto, S., H. Hikasa, H. Ono, and M. Taira. 2003. Molecular link in the sequential induction of the Spemann organizer: direct activation of the cerberus gene by Xlim-1, Xotx2, and Siamois, immediately downstream from Nodal and Wnt signaling. Dev. Biol. 257:190-204. [DOI] [PubMed] [Google Scholar]
- 51.Yang, J., C. Tan, R. S. Darken, P. A. Wilson, and P. S. Klein. 2002. B-catenin/Tcf-regulated transcription prior to the midblastula transition. Development 129:5743-5752. [DOI] [PubMed] [Google Scholar]
- 51a.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10:1443-1454. [DOI] [PubMed] [Google Scholar]
- 52.Zaraisky, A. V. Ecochard, O. V. Kazanskaya, S. A. Lukyanov, I. V. Fesenko, and A.-M. Duprat. 1995. The homeobox gene XANF-1 may control development of the Spemann organizer. Development 121:3839-3847. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, J., D. W. Houston, M. L. King, C. Payne, C. Wylie, and J. Heasman. 1998. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94:515-524. [DOI] [PubMed] [Google Scholar]