Abstract
Transcription activation in yeast (Saccharomyces cerevisiae) involves ordered recruitment of transcription factor complexes, such as TFIID, SAGA, and Mot1p. Previously, we showed that both Mot1p and Taf1p are recruited to the HXT2 and HXT4 genes, which encode hexose transporter proteins. Here, we show that SAGA also binds to the HXT2 and HXT4 promoters and plays a pivotal role in the recruitment of Mot1p and Taf1p. The deletion of either SPT3 or SPT8 reduces Mot1p binding to HXT2 and HXT4. Surprisingly, the deletion of GCN5 reduces Taf1p binding to both promoters. When GCN5 is deleted in spt3Δ or spt8Δ strains, neither Mot1p nor Taf1p binds, and this results in a diminished recruitment of TATA binding protein and polymerase II to the HXT4 but not the HXT2 promoter. This is reflected by the SAGA-dependent expression of HXT4. In contrast, SAGA-independent induction of HXT2 suggests a functional redundancy with other factors. A functional interplay of different SAGA subunits with Mot1p and Taf1p was supported by phenotypic analysis of MOT1 SAGA or TAF1/SAGA double mutant strains, which revealed novel genetic interactions between MOT1 and SPT8 and between TAF1 and GCN5. In conclusion, our data demonstrate functional links between SAGA, Mot1p, and TFIID in HXT gene regulation.
Yeast cells respond to environmental stress by changing their transcription profiles. Changes in the environment are relayed via a cascade of signal transduction pathways to transcriptional activators. These activators recruit distinct cofactors which can modify the chromatin organization of promoter regions, enabling association of the mediator complex to the core promoter and subsequent recruitment of the TATA binding protein (TBP) and the basal transcription machinery (36). The recruitment of different activities at a given time during transcription activation is a gene-specific event (14).
Recent analysis has indicated that yeast can have different requirements for the TFIID or SAGA coactivator complexes. They interact with activators, possess histone acetyltransferase (HAT) activity, and facilitate TBP binding to promoter sites (24). SAGA and TFIID share a common set of TBP-associated factors (TAFs) (25), which are required for the integrity of both complexes (33, 57). In contrast, TBP is an integral part of the TFIID complex but not of SAGA. However, TBP shows multiple genetic interactions with the SAGA components SPT3 and SPT8 (20, 21) and physically interacts with Spt8p (50, 55). An alternative SAGA-like complex, SALSA/SLIK, contains a truncated form of Spt7p and lacks Spt8p (48). Moreover, SAGA shares the Ada1p/Gcn5p HAT module with the ADA complex (19).
In vivo protein-DNA cross-linking studies classified yeast promoters as either TAF dependent or TAF independent based on the ratio of TBP/TAF binding to promoter sites (29, 34). Genome-wide expression analysis of various strains carrying mutations in genes encoding subunits of TFIID and SAGA also allowed classification into SAGA-dependent genes and TFIID-dependent genes (28, 32). Interestingly, consensus TATA elements which constitute the optimal binding site for TBP are overrepresented in SAGA-dependent genes (5). This suggests that TFIID and SAGA complexes are recruited to different sets of target promoters via different molecular mechanisms. SAGA is specifically recruited to upstream activator sequences (UAS) (8, 9, 31). In contrast, TFIID is specifically recruited to core promoter regions which require TAF subunits that either interact with activators or have specificity for promoter sequences (11).
Mot1p has initially been characterized as a global negative regulator of transcription (16, 42, 44). Mot1p has been found in a stable complex with TBP (43), and the Mot1p/TBP complex binds TATA DNA with high affinity (26). The C-terminal ATPase domain of Mot1p can disrupt Mot1p-TBP-TATA ternary complexes in vitro upon addition of ATP (2). Thus, Mot1p can exert its negative role in transcription by disruption of TBP-TATA complexes in an ATP-dependent manner (41). Genome-wide expression analysis of two different mot1 mutant strains shows that Mot1p has both negative and positive effects on transcription regulation (1, 15, 22). In addition, in vivo protein-DNA cross-linking studies show that Mot1p binds to heat shock, copper-regulated, or salt-inducible promoter regions after their activation, which correlates with TBP and RNA polymerase II (Pol II) binding to these promoters (22, 23). Moreover, the promoters of the heat shock genes SSA3, SSA4, HSP104, and CTT1 and the copper-inducible CUP1 gene have been described as TAF independent (29). This suggests a positive role of Mot1p in the activation of these stress-regulated genes. Moreover, it was proposed that stress conditions activate the Mot1p/TBP complex (23). In agreement with these results, we found that Mot1p is involved in recruitment of TBP to the HXT2 and HXT4 genes. TBP recruitment was severely compromised in two different mot1 mutant strains but only weakly in a taf1 mutant (1).
The HXT2 and HXT4 genes are part of a family of hexose transporters comprising 18 different genes in yeast. HXT2 and HXT4 are high-affinity transporters which are transcribed under conditions of low glucose (37, 38, 56). Under conditions of high glucose, the HXT2 and HXT4 genes are repressed by Mig1p and Rgt1p, which recruit the general repressor complex Tup1p/Ssn6p. Under low-glucose conditions, the Snf1p kinase phosphorylates Mig1p, which leads to derepression of the HXT2 and HXT4 genes (37) and subsequent recruitment of Taf1p, Mot1p, TBP, and Pol II (1). How these factors are recruited to the HXT2 and HXT4 promoters remains an open question. Possibly, the SAGA complex is involved in this process. Shifting cells to low glucose concentrations could be regarded as a stress condition, and SAGA has been proposed to regulate stress-dependent genes (28). Moreover, MOT1 displays genetic interactions with SPT3 (12, 35), a component of SAGA. In addition, it has been shown that Spt3p and Mot1p are recruited to the GAL1 promoter in an interdependent manner (53).
Here, we show that SAGA is recruited to the HXT2 and HXT4 promoters upon activation by low glucose. In addition, deletion of the SPT3 or SPT8 subunit affects recruitment of Mot1p. In contrast, Taf1p recruitment is dependent on the Gcn5p module of SAGA. Our results provide novel insight into the interplay of SAGA, TFIID, and Mot1p on activated promoters.
MATERIALS AND METHODS
Yeast strains.
The Saccharomyces cerevisiae strains used in this study are listed in Table 1, except for FHY59, a taf1ts2 strain, and the congenic wild-type strain (54). The COy001 strain represents the W303-derived DY150 strain purchased from the American Type Culture Collection (Manassas, VA). All procedures were performed according to standard methods (10). The HA3-MOT1 (DP107) and HA3-TAF1 (YBY838) tagged strains have been described previously (3, 43). Single or double SAGA mutant strains were constructed as described previously (47). However, the gcn5Δ strain was constructed using a GCN5 disruption cassette (51). To construct an spt3Δ spt8Δ strain, COy015 (spt3Δ::URA3) was grown on 5-fluoroorotic acid plates to select for colonies which lost the URA3 cassette. Subsequently, the obtained strain was retransformed to replace the SPT8 coding region (47). All deletions were verified by PCR analysis with primers corresponding to both wild-type and disruption alleles. SPT20-TAP and SPT3-TAP strains were constructed as described previously (46). Proper integration was verified by Western blot analysis and by PCR analysis with primers corresponding to both the wild-type and the tagged allele. To introduce the mot1-1 allele in the W303 background, a 292-bp fragment of the mot1-1 allele, harboring a Trp1730Stop mutation, was amplified by PCR (47) and transformed into the COy001 strain. Transformants carrying the mot1-1 allele were identified based on a temperature-sensitive (TS) phenotype by growth at 37°C. From these clones, the 3′ end of MOT1 was PCR amplified and sequenced to verify the mot1-1 mutation. To test for genetic interactions between SAGA and MOT1, single mutant strains were crossed and sporulated, followed by tetrad analysis to check for proper marker segregation. Sequences of oligonucleotides used in this study are available upon request.
TABLE 1.
List of yeast strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| COy001 | MATα leu2-3,112 his3-11,15 trp1-1 can1-100 ade2-1 ura3-1 | This paper |
| COy015 | Isogenic to COy001 except spt3Δ::URA3 | This paper |
| COy017 | Isogenic to COy001 except ada2Δ::URA3 | This paper |
| COy019 | Isogenic to COy001 except spt7Δ::URA3 | This paper |
| COy021 | Isogenic to COy001 except spt8Δ::URA3 | This paper |
| COy023 | Isogenic to COy001 except spt20Δ::URA3 | This paper |
| COy026 | Isogenic to COy001 except gcn5Δ::KANA | This paper |
| COy054 | Isogenic to COy001 except spt3Δ::URA3 gcn5Δ::KANA | This paper |
| COy055 | Isogenic to COy001 except spt8Δ::URA3 gcn5Δ::KANA | This paper |
| COy056 | Isogenic to COy001 except spt3Δ spt8Δ::URA3 | This paper |
| COy059 | Isogenic to COy001 except mot1-1::URA3 | This paper |
| DPY107 | MATaura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 leu2-Δ1 mot1::TRP1::flu3::MOT1 | 43 |
| COy089 | Isogenic to DPY107 except spt3Δ::URA3 | This paper |
| COy091 | Isogenic to DPY107 except spt8Δ::URA3 | This paper |
| JCA001 | Isogenic to DPY107 except gcn5Δ::KANA | This paper |
| COy095 | Isogenic to DPY107 except spt3Δ::URA3 gcn5Δ::KANA | This paper |
| COy096 | Isogenic to DPY107 except spt8Δ::URA3 gcn5Δ::KANA | This paper |
| COy097 | Isogenic to DPY107 except spt3Δ spt8Δ::URA3 | This paper |
| YBY838 | MATaura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 leu2-Δtaf11Δ::TRP1 pRS313-3Flu-TAF130-His6-FLAG (HIS CEN) | 3 |
| COy098 | Isogenic to YBY838 except spt3Δ::URA3 | This paper |
| COy100 | Isogenic to YBY838 except spt8Δ::URA3 | This paper |
| JCA002 | Isogenic to YBY838 except gcn5Δ::KANA | This paper |
| COy104 | Isogenic to YBY838 except spt3Δ::URA3 gcn5Δ::KANA | This paper |
| COy105 | Isogenic to YBY838 except spt8Δ::URA3 gcn5Δ::KANA | This paper |
| COy106 | Isogenic to YBY838 except spt3Δ spt8Δ::URA3 | This paper |
| COy142 | Isogenic to COy001 except SPT20::TAP::TRP1 | This paper |
| COy140 | Isogenic to COy001 except SPT3::TAP::TRP1 | This paper |
| COy036 | Isogenic to COy001 except mot1-1::URA3 SPT20::TAP::TRP1 | This paper |
| COy182 | Isogenic to FHY58 except spt3Δ::URA3 | This paper |
| COy184 | Isogenic to FHY58 except spt8Δ::URA3 | This paper |
| COy180 | Isogenic to FHY58 except gcn5Δ::KANA | This paper |
| COy181 | Isogenic to FHY59 except spt3Δ::URA3 | This paper |
| COy183 | Isogenic to FHY59 except spt8Δ::URA3 | This paper |
| COy179 | Isogenic to FHY59 except gcn5Δ::KANA | This paper |
| COy185 | Isogenic to COy001 except mot1-1 spt3Δ::URA3 | This paper |
| COy187 | Isogenic to COy001 except mot1-1 gcn5Δ::KANA | This paper |
Growth conditions.
For glucose concentration shift experiments, cells were grown in SC medium containing 4% glucose. When cells had reached mid-log phase (optical density at 600 nm = 0.55 to 0.6), 250 ml of culture was cross-linked for 20 min at room temperature by addition of 1% formaldehyde (corresponding to time t = 0). The remaining cells were harvested by centrifugation for 6 min at 1,700 × g in a Sorvall SLA3000 rotor, resuspended in a small volume of SC with 4% glucose, and diluted in SC without glucose to adjust the glucose concentration to 0.1%. Cells were incubated at 30°C and subjected to cross-linking at the indicated time points.
For RNA analysis, cells were grown in SC medium containing 4% glucose. When cells had reached mid-log phase (optical density at 600 nm = 0.55 to 0.6), 10 ml of the culture was directly added to 25 ml liquid N2 (t = 0). After shifting to low glucose, 10 ml of the culture was removed at the indicated time points and was frozen directly in liquid N2. After thawing, cells were collected by centrifugation, refrozen in liquid N2, and stored at −80°C upon further processing.
Northern blotting.
RNA isolation and hybridization conditions were described previously (1). Oligonucleotide sequences and the labeling procedure used for HXT genes have been described previously (17). A 1-kb fragment of the ACT1 coding region (spanning the region +324 to +1347) was used to analyze variations in mRNA loading. The ACT1 DNA fragment was labeled with the RediprimeII system according to the manufacturer's protocol (Amersham). PhosphorImager quantification of HXT2 and HXT4 mRNA signals has been described previously (1). Data from duplicate experiments were used for this quantification.
Chromatin immunoprecipitation (ChIP) assay.
Chromatin extracts (CE) were prepared as previously described (1). CE (200 μl) were used for immunoprecipitation (IP), and 10 μl CE was used for input control preparation. IPs were performed with 25 μl of Prot G-agarose beads (Roche) prebound to 10 μg anti-yTBP, 30 μg antihemagglutinin (anti-HA) antibodies (12CA5), or 10 μl anti-Pol II CTD monoclonal antibody (8WG16) or with 30 μl immunoglobulin G Sepharose 6 fast flow beads (Amersham). IPs were performed as described previously (1). After elution, cross-links were reversed by incubating the eluates with 200 μg proteinase K for 2 h at 37°C and overnight at 65°C. DNA from input samples was prepared similarly. After DNA purification from the eluates using Qiaquick columns (QIAGEN), DNA was analyzed by multiplex PCR (1).
RESULTS
Transcriptional induction of HXT4, but not HXT2, depends on the SAGA complex.
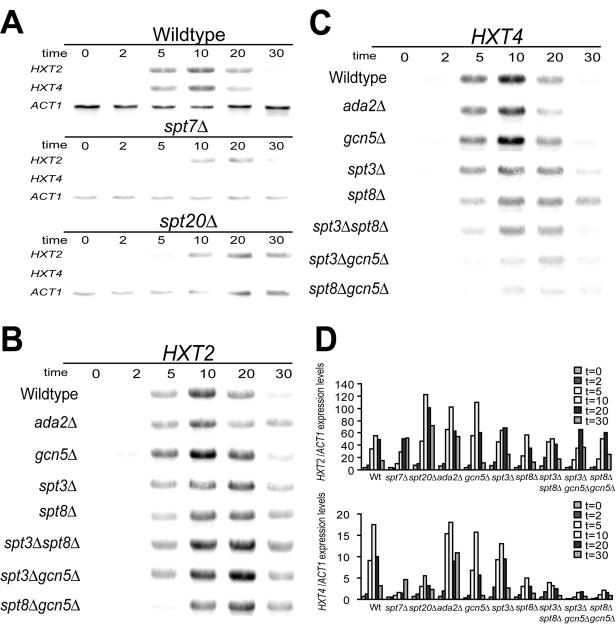
The SAGA complex consists of multiple subunits involved in different aspects of its coactivator and histone acetyltransferase functions (49). Biochemical and structural analysis showed that the Spt7p and Spt20p subunits are essential for the structural integrity of the complex (50, 57). In order to test whether SAGA is involved in transcription activation of the HXT2 and HXT4 genes, we analyzed their expression in yeast strains with deletions of specific SAGA subunits after shifting to low glucose concentrations. To allow the analysis of early time points, we devised a rapid cell harvesting protocol, which involves the instantaneous freezing of cells (see Materials and Methods). In wild-type yeast cells, both HXT2 and HXT4 mRNAs accumulated, peaking at 10 min after the shift to 0.1% glucose (Fig. 1A and D). In contrast, spt7Δ or spt20Δ mutant cells were severely compromised in HXT4 induction. Deletion of these SAGA subunits only mildly affected HXT2 activation. Interestingly, HXT2 induction seemed to be somewhat delayed in the spt20Δ and spt7Δ strains (Fig. 1A and D; see also the supplemental material).
FIG. 1.
Transcription of HXT4 but not HXT2 is affected in SAGA mutant strains. (A) Kinetics of mRNA expression of HXT genes in wild-type, spt7Δ, and spt20Δ strains upon a shift to low glucose. Cells were grown in 4% glucose and shifted to 0.1% glucose for 0, 2, 5, 10, 20, and 30 min before collection (see Materials and Methods). RNA samples from wild-type, spt7Δ, and spt20Δ strains were processed in parallel and analyzed on a single Northern blot to ensure direct comparison of hybridization signals. HXT mRNAs were detected using oligonucleotide probes described previously (1, 17). ACT1 probes were used us an internal loading control. Data shown are representative of at least two independent experiments. (B and C) Kinetics of mRNA expression of the HXT2 gene (B) or the HXT4 gene (C) in various SAGA mutant strains. RNA samples from wild-type, ada2Δ, spt3Δ, gcn5Δ, spt8Δ, spt3Δ gcn5Δ, spt8Δ gcn5Δ, and spt3Δ spt8Δ strains were analyzed on single Northern blots as in panel A. Reprobing of the blots with the ACT1 probe verified equal mRNA loading (data not shown). (D) Quantification of HXT2 and HXT4 mRNAs. HXT hybridization signals were quantified by a PhosphorImager and are expressed relative to ACT1 mRNA signals.
To investigate contributions of the different SAGA modules, we constructed additional yeast deletion strains to compare transcriptional induction of the HXT2 and HXT4 genes (Fig. 1B, C, and D and the supplemental material). As expected, HXT2 mRNA induction was not affected by the deletion of individual SAGA modules, but induction shows a delay similar to spt20Δ cells (Fig. 1B and D; see also the supplemental material). Removal of the HAT activity by deletion of either GCN5 or ADA2 did not affect the induction of HXT4 mRNA. In contrast, the deletion of either SPT3 or SPT8, which comprise the TBP module of SAGA (20, 21), resulted in decreased HXT4 transcription. When spt3Δ spt8Δ cells were analyzed, we found that this double deletion did not further reduce HXT4 induction. The observed reduction in HXT4 transcription in spt8Δ cells shows that SAGA is involved and that alternative complexes, such as the SALSA/SLIK or ADA complexes, do not play a role (19, 48). In contrast, in gcn5Δ spt3Δ or gcn5Δ spt8Δ cells, expression was further reduced to a level comparable to that seen in spt7Δ or spt20Δ cells.
Taken together, these observations indicate that SAGA plays an important role in HXT4 gene induction after shifting to low glucose conditions. The Spt3p/Spt8p TBP module of SAGA seems to be most important in this. The contribution of its HAT module (Gcn5p-Ada2p-Ada3p) is apparent only in strains already compromised in this TBP module.
Transcriptional activation of HXT2 and HXT4 coincides with SAGA recruitment.
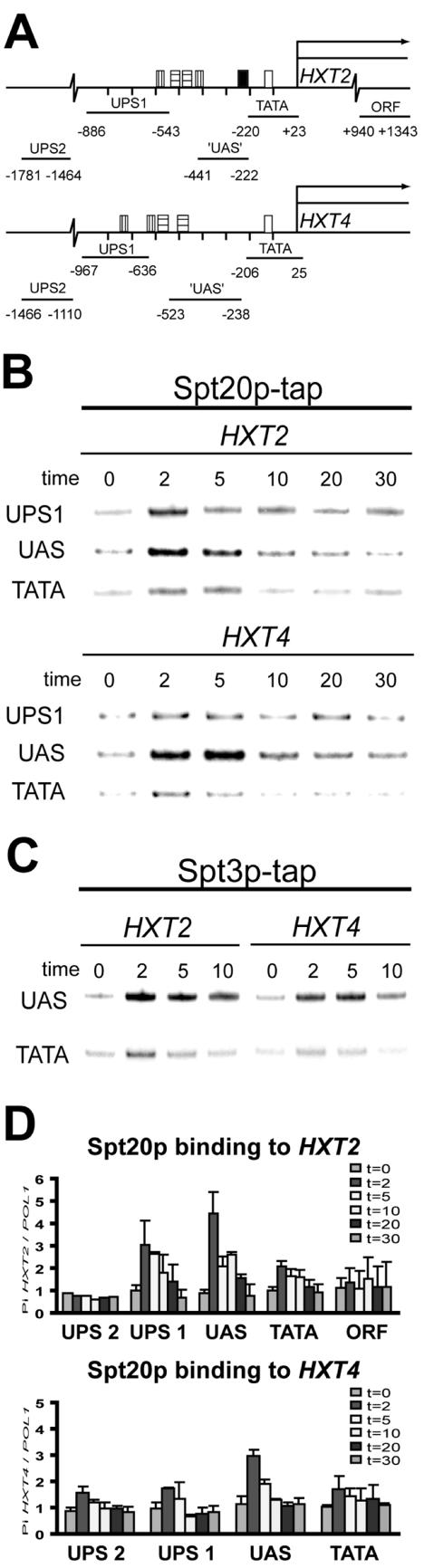
It has been shown that the SAGA coactivator complex can bind to UAS regions of target promoters upon their activation (4, 8, 13, 31). To investigate whether SAGA is directly involved in the activation of the HXT genes, we tested the association of the architectural Spt20p and TBP-interacting Spt3p subunits of SAGA during activation of the HXT2 and HXT4 promoters. This was investigated by ChIP experiments employing yeast strains expressing tandem affinity purification (TAP)-tagged versions of Spt20p or Spt3p (Table 1). The TAP-tagged strains were grown in SC medium containing 4% glucose and shifted to SC medium containing 0.1% glucose to induce the HXT2 and HXT4 genes. Subsequently, formaldehyde-cross-linked chromatin was isolated at various times after the shift to low glucose. Immunopurified chromatin was analyzed by multiplex PCR using primer sets that amplify the TATA and upstream region of the HXT genes (Fig. 2A) or an intragenic fragment of the POL1 gene as a normalization control (30). Directly after shifting to 0.1% glucose, binding of Spt20p and Spt3p to the upstream regions of both HXT2 and HXT4 is specifically increased and localized to −441/−222 and −523/−238 of HXT2 and HXT4, respectively (Fig. 2B). A putative activator colocalizes the HXT2 region (39), but this is not known yet for the HXT4 fragment. Spt20p and Spt3p association is transient and can already be detected 2 minutes after a shift to low glucose. This precedes the induction of HXT mRNA expression (compare Fig. 1A). After 5 minutes, Spt20p and Spt3p start to decrease to background levels. The rapid recruitment of SAGA subunits coincides with Mot1p binding kinetics (1). Mot1p becomes associated to the TATA region of the HXT2 and HXT4 promoters 2 minutes after the shift to low glucose and decreases after 5 to 10 minutes.
FIG. 2.
SAGA specifically binds to the upstream region of both HXT2 and HXT4 genes. (A) HXT promoter structure and location of primers used to analyze Spt20p and Spt3 binding. Boxes indicate bind-ing sites for Mig1p (vertical stripes), Rgt1p (horizontal stripes), putative UAS (filled), and TBP (open) (39). Lines represent the DNA fragment, which was amplified in a multiplex PCR analysis. Numbers represent the exact location of HXT2 and HXT4 primers relative to the start site of the coding region. (B and C) Cells expressing a TAP-tagged version of Spt20p (B) or Spt3p (C) were subjected to a glucose shift, and cross-linking was initiated by addition of formaldehyde at the indicated time points. Input and immunoprecipitated DNA was analyzed by multiplex PCR with primers spanning the TATA box (TATA), putative UAS, or further upstream (UPS1) regions of the HXT genes as indicated. (D) Quantification of ChIP signals over the HXT2 and HXT4 genes. Radioactive signals of the indicated PCR fragments were quantified with a PhosphorImager. The signals are expressed relative to the POL1 signal, which was included as a negative control in the multiplex PCR analysis.
In conclusion, these experiments show that SAGA is recruited to both HXT2 and HXT4 promoters under conditions of activation.
Mot1p and Taf1p recruitment to HXT genes depends on different modules of SAGA.
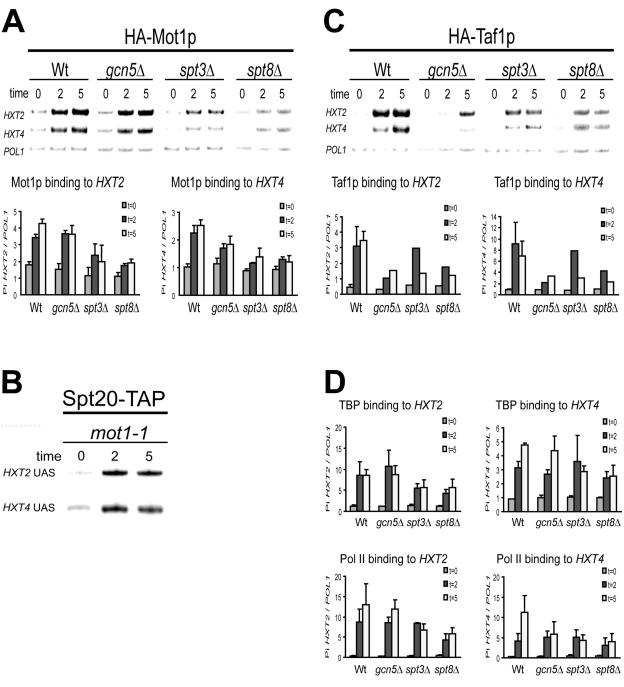
We reported previously that Mot1p and the Taf1p subunit of TFIID are recruited to the HXT2 and HXT4 promoters upon a change to low glucose concentrations (1). Mutations in MOT1 affect TBP recruitment, whereas mutations in TAF1 have little effect (1). These findings and those described above suggest that SAGA could be involved in Mot1p and/or Taf1p recruitment. In addition, observations made in other experimental systems showed that Spt3p and Spt8p can be involved in the recruitment of TBP (7, 8, 18, 31). Spt3p and Gcn5p were also implicated in the recruitment of Mot1p to the activated GAL1 promoter (53). To test SAGA involvement in Mot1p and or Taf1p recruitment, we constructed several SAGA mutant strains expressing HA-tagged versions of Mot1p (43) or of Taf1p (3). Subsequently, recruitment of these factors, TBP, and Pol II binding to the activated HXT promoters was determined by using ChIP assays (see Materials and Methods).
Mot1p was recruited within 2 minutes to the HXT2 and HXT4 promoters in a wild-type strain, in agreement with our previous findings (Fig. 3A) (1). In contrast, Mot1p recruitment to both promoters was severely impaired in the spt3Δ and spt8Δ cells and reduced only slightly in the gcn5Δ strain (Fig. 3A). To test whether recruitment of SAGA also depends on Mot1p, we analyzed Spt20p binding in the mot1-1 strain (Fig. 3B), which is severely impaired in TBP recruitment (1). We found that Spt20p was recruited normally to the HXT2 and HXT4 promoters in the mot1-1 mutant. Thus, in the case of the HXT promoters, the Spt3p-Spt8p module of SAGA is essential for efficient Mot1p recruitment.
FIG. 3.
Binding kinetics of SAGA, Mot1p, Taf1p, TBP, and Pol II to HXT genes in SAGA and MOT1 mutant strains after a shift to low glucose. (A) Representative PCR and PhosphorImager quantification of Mot1p binding to HXT genes in wild-type (Wt), gcn5Δ, spt3Δ, and spt8Δ cells. The top panel shows multiplex PCR analysis in which POL1 primers have been included as a normalization control. Primers amplifying HXT2 and HXT4 are specific for the core promoter region as indicated for Fig. 2A. Each sample was analyzed at least in duplo. The lower panel displays the quantification. Factor binding is expressed as HXT/POL1 ratios, as described previously (1). (B) Analysis of Spt20p binding to HXT genes in a mot1-1 mutant strain after a shift to low glucose. (C) Taf1p binding to HXT in Wt, gcn5Δ, spt3Δ, and spt8Δ cells. (D) TBP and Pol II recruitment to HXT genes in Wt, gcn5Δ, spt3Δ, and spt8Δ strains.
In contrast to Mot1p, the deletion of GCN5 severely impaired Taf1p recruitment (Fig. 3C). Taf1p recruitment was also reduced in spt8Δ and to a lesser extent spt3Δ cells. To test whether the impaired recruitment of Mot1p and Taf1p correlates with reduced preinitiation complex formation on HXT start site regions, we analyzed TBP and Pol II recruitment to these promoters. As expected (1), the kinetics of TBP recruitment to the activated HXT promoters is very rapid and coincides with Mot1p and Taf1p binding (Fig. 3D). Surprisingly, TBP and Pol II association with both the HXT2 and HXT4 promoters is only mildly affected by the deletion of the SAGA subunits (Fig. 3D). The strongest reduction, however, is observed in the spt8Δ strain, which also displays the strongest effect on mRNA accumulation. The weakest effects on TBP recruitment are observed in the gcn5Δ strain, which displays a severe reduction in Taf1p recruitment. This is in agreement with observations that Taf1p plays a minor role in TBP recruitment to activated HXT promoters (1).
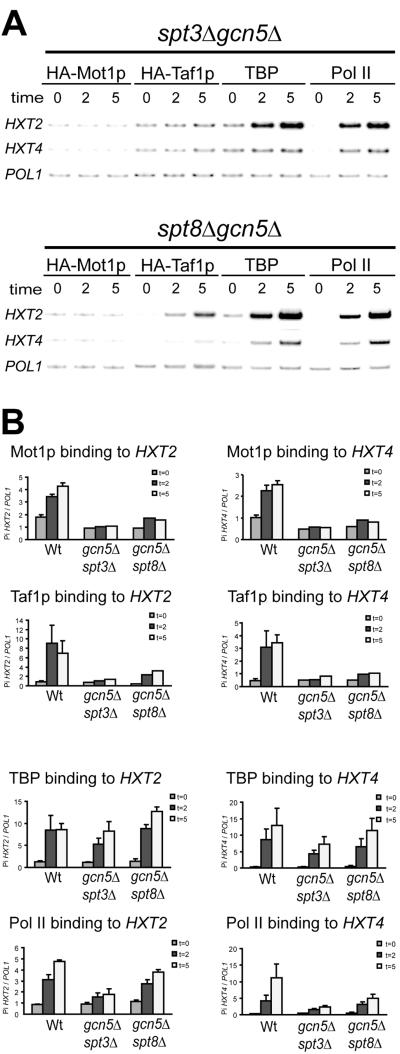
Different modules of SAGA cooperate in Mot1p and Taf1p recruitment to HXT genes.
The observations that different SAGA subunits are involved in the recruitment of Mot1p and Taf1p prompted us to test whether different modules of SAGA cooperate in the recruitment of Mot1p and Taf1p. To this end, we constructed gcn5Δ spt3Δ and gcn5Δ spt8Δ strains expressing either HA-Mot1p or HA-Taf1p. Subsequent ChIP analysis showed that Mot1p and Taf1p recruitment was absent or strongly reduced to both HXT promoters in gcn5Δ spt3Δ and gcn5Δ spt8Δ cells (Fig. 4). These findings indicate that Gcn5p can work in concert with Spt8p and Spt3p to recruit Mot1p to the HXT2 and HXT4 promoters. In addition, the deletion of SPT3 but not SPT8 further reduces Gcn5p-dependent Taf1p recruitment (Fig. 4). Next we analyzed TBP and Pol II recruitment in these double mutant strains. Surprisingly, TBP recruitment to HXT2 was not affected, and the efficiency of TBP recruitment to HXT4 was only mildly reduced (Fig. 4B). Therefore, in the absence of a functional SAGA complex, TBP does not absolutely require Mot1p or the TFIID complex to associate with the HXT2 and HXT4 promoters during their activation by a shift to low glucose. In contrast to this, Pol II recruitment to HXT4 is severely reduced, but little effect is observed on Pol II binding to HXT2 (Fig. 4B). This parallels mRNA accumulation of these genes upon a shift to low glucose (Fig. 1B). These findings also suggest that TBP complexes formed on the HXT4 promoter are not functional. In conclusion, disruption of both the Gcn5p HAT and Spt3p/Spt8p TBP modules of SAGA prevent Mot1p recruitment to the HXT promoters.
FIG. 4.
Binding kinetics of Mot1p, Taf1p, TBP, and Pol II to HXT genes in SAGA double deletion strains. (A) Recruitment of Mot1p, Taf1p, TBP, and Pol II in gcn5Δ spt3Δ and gcn5Δ spt8Δ cells after a shift to low glucose. Multiplex PCR signals of immunoprecipitated DNA as indicated above the lanes are displayed for the TATA-containing fragments of HXT2 and HXT4 as described for Fig. 2A. (B) PhosphorImager quantification of Mot1p, Taf1p, TBP, and Pol II binding to the indicated HXT promoters.
Genetic interactions of MOT1 or TAF1 with SAGA subunits.
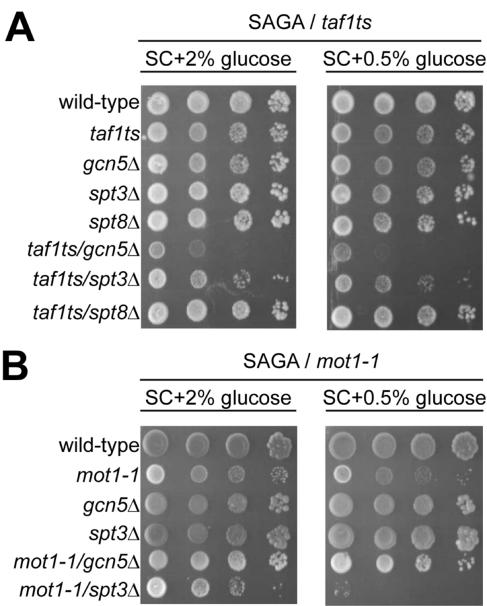
Differential SAGA subunit requirement as observed for the HXT promoters suggests separate pathways for Mot1p and Taf1p recruitment. We decided to investigate whether this could be a general phenomenon by testing for genetic interactions of MOT1 and TAF1 with different SAGA modules. The deletion of GCN5 in taf1ts2 cells elicited a severe growth defect on SC plates containing 2% glucose (Fig. 5A, row 6). When these gcn5Δ/taf1ts2 mutant cells were spotted on SC plates containing 0.5% glucose, the growth defect was even more pronounced. In contrast, spt8Δ/taf1ts2 and spt3Δ/taf1ts cells grew like the wild type and displayed a very mild defect, respectively (Fig. 5A, rows 7 and 8). In order to cross the mot1-1 mutant with SAGA mutant strains in the W303 background, we first created the mot1-1 allele in this background (see Materials and Methods). In contrast to taf1ts2 cells, we noted that mot1-1 cells already displayed a growth defect at a lower glucose concentration (Fig. 5, compare panels A and B). The mot1-1 strain was crossed with the spt3Δ, spt8Δ, and gcn5Δ mutant strains. Resulting diploids were subjected to sporulation to obtain double disruptant strains. In the case of mot1-1/spt8Δ, analysis of 21 tetrads revealed synthetic lethality between mot1-1 and spt8Δ. The other combinations were viable and were analyzed for growth at different temperatures and glucose concentrations in synthetic media. The deletion of SPT3 in mot1-1 cells created a strong growth defect, which was most apparent at lower glucose concentrations (Fig. 5B). This agrees with earlier reports of spt3Δ/mot1-1 cells as severely growth defective (12, 35). In contrast, the deletion of GCN5 had no effect on mot1-1 mutant cells.
FIG. 5.
Genetic interactions between SAGA, MOT1, and TAF1 during conditions of stress. (A) Wild-type (FHY58) and SAGA/TAF1 or (B) wild-type (W303) and SAGA/MOT1 mutant strains (as indicated) were serially diluted (10-fold steps) and grown on SC plus 2% glucose or SC plus 0.5% glucose. Cells were grown for 4 days at 33°C.
The observed growth defect of gcn5Δ/taf1ts2 cells suggests a Gcn5p-Taf1p pathway. In contrast, mot1-1 cells do not depend on Gcn5p but rather on Spt3p and Spt8p, suggesting the existence of a separate Spt3p/Spt8p-Mot1p pathway.
DISCUSSION
In this paper we showed that SAGA is recruited to the activated HXT2 and HXT4 promoters and that SAGA is essential for the recruitment of Mot1p and the Taf1p subunit of TFIID. The deletion of the Spt3p/Spt8p TBP module of SAGA affected predominantly the recruitment of Mot1p, whereas the deletion of GCN5 selectively impaired Taf1p recruitment. When both the Spt3p/Spt8p module and the Gcn5p HAT module were absent, Mot1p and Taf1p were not recruited. Surprisingly, TBP recruitment is relatively unaffected in such SAGA mutant strains. In contrast, Pol II association to the HXT4 but not the HXT2 promoter is reduced, which is reflected by differences in HXT2 and HXT4 mRNA accumulation. Functional interactions of SAGA, Mot1p, and Taf1p were supported by phenotypic analyses of double mutant strains grown under normal and glucose-restricted conditions. A mot1-1 strain shows phenotypes with spt8Δ and spt3Δ alleles but not with gcn5Δ. Conversely, a taf1ts2 TS strain displays a synthetic growth phenotype with gcn5Δ.
Recruitment of the SAGA complex to HXT genes.
HXT2 and HXT4 encode high-affinity hexose transporters and are transcribed under low glucose concentrations (38). We used activation of these genes as a model system to investigate transcriptional regulation by Mot1p and Taf1p. Our previous work showed that both of these TBP-interacting proteins can be recruited to the HXT2 and HXT4 promoters and that Mot1p is essential for TBP recruitment (1). Relatively little is known about positively acting transcription factors involved in activation of these HXT genes. While SAGA is clearly recruited to both HXT promoters, it remains unclear which gene-specific activators are responsible for this. Deletion analysis of the HXT2 promoter identified a UAS element located between −291 and −218 relative to the start of the gene (39). Thus, one possible mechanism for SAGA recruitment is through the activator(s) binding to this region. Alternatively, activation of the Tup1p/Ssn6p complex mediates SAGA binding to the HXT promoters. It has been shown that under stress conditions, the Tup1p/Ssn6p corepressor complex transforms into a coactivator, which facilitated SAGA binding to GAL1 and several osmotic stress-induced genes (40, 45). In this case, SAGA recruitment required the Cti6p protein (40). Possibly, Cti6p plays a similar role for the HXT genes studied here.
It is also important to note that although recruited to the HXT2 promoter, the SAGA complex is not required for transcriptional activation of this gene. This indicates a functional redundancy of SAGA, which is not apparent in HXT4 transcription. This could either be due to other coactivators present at the HXT2 promoter that substitute for SAGA function or be due to a different chromatin organization of this gene. In either case, a different requirement for SAGA could be beneficial for yeast cells, as it allows differential regulation of HXT2 and HXT4 genes.
Recruitment of Mot1p and Taf1p by distinct SAGA modules.
The described molecular function of SAGA includes recruitment of TBP to the transcription start sites of SAGA-dependent promoters via Spt3p and Spt8p (4, 7, 8, 31) and chromatin modification via the histone acetyltransferase activity of Gcn5p (4). A recently determined low-resolution structure of the SAGA complex (57) shows that Spt3p/Spt8p is organized in a separate module, termed domain V. In contrast, Gcn5p localizes to a central position in SAGA and faces a cleft, which could accommodate a nucleosome-like particle (52, 57).
Our results demonstrate that Gcn5p is essential for Taf1p recruitment, whereas the Spt3p/Spt8p module is most important for Mot1p. This suggests that these factors are differentially recruited. Taf1p recruitment via the Gcn5p module may involve its HAT activity, which could result in recruitment of other bromodomain-containing factors, such as the Swi/Snf complex, or in stabilization of SAGA binding (27). However, Gcn5p-dependent Taf1p recruitment seems less important for HXT4 transcription, as induction of this gene is not affected in gcn5Δ or ada2Δ cells (Fig. 1B). In contrast, the Spt3p/Spt8p module, which is primarily responsible for Mot1p recruitment, is essential for efficient induction of HXT4 transcription. This agrees with earlier findings that Mot1p is primarily responsible for TBP recruitment to HXT promoters (1).
A connection between Mot1p and SAGA components was also observed during induction of GAL1 transcription. In this case, Mot1p recruitment depends on Spt3p and Gcn5p (53). For GAL1, this is interdependent, because SAGA was not recruited in the mot1-1 strain. In contrast, for the HXT promoters Gcn5p is not essential for Mot1p recruitment. In addition, the recruitment of SAGA was not affected in mot1-1 cells. Thus, although Mot1p recruitment to GAL1 and the HXT genes depends on SAGA, different mechanisms which may reflect differences in activator and/or promoter context are involved.
In light of our earlier findings of Mot1p-dependent TBP recruitment (1), it was unexpected that recruitment of TBP is only mildly affected in strains lacking both Gcn5p and the Spt3p/Spt8p module (Fig. 4B). How can it be explained that in the absence of detectable Taf1p or Mot1p, TBP is still recruited to the HXT promoters? It was suggested that Spt3p and Spt8p play a dual role in transcription and can inhibit TBP binding (6). Possibly, in wild-type cells recruitment of TBP requires TBP-associated factors, like Mot1p and Taf1p, to overcome inhibition by Spt3p/Spt8p. In gcn5Δ spt3Δ and gcn5Δ spt8Δ strains, the recruitment of Mot1p and Taf1p is abolished but inhibition of TBP association would also be relieved. These observations also strengthen the notion that yeast cells contain pools of TBP, which are not in a TFIID or Mot1p complex (23), and argue against the model that SAGA and TFIID together account for all transcription (28). How TBP becomes recruited in the absence of functional SAGA, Taf1p, or Mot1p remains an open question. But the results with the HXT4 promoter suggest that SAGA can be required at a post-TBP recruitment step as Pol II recruitment is abolished (Fig. 4B). This view is supported by recent findings that Spt8p can contact TFIIA and Taf4p of TFIID (55). Possibly, these Spt8p interactions may play a role in the activation of HXT4.
Interplay between SAGA, Mot1p, and Taf1p in transcriptional regulation.
The finding that the recruitment of Taf1p (and presumably TFIID) to the HXT genes is dependent on the Gcn5p HAT module of SAGA is rather unexpected in light of recent reports (5, 28), which grouped promoters into TATA-less and TFIID dominated or TATA containing and SAGA dominated. The latter (and smaller) group consists for the most part of stress-induced genes (28). In contrast, previous genome-wide mRNA expression studies indicated that SAGA and TFIID are functionally redundant, suggesting that they serve overlapping sets of promoters (32). This is in better agreement for the HXT genes, as we found that both SAGA and TFIID are recruited to the HXT2 and HXT4 promoters but that only SAGA is functionally required for HXT4. Strikingly, both promoters contain TATA consensus sequences (5) and both would be classified as SAGA dominated. Also, the genetic interaction between gcn5Δ and the taf1 TS allele (Fig. 5A) suggests cooperation between TFIID and SAGA complexes rather than completely separated transcriptional pathways.
The connection between Mot1p and SAGA could extend beyond the GAL1 and HXT genes and have general implications for transcriptional regulation. Several lines of evidence support this suggestion. First, Mot1p- and SAGA-dependent genes are involved in stress responses (23, 28). Second, MOT1 genetically interacts with SPT3 (12, 35) and SPT8 (this study). Third, a recent genome-wide localization study suggested direct involvement of Mot1p in regulation of the SAGA pathway (58).
While our analysis of the HXT4 gene indicates that SAGA and Mot1p functionally cooperate, it was suggested that Mot1p rather acts as a negative factor for SAGA-dominated stress-induced genes (58). This is also at odds with results showing that Mot1p and TFIIB can cooccupy stress-induced promoters (23). Clearly, more detailed studies involving more promoters are required to clarify whether the cooperation between Mot1p and SAGA on the HXT4 promoter is a general phenomenon.
Altogether, our study revealed mechanistic insight into the regulation of hexose transporters in yeast. In addition, we demonstrated functional interplay between SAGA, Mot1p, and TFIID in glucose-regulated transcription, and our conclusions provide a framework to study the interaction of these pivotal transcription regulators at other stress-regulated genes.
Supplementary Material
Acknowledgments
We are grateful to members of the Timmers laboratory for helpful discussions and technical advice. We thank F. Holstege and P. Pijnappel for providing 12CA5 monoclonal antibody. We also thank J.-C. Andrau for providing yeast strains, P. A. Weil for providing anti-TBP antibodies and yeast strains, G. Thireos for supplying the GCN5 disruption cassette, and J. Thorner for sharing unpublished information.
H.T.M.T. and C.J.C.V.O. were supported by grants from The Netherlands Organization for Scientific Research (NWO-MW Pionier 900-98-142) and the European Union (Improving Human Potential RTN2-2001-00026).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Andrau, J.-C., C. J. C. van Oevelen, H. A. A. M. van Teeffelen, P. A. Weil, F. C. P. Holstege, and H. T. M. Timmers. 2002. Mot1 is essential for TBP recruitment to selected promoters during in vivo gene activation. EMBO J. 21:5173-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auble, D. T., K. E. Hansen, C. G. F. Mueller, W. S. Lane, J. Thorner, and S. Hahn. 1994. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 8:1920-1934. [DOI] [PubMed] [Google Scholar]
- 3.Bai, Y., G. M. Perez, J. M. Beechem, and P. A. Weil. 1997. Structure-function analysis of TAF130: identification and characterization of a high-affinity TATA-binding protein interaction domain in the N terminus of yeast TAF(II)130. Mol. Cell. Biol. 17:3081-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbaric, S., H. Reinke, and W. Horz. 2003. Multiple mechanistically distinct functions of SAGA at the PHO5 promoter. Mol. Cell. Biol. 23:3468-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basehoar, A. D., S. J. Zanton, and B. F. Pugh. 2004. Identification and distinct regulation of yeast TATA box-containing genes. Cell 116:699-709. [DOI] [PubMed] [Google Scholar]
- 6.Belotserkovskaya, R., D. E. Sterner, M. Deng, M. H. Sayre, P. M. Lieberman, and S. L. Berger. 2000. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol. Cell. Biol. 20:634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaumik, S. R., and M. R. Green. 2002. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22:7365-7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaumik, S. R., and M. R. Green. 2001. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 15:1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, C. E., L. Howe, K. Sousa, S. C. Alley, M. J. Carrozza, S. Tan, and J. L. Workman. 2001. Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science 292:2333-2337. [DOI] [PubMed] [Google Scholar]
- 10.Burke, D., D. Dawson, T. Stearns, and Cold Spring Harbor Laboratory. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual, 2000 ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Chen, B.-S., and M. Hampsey. 2002. Transcription activation: unveiling the essential nature of TFIID. Curr. Biol. 12:R620-R622. [DOI] [PubMed] [Google Scholar]
- 12.Collart, M. A. 1996. The NOT, SPT3, and MOT1 genes functionally interact to regulate transcription at core promoters. Mol. Cell. Biol. 16:6668-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 14.Cosma, P. A. 2002. Ordered recruitment: gene-specific mechanism of transcription activation. Mol. Cell 10:227-236. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta, A., R. P. Darst, K. J. Martin, C. A. Afshari, and D. T. Auble. 2002. Mot1 activates and represses transcription by direct, ATPase-dependent mechanisms. Proc. Natl. Acad. Sci. USA 99:2666-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis, J. L., R. Kunisawa, and J. Thorner. 1992. A presumptive helicase (MOT1 gene product) affects gene expression and is required for viability in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 12:1879-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diderich, J. A., M. Schepper, P. van Hoek, M. A. Luttik, J. P. van Dijken, J. T. Pronk, P. Klaassen, H. F. Boelens, M. J. de Mattos, K. van Dam, and A. L. Kruckeberg. 1999. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J. Biol. Chem. 274:15350-15359. [DOI] [PubMed] [Google Scholar]
- 18.Dudley, A. M., C. Rougeulle, and F. Winston. 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberharter, A., D. E. Sterner, D. Schieltz, A. Hassan, J. R. Yates III, S. L. Berger, and J. L. Workman. 1999. The ADA complex is a distinct histone acetyltransferase complex in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6621-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenmann, D. M., K. M. Arndt, S. L. Ricupero, J. W. Rooney, and F. Winston. 1992. SPT3 interacts with TFIID to allow normal transcription in Saccharomyces cerevisiae. Genes Dev. 6:1319-1331. [DOI] [PubMed] [Google Scholar]
- 21.Eisenmann, D. M., C. Chapon, S. M. Roberts, C. Dollard, and F. Winston. 1994. The Saccharomyces cerevisiae SPT8 gene encodes a very acidic protein that is functionally related to SPT3 and TATA-binding protein. Genetics 137:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisberg, J. V., Z. Moqtaderi, L. Kuras, and K. Struhl. 2002. Mot1 associates with transcriptionally active promoters and inhibits association of NC2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:8122-8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisberg, J. V., and K. Struhl. 2004. Cellular stress alters the transcriptional properties of promoter-bound Mot1-TBP complexes. Mol. Cell 14:479-489. [DOI] [PubMed] [Google Scholar]
- 24.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 25.Grant, P. A., D. E. Sterner, L. J. Duggan, J. L. Workman, and S. L. Berger. 1998. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 8:193-197. [DOI] [PubMed] [Google Scholar]
- 26.Gumbs, O., A. M. Campbell, and P. A. Weil. 2003. High-affinity DNA binding by a Mot1p-TBP complex: implications for TAF-independent transcription. EMBO J. 22:3131-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan, A. H., K. E. Neely, and J. L. Workman. 2001. Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell 104:817-827. [DOI] [PubMed] [Google Scholar]
- 28.Huisinga, K. L., and B. F. Pugh. 2004. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 13:573-585. [DOI] [PubMed] [Google Scholar]
- 29.Kuras, L., P. Kosa, M. Mencia, and K. Struhl. 2000. TAF-containing and TAF-independent forms of transcriptionally active TBP in vivo. Science 288:1244-1248. [DOI] [PubMed] [Google Scholar]
- 30.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399:609-613. [DOI] [PubMed] [Google Scholar]
- 31.Larschan, E., and F. Winston. 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15:1946-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, T. I., H. C. Causton, F. C. Holstege, W. C. Shen, N. Hannett, E. G. Jennings, F. Winston, M. R. Green, and R. A. Young. 2000. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405:701-704. [DOI] [PubMed] [Google Scholar]
- 33.Leurent, C., S. L. Sanders, M. A. Demeny, K. A. Garbett, C. Ruhlmann, P. A. Weil, L. Tora, and P. Schultz. 2004. Mapping key functional sites within yeast TFIID. EMBO J. 23:719-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, X. Y., S. R. Bhaumik, and M. R. Green. 2000. Distinct classes of yeast promoters revealed by differential TAF recruitment. Science 288:1242-1244. [DOI] [PubMed] [Google Scholar]
- 35.Madison, J. M., and F. Winston. 1997. Evidence that Spt3 functionally interacts with Mot1, TFIIA, and TATA-binding protein to confer promoter-specific transcriptional control in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orphanides, G., and D. Reinberg. 2002. A unified theory of gene expression. Cell 108:439-451. [DOI] [PubMed] [Google Scholar]
- 37.Ozcan, S., and M. Johnston. 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63:554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozcan, S., and M. Johnston. 1995. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell. Biol. 15:1564-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozcan, S., and M. Johnston. 1996. Two different repressors collaborate to restrict expression of the yeast glucose transporter genes HXT2 and HXT4 to low levels of glucose. Mol. Cell. Biol. 16:5536-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papamichos-Chronakis, M., T. Petrakis, E. Ktistaki, I. Topalidou, and D. Tzamarias. 2002. Cti6, a PHD domain protein, bridges the Cyc8-Tup1 corepressor and the SAGA coactivator to overcome repression at GAL1. Mol. Cell 9:1297-1305. [DOI] [PubMed] [Google Scholar]
- 41.Pereira, L. A., M. P. Klejman, and H. T. M. Timmers. 2003. Roles for BTAF1 and Mot1p in dynamics of TATA-binding protein and regulation of RNA polymerase II transcription. Gene 315:1-13. [DOI] [PubMed] [Google Scholar]
- 42.Piatti, S., R. Tazzi, A. Pizzagalli, P. Plevani, and G. Lucchini. 1992. Control of DNA synthesis genes in budding yeast: involvement of the transcriptional modulator MOT1 in the expression of the DNA polymerase α gene. Chromosoma 102:107-113. [DOI] [PubMed] [Google Scholar]
- 43.Poon, D., A. M. Campbell, Y. Bai, and P. A. Weil. 1994. Yeast TAF170 is encoded by MOT1 and exists in a TATA box-binding protein (TBP)-TBP-associated factor complex distinct from transcription factor IID. J. Biol. Chem. 269:23135-23140. [PubMed] [Google Scholar]
- 44.Prelich, G., and F. Winston. 1993. Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics 135:665-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proft, M., and K. Struhl. 2002. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell 9:1307-1317. [DOI] [PubMed] [Google Scholar]
- 46.Puig, O., F. Caspary, G. Rigaut, B. Rutz, E. Bouveret, E. Bragado-Nilsson, M. Wilm, and B. Seraphin. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218-229. [DOI] [PubMed] [Google Scholar]
- 47.Reid, R. J., M. Lisby, and R. Rothstein. 2002. Cloning-free genome alterations in Saccharomyces cerevisiae using adaptamer-mediated PCR. Methods Enzymol. 350:258-277. [DOI] [PubMed] [Google Scholar]
- 48.Sterner, D. E., R. Belotserkovskaya, and S. L. Berger. 2002. SALSA, a variant of yeast SAGA, contains truncated Spt7, which correlates with activated transcription. Proc. Natl. Acad. Sci. USA 99:11622-11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya, L. A. Pacella, F. Winston, J. L. Workman, and S. L. Berger. 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Syntichaki, P., I. Topalidou, and G. Thireos. 2000. The Gcn5 bromodomain co-ordinates nucleosome remodelling. Nature 404:414-417. [DOI] [PubMed] [Google Scholar]
- 52.Timmers, H. T. M., and L. Tora. 2005. SAGA unveiled. Trends Biochem. Sci. 30:7-10. [DOI] [PubMed] [Google Scholar]
- 53.Topalidou, I., M. Papamichos-Chronakis, G. Thireos, and D. Tzamarias. 2004. Spt3 and Mot1 cooperate in nucleosome remodeling independently of TBP recruitment. EMBO J. 23:1943-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker, S. S., J. C. Reese, L. M. Apone, and M. R. Green. 1996. Transcription activation in cells lacking TAF(II)s. Nature 383:185-188. [DOI] [PubMed] [Google Scholar]
- 55.Warfield, L., J. A. Ranish, and S. Hahn. 2004. Positive and negative functions of the SAGA complex mediated through interaction of Spt8 with TBP and the N-terminal domain of TFIIA. Genes Dev. 18:1022-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wieczorke, R., S. Krampe, T. Weierstall, K. Freidel, C. P. Hollenberg, and E. Boles. 1999. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 31:123-128. [DOI] [PubMed] [Google Scholar]
- 57.Wu, P. Y., C. Ruhlmann, F. Winston, and P. Schultz. 2004. Molecular architecture of the S. cerevisiae SAGA complex. Mol. Cell 15:199-208. [DOI] [PubMed] [Google Scholar]
- 58.Zanton, S. J., and B. F. Pugh. 2004. Changes in genomewide occupancy of core transcriptional regulators during heat stress. Proc. Natl. Acad. Sci. USA 101:16843-16848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.