Abstract
Short chain fatty acids (SCFA) exist in dietary foods and are produced by the fermentation of gut microbiota, and are considered an important element for regulating host health. Through blood circulation, SCFA produced in the gut and obtained from foods have an impact on the intestinal health as well as vital organs of the host. It has been recognized that the gut is the “vital organ” in the host. As the gut microbial metabolites, SCFA could create an “axis” connecting the gut and to other organs. Therefore, the “gut-organ axes” have become a focus of research in recent years to analyze organism health. In this review, we summarized the sources, absorption properties, and the function of SCFA in both gut and other peripheral tissues (brain, kidney, liver, lung, bone and cardiovascular) in the way of “gut-organ axes”. Short chain fatty acids exert both beneficial and pathological role in gut and other organs in various ways, in which the beneficial effects are more pronounced. In addition, the beneficial effects are reflected in both preventive and therapeutic effects. More importantly, the mechanisms behinds the gut and other tissues provided insight into the function of SCFA, assisting in the development of novel preventive and therapeutic strategies for maintaining the host health.
Keywords: Short chain fatty acid, Intestinal barrier, Gut-organ axis, Host health, Beneficial effect, Pathological effect
1. Introduction
The intestine has long been regarded as the most important organ for the human body to defend against external contaminants. The intestinal epithelium containing three different cell types (enterocytes, Paneth cells, and goblet cells), which are considered to be the first line of defense (Gao et al., 2020). Additionally, the intestinal microbiota colonizing the gut also plays a crucial role in maintaining the health condition of healthy. Firmicutes and Bacteroidetes dominate intestinal microbiota, accounting for almost 80% of the total population (Foster and Neufeld, 2013). Links between the gut microbiota and numerous metabolic diseases, such as diabetes, obesity, and nervosa, have been reported (Fan and Pedersen, 2021).
The intestinal microbiota produces various metabolites for intestinal cells, including short-chain fatty acids (SCFA), lactate, deoxycholic acid (DCA), and tryptophan metabolites, which could exert beneficial effects on the host (Nicolas and Chang, 2019). Researchers have been particularly interested in the bioactive role that SCFA play. Short chain fatty acids could be utilized for energy by intestinal cells or released into the portal vein circulation for utilization by peripheral tissues and provide 10% of the daily caloric demand of the human body (Alexander et al., 2019). In addition, as key bacterial metabolites, SCFA could affect various organs, including brain and liver (Koh et al., 2016). Recently, the concept of “gut-organ axes” has been proposed to help create innovative diagnosis and therapeutic approaches (Ahlawat et al., 2021). Therefore, the present study summarized the potential effects of SCFA in human health as well as the underlying cause-effect mechanisms in theoretically based on “gut-organ axes".
2. Exogenous intake, endogenous biosynthesis, and absorption of SCFA
To explore the impact of SCFA on the body health, we must understand the sources and absorption mechanism of SCFA firstly. There are currently limited reviews on the content of SCFA in food as most studies focus on the production of SCFA by intestinal microbiota fermentation. Therefore, we summarize the sources and absorption of SCFA in the body, especially the contents of SCFA in milk, which have not been reported systematically in the previous studies. These findings will be explained in detail in the following contents.
2.1. Biosynthesis and intake of SCFA
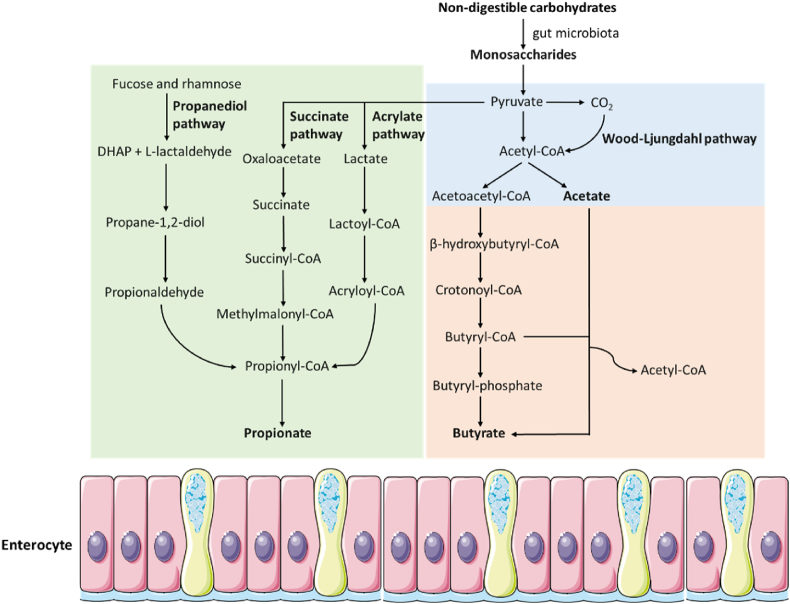
There are two sources from which the intestinal epithelium can obtain SCFA: one is bacterial fermentation (main source), and the other one is direct intake via food (Koh et al., 2016; Zhao et al., 2022). Short chain fatty acids are the primary fermentation by-products of non-digestible carbohydrates (NDC), which are digested by the gut microbiota in the cecum and colon after evading digestion in the small intestine. The main SCFA produced are acetate, propionate and butyrate, with acetate accounting for 60% to 70% of the total SCFA (Morrison and Preston, 2016). The acetate-producing bacteria are widely distributed, such as Akkermansia muciniphila, Ruminococcus spp. and Bacteroides spp. while the bacteria producing propionate (Bacteroides spp. and Ruminococcus obeum etc), and butyrate (Coprococcus comes and Roseburia spp.) are highly conserved and specific (Zeng et al., 2019). As illustrated in Fig. 1, a variety of factors, including nutrition and intestinal microbiota composition, have an impact on the biosynthesis and quantity of SCFA. The two metabolic pathways that gut microbiota uses to produce acetate are mainly formed by hydrolysis of acetyl-CoA and Wood-Ljungdahl pathway (Ragsdale and Pierce, 2008). There are three different ways for the colonic bacteria to produce propionate, including the succinate, acrylate and propanediol pathways, where deoxyhexose sugars are used as substrates (Scott et al., 2006). There are two potential routes to produce butyrate in the gut. The first is the classical pathway, which involves butyryl-CoA being converted to butyrate via phosphor-transbutyrylase and butyrate kinase. Another pathway is the conversion of butyryl-CoA to butyrate via butyryl-CoA:acetate CoA-transferase (den Besten et al., 2013; Duncan et al., 2002). When less fermentable fiber is available, the microbes also use amino acids in the diet to produce SCFA (Wall et al., 2009). Milk, as an important human food source, contains a considerable amount of SCFA (Zhao et al., 2022). The concentrations of SCFA in milk are listed in Table 1.
Fig. 1.
The endogenous biosynthesis of short chain fatty acids. DHAP = dihydroxyacetone phosphate.
Table 1.
The list of short chain fatty acid concentrations in milk.
| Item | Sample | Detection method | Concentration | Unit of meaure | Reference |
|---|---|---|---|---|---|
| Butyrate | Human milk collected from China (n = 90) | Thin-layer chromatography | 0.06 ± 0.01 in Zhengzhou, 0.03 ± 0.01 in Wuhan, and 0.07 ± 0.03 in Harbin | % of triglycerides | Chen et al. (2020) |
| Caproate | Human milk collected from China (n = 90) | Thin-layer chromatography | 0.01 ± 0.00 in Zhengzhou, 0.01 ± 0.00 in Wuhan, and 0.03 ± 0.01 in Harbin | % of triglycerides | Chen et al. (2020) |
| Butyrate | Human milk collected from China (n = 180) | Ultra-high-performance supercritical fluid chromatography | 0.06 ± 0.06 in full-term colostrum milk, 0.07 ± 0.05 in full-term transitional milk, and 0.24 ± 0.20 in full-term mature milk | mg/g milk fat | Dai et al. (2020) |
| Formate | Human milk collected from Australia, Japan, Norway, South Africa, and USA (n = 109) | Nuclear magnetic resonance analysis | Range: 15.2–4960.3 Median: 43.7 |
μM | Stinson et al. (2020) |
| Acetate | Human milk collected from Australia, Japan, Norway, South Africa, and USA (n = 109) | Nuclear magnetic resonance analysis | Range: 13.5–4307.7 Median: 46.8 |
μM | Stinson et al. (2020) |
| Butyrate | Human milk collected from Australia, Japan, Norway, South Africa, and USA (n = 109) | Nuclear magnetic resonance analysis | Range: 4.8–409.5 Median: 95.6 |
μM | Stinson et al. (2020) |
| Butyrate | Human milk collected from United Kingdom (n = 102) | Gas chromatography-mass spectrometry | Range: 0–3.5 | mg/100 mL | Prentice et al. (2019) |
| Acetate | Milk collected from Canada (n = 16, four replicates from each type of milk) | Liquid chromatography-tandem mass spectrometry | 38.0 ± 1.0 in skim milk, 37.0 ± 1.0 in 1% milk, 46.0 ± 1.0 in 2% milk, 43.0 ± 1.0 in 3.25% milk | μΜ | Foroutan et al. (2019) |
| Propionate | Milk collected from Canada (n = 16, 4 replicates from each type of milk) | Liquid chromatography-tandem mass spectrometry | 1.0 ± 0.1 in skim milk, 2.94 ± 0.12 in 1% milk, 2.0 ± 0.1 in 2% milk, 2.0 ± 0.1 in 3.25% milk | μΜ | Foroutan et al. (2019) |
| Butyrate/iso butyrate | Milk collected from Canada (n = 16, 4 replicates from each type of milk) | Liquid chromatography-tandem mass spectrometry | 37.0 ± 1.0 in skim milk, 34.9 ± 10.2 in 1% milk, 24.0 ± 1.0 in 2% milk, 27.0 ± 1.0 in 3.25% milk | μΜ | Foroutan et al. (2019) |
| Valerate/iso valerate | Milk collected from Canada (n = 16, 4 replicates from each type of milk) | Liquid chromatography-tandem mass spectrometry | 1.8 ± 0.1 in skim milk, 4.0 ± 0.2 in 1% milk, 4.18 ± 0.03 in 2% milk, 4.91 ± 0.13 in 3.25% milk | μΜ | Foroutan et al. (2019) |
| Caproate | Milk collected from Canada (n = 16, 4 replicates from each type of milk) | Liquid chromatography-tandem mass spectrometry | 1.8 ± 0.1 in skim milk, 4.0 ± 0.2 in 1% milk, 4.18 ± 0.03 in 2% milk, 4.91 ± 0.13 in 3.25% milk | μΜ | Foroutan et al. (2019) |
| Butyrate | Milk collected from China. Human (n = 1), cow (n = 15), goat (n = 15), yak (n = 38), buffalo (n = 9), camel (n = 12) | Gas chromatography -flame ionization detection | 0.10 ± 0.01 in human milk, 1.23 ± 0.02 in cow milk, 1.62 ± 0.03 in buffalo milk, 0.93 ± 0.02 in goat milk, 2.05 ± 0.03 in yak milk, and 12.2 ± 0.09 in camel milk | g/100 g total fatty acids | Teng et al. (2017) |
| Butyrate | Milk collected from Brasil (n = 12) | Gas chromatography | 3.53 ± 0.95 in raw milk, 2.87 ± 0.46 in pasteurized milk, and 2.86 ± 0.40 in ultra high temperature treated milk | % total fatty acids | Pestana et al. (2015) |
2.2. SCFA absorption
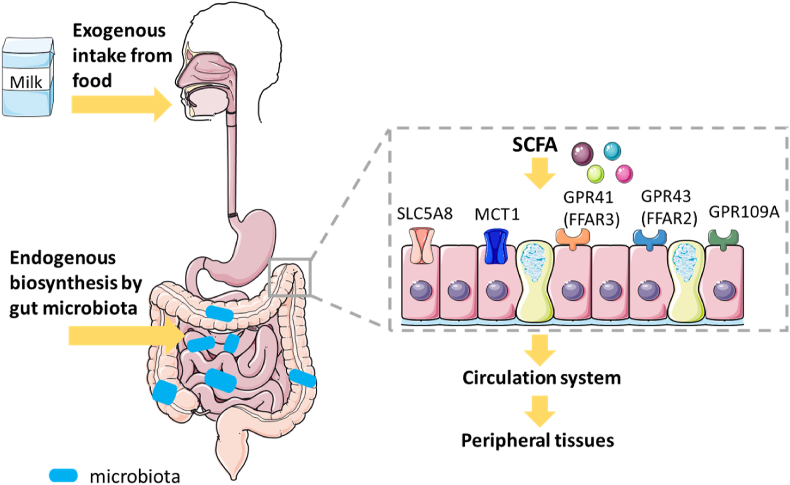
The concentration of SCFA varied among different intestinal segments, with the cecum and proximal colon having the highest concentration (70–140 mM), and the distal colon having a lower concentration (20–140 mM), respectively (Sun et al., 2017). This difference results from the absorption characteristics of SCFA with various transporters. Due to their hydrophobicity, as shown in Fig. 2, SCFA could be easily absorbed through the apical membrane of colon cells via nonionic diffusion (passive diffusion) (McNabney and Henagan, 2017). In addition, H+-dependent monocarboxylate transporter 1 (MCT1) and sodium-coupled monocarboxylate transporters (SCMT) are also important mediators of SCFA absorption. Solute carrier family 5, member 8 (SLC5A8) is a crucial component of SCMT (Liu et al., 2018; Silva et al., 2020). Furthermore, G-protein coupled receptor (GPR) 41, GPR43 and GPR109A are the receptors, GPR41 and GPR43 later known as free fatty acid receptors (FFAR)3 and FFAR2, which could activate SCFA (Bolognini et al., 2016). Butyrate is consumed in the colon as the preferred energy source for colon cells. Acetate and propionate subsequently enter the portal vein, while propionate is metabolized in the liver, acetate becomes the SCFA with the highest concentration in the peripheral circulation (Koh et al., 2016). The effects of SCFA on host health, including gut and other organs, are outlined in the following sections.
Fig. 2.
The sources and absorption of SCFA. The sources of SCFA in body includes exogenous intake from food, such as milk, and endogenous biosynthesis of non-digestible carbohydrates by gut microbiota in the cecum and colon. The transporters including SLC5A8, MCT1 and the receptors including GPR41 (FFAR3), GPR43 (FFAR2), and GPR109A are involved in the absorption of SCFA in the intestine. The absorbed SCFA enter the portal vein, and then dispersed to peripheral tissues, including brain, kidney, liver, lung, bone and cardiovascular tissues. SCFA = short chain fatty acids; SLC5A8 = solute carrier family 5 member 8; MCT1 = monocarboxylate transporter 1; GPR41 = G-protein coupled receptor 41; GPR43 = G-protein coupled receptor 43; GPR109A = G-protein coupled receptor 109A; FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3.
3. SCFA have both beneficial and pathological effects on gut health
The intestinal barrier is crucial for the body against external pollutants, which consists of physical, chemical, immunological and microbial barriers (Gao et al., 2020). On the basis of recently reported studies (Ma et al., 2022), we suggest that SCFA play a dual effect on intestinal health in these four (physical, chemical, immunological and microbial barriers) aspects.
According to a published study, intestinal development and the expression of tight junction (TJ)-related genes were both improved in the small intestine and colon of weaned piglets when acetate, propionate, and butyrate were infused into the stomach (Diao et al., 2019). An in vitro study showed that acetate, propionate, and butyrate improved the impaired intestinal TJ integrity through the AMP-activated protein kinase (AMPK) signaling pathway (Eamin et al., 2013). The findings in mice and HCT116 cells clarified that butyrate reduced intestinal permeability and enhanced the TJ-related gene level in a hypoxiainducible factor-1 (HIF-1)-dependent manner (Fachi et al., 2019). Results from IPEC-J2 cells showed that butyrate had a favorable effect on TJ gene expression (Ma et al., 2012). Butyrate exposure at 0.5 to 2.5 mM boosted the expression of zonula occludens-1 (ZO-1) and occludin in the IPEC-J2 cells, but not at the concentration of 5 mM (Saleri et al., 2022). However, there was no discernible variation in mRNA levels of ZO-1 and occludin when Caco-2 cells were exposed to acetate, propionate, and butyrate (Xi et al., 2022). While Caco-2 cells exposed to 5 mM butyrate for 48 h led to increased intestinal permeability and apoptosis proportion (Huang et al., 2014). Therefore, SCFA have both positive and negative effects on intestinal physical barrier by regulating the survival and cellular junctions of intestinal cells.
For the intestinal chemical barrier, it has been reported that propionate and butyrate upregulated the secretion of mucin (MUC) 2 in LS174T cells (Burger-van Paassen et al., 2009). This result was consistent with findings demonstrating that SCFA could stimulate intestinal MUC1 and MUC2 gene expression (Diao et al., 2019). Mucin (MUC2, MUC3 and MUC5AC) gene expression can be induced by butyrate in a variety of patterns (Bai et al., 2010; Gaudier et al., 2004). However, the supplementation of SCFA did not affect the MUC1 and MUC2 mRNA expression in piglets (Zhou et al., 2020). Although the MUC2 gene expression was enhanced in mouse colon exposed to 100 mM butyrate, the mucus thickness was decreased (Gaudier et al., 2009). Hence, the supplementation of SCFA bi-directionally regulates the intestinal chemical barrier mainly via modulating the expression of MUC related genes.
For immunological barrier, a reported review demonstrated that SCFA performed anti-inflammatory effects in the intestine through activating GPR, including GPR41, GPR43, and GPR109A (Venegas et al., 2019). The inflammatory response with inhibiting interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) expression was ameliorated in mice administered butyrate in drinking water and RAW 246.7 cells (Chen et al., 2018). Butyrate also reduced the secretion of pro-inflammatory cytokines in Caco-2 cells, COLO 205 cells, RAW 264.7 cells, and peritoneal macrophages in IL-10-deficient mice (Iraporda et al., 2015; Lee et al., 2017). However, broiler chicks fed formate had a much higher number of spleen lymphocytes (Ragaa et al., 2016). In the mice model, SCFA promoted IL-8 and IL-10 production (Singh et al., 2014; Sun et al., 2018), and the level of IL-22 produced by CD4+ cells through GPR41 (Yang et al., 2020b). The gene expression of IL-1β and IL-17A was elevated in mice administered 80 mM butyrate via enemas (Jiminez et al., 2017). In addition, butyrate inclusion in colostrum caused the immunoglobulin (Ig) G level in calf serum to decrease (Hiltz and Laarman, 2019). Accordingly, SCFA play anti-inflammatory or pro-inflammatory effects mainly through regulating the expression of cytokines.
For the microbial barrier, weaned pigs fed diets containing 6.4 g/kg formate had a lower abundance of Lactobacillus, Parvimonas, and Leuconostoc (Luise et al., 2017). After calves administering milk replacer with butyrate, the imbalance of intestinal health-related Mogibacterium in cecum was restored (O'Hara et al., 2018). The combination of formate and essential oils drastically reduced the counts of Salmonella and Clostridium in the intestine of broiler chickens (Pathak et al., 2017). The neonatal piglets administrated with butyrate decreased the abundance of Lactobacillus in the colon on day 8, although there were only minor alterations in community structure of the intestinal microbiota (Xu et al., 2016). Short chain fatty acids were able to reduce intestinal pH and block the biosynthesis of harmful metabolites by bacteria (Ma et al., 2022). Therefore, SCFA have the ability to inhibit harmful microbiota and maintain intestinal barrier health.
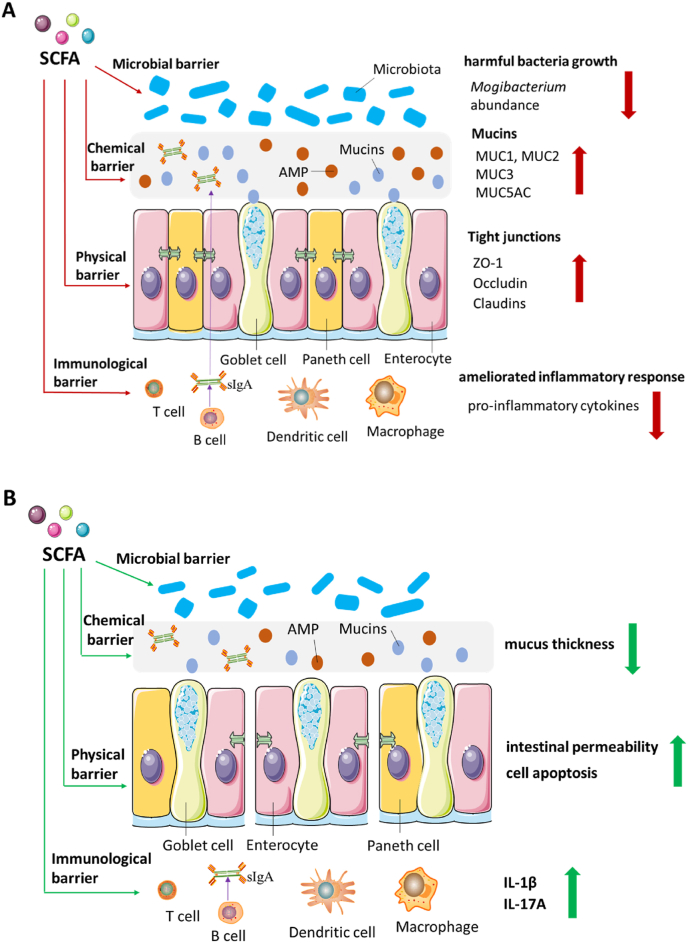
In conclusion, SCFA have positive effects on intestinal health, which are primarily reflected in increasing the expression of TJ proteins including ZO-1, claudins and occludin (physical barrier), promoting the expression of MUC-related genes (chemical barrier), decreasing the expression of pro-inflammatory cytokines (immunological barrier) and inhibiting the growth of harmful microbiota (microbial barrier). Correspondingly, the pathological effects of SCFA are reflected in the increased intestinal permeability, decreased mucus thickness and elevated cytokines (Fig. 3).
Fig. 3.
The double-sided role of SCFA in intestinal health on physical, chemical, immunological, and microbial barrier. (A) The beneficial effects of SCFA on intestinal barrier include increased expression level of tight junction proteins, mucins and decreased harmful bacterial growth as well as the pro-inflammatory cytokines expression. (B) The negative effects of SCFA on intestinal barrier include increased intestinal permeability and pro-inflammatory cytokines as well as the decreased mucus thickness. SCFA = short chain fatty acids; AMP = antimicrobial peptides; MUC = mucin; ZO-1 = zonula occludens-1; sIgA = secretory immunoglobulin A; IL-1β = interleukin-1β; IL-17A = interleukin-17A.
Compared with monogastric species (humans, rats, mice, rabbits etc.), ruminant animals (calves, goats, lambs etc.) have an advantage in digesting plant biomass (Hungate, 1966). This advantage comes from the rumen, which occupies for estimated 80% of the total volume of the gastrointestinal tract (Oh et al., 1972). It has been reviewed that exogenous butyrate had beneficial effects on the development of the ruminal epithelium (Niwińska et al., 2017). The ruminal papillae are important for the nutrient absorption in the ruminant epithelium (Gabel et al., 2002). Compared with animals receiving a normal diet, 0.3% sodium butyrate introduction in dry matter led to increased papillary length in calves (Górka et al., 2009, 2011a, 2011b; Kato et al., 2011) and lambs (Cavini et al., 2015). Similar results were also shown in goats, where an intraluminal butyrate infusion increased the papillary size, density and surface area (Malhi et al., 2013). The potential mechanisms of butyrate enhancing the papillary development could be explained by inhibiting cell apoptosis (Mentschel et al., 2001), activating the nuclear factors (Naeem et al., 2012), and regulating energy delivery (Kuzinski et al., 2012). However, the reports on whether butyrate administration could lead to improvements in calf rearing are still conflicting (Niwińska et al., 2017). Overall, SCFA exerted a double-sided role in both monogastric and ruminant animals.
4. SCFA in gut-organ axes: communication bridge within the body
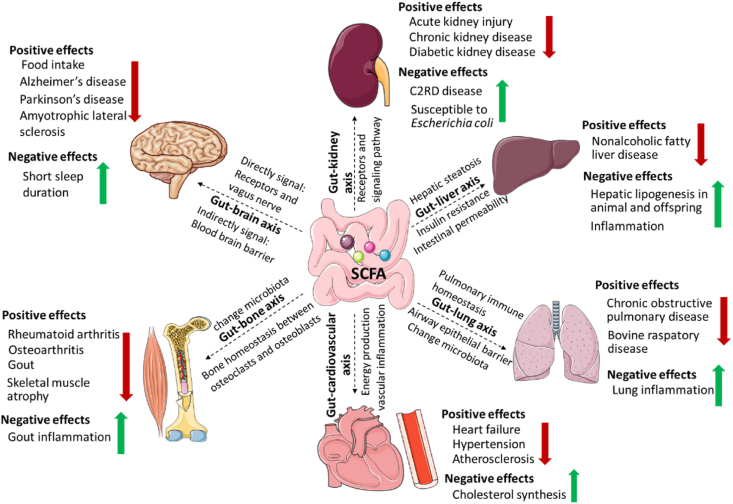
The controversial effects of SCFA on cancer, obesity and diabetes have been well documented (Cong et al., 2022; Liu et al., 2018). Recently, attention has been drawn to the relationship between SCFA and the health function of the whole body, including the peripheral system (de Vos et al., 2022). Short chain fatty acids are mainly produced by the intestinal microbiota, and the connections between the gut and other organs may be facilitated by gut microbial metabolites in the form of “axes” (Welch et al., 2022). The concept of “gut-organ axes” is an emerging model that can be used to both diagnose and treat diseases (Ahlawat et al., 2021). Therefore, in the current study, we will summarize the effects of SCFA on human health through the “gut-organ axes” (Fig. 4).
Fig. 4.
The double-sided role of SCFA in host health through “gut-organ axes”. The communication between gut and organ linked by SCFA includes “gut-brain axis”, “gut-kidney axis”, “gut-liver axis”, “gut-lung axis”, “gut-bone axis” and “gut-cardiovascular axis”. SCFA = short chain fatty acids.
4.1. SCFA in gut-brain axis
Recent studies have shown the effects of SCFA from gut microbiota on the gut-brain axis (Bruning et al., 2020; O'Riordan et al., 2022; Silva et al., 2020). It is clear that SCFA and their metabolites generated in the gut have strong biologically active properties, exerting both beneficial and pathological effects on the brain (Table 2). Short chain fatty acids communicate with the brain directly through their receptors and the vagus nerve. Both the central and peripheral nervous systems have neuronally expressed receptors of SCFA. The presence of SCFA receptor FFAR3 was identified in brain endothelial cells (Hoyles et al., 2018). Activation of FFAR leads to the inhibition of appetite-inducing hypothalamic activity in neuropeptide Y-expressing neurons, which is related to changes in circadian rhythm and appetite (Silva et al., 2020). It also demonstrated that acute oral, rather than intravenous, butyrate could lower the activity of hypothalamic neurons expressing neuropeptide Y, which would inhibit appetite in mice (Li et al., 2018). The results of 500 mg/kg 11C-acetate and PET-CT scanning showed that colonic acetate could be taken up by the brain, and suppressed appetite in C57BL/6 mice (Frost et al., 2014). In addition, intraperitoneal injection of SCFA may also, to variable degrees, diminish appetite. Butyrate is the most effective SCFA, followed by propionate and acetate. This effect was attenuated after the ablation of the vagus nerve, indicating that suvagal afferents were implicated in the inhibition of food intake by SCFA (Goswami et al., 2018). It has been reported that the reduced appetite has been proven to be beneficial for a variety of metabolic diseases, including prevention of obesity, dyslipidemia, and hepatic steatosis development, which promotes host health (Wang et al., 2021).
Table 2.
The studies on two-sided role of SCFA in host health via “gut-brain axis” establishing the connection between gut and brain.
| Item | Model/subject | Administration route | Dose and duration | Outcomes | Effects type | Reference |
|---|---|---|---|---|---|---|
| – | 30 autistic subjects from China, 20 healthy volunteers as controls | – | – | ↓ Fecal acetate and butyrate, as well as butyrate-producing taxa in ASD subjects | Beneficial effect | Liu et al., 2019a, Liu et al., 2019b |
| Butyrate, SCFA-produced bacteria | C57BL/6J germ-free adult mice, sterile water as control | Oral gavage | Butyrate (1 g/kg body weight per day) for 3 d, Clostridium tyrobutyricum and Bacteroides thetaiotaomicron for 2 weeks |
↓ Blood–brain barrier permeability; ↑ occludin expression in the frontal cortex and hippocampus | Beneficial effect | Braniste et al. (2014) |
| Acetate | C57BL/6 mice, saline as control | Intraperitoneal injection | 500 mg/kg, 1, and 2 h | ↓ Acute food intake; ↑ anorectic neuropeptide expression profile | Beneficial effect | Frost et al. (2014) |
| SCFA | C57BL/6J mice, saline as control | Intraperitoneal injection | Acetate (6 mmol/kg), propionate (6 mmol/kg), butyrate (6 mmol/kg) for 0.5, 1, and 3 h | ↓ Food intake | Beneficial effect | Goswami et al. (2018) |
| SCFA | C57BL/6 mice SAE model, saline as control | Intragastrical administration before the model establishment | SCFA (acetate: propionate: butyrate at a ratio of 3:1:1) at 500 mg/kg body for 7 d | ↓ Behavioral impairment and neuronal degeneration; ↓IL-1β and IL-6 levels in SAE mice brain | Beneficial effect | Liu et al. (2021) |
| – | Germ-free C57BL/6 mice | Fecal transplant gavage from aged or young male C57BL/6 mice into germ-free male mice at d 7, 14, 30, and 60 | – | ↓ Fecal acetate, propionate, and butyrate in aged mice with an aged microbiome | Beneficial effect | Lee et al. (2020a) |
| Butyrate | Sprague–Dawley rats cerebral ischemia model, saline as control | Intragastrical administration after ischemic modeling | Butyrate (30 mg/kg), 14 d | ↓ Volume of the cerebral infarction; ↓ cerebral edema; ↓ intestinal integrity | Beneficial effect | Chen et al. (2019) |
| Butyrate | E3L.CETP mice received subdiaphragmatic vagotomy surgery, and sham surgery as controls | Intragastric gavage, intravenous injection after a recovery period of 1 week after the surgery | 5% (wt/wt), 7 weeks | ↓ Food intake; ↓ activity of orexigenic neurons for acute oral administration | Beneficial effect | Li et al. (2018) |
| SCFA | Human THP-1 cells with LPS (20 ng/mL) plus IFN-γ (100 U/mL) | SCFA were added to THP-1 cells 15 min before their stimulation with LPS plus IFN-γ, and then incubation 48 h | Acetate (5-500 μM), propionate (5-500 μM), butyrate (5-500 μM), formate (5-500 μM), and valerate (5-500 μM) for 48 h | ↓ IL-1β, monocyte MCP-1, and TNF-α secretion | Beneficial effect | Wenzel et al. (2020) |
| – | Aged stroke mice | FTG from aged or young male C57BL/6 mice into aged stroke mice for 3 d | – | ↑ SCFA-producers in young mice; ↓ poststroke neurological deficits, inflammation in aged stroke mice; ↑ gut, brain and plasma SCFA concentrations in aged stroke mice | Beneficial effect | Lee et al. (2020b) |
| Butyrate | N9 microglial cells, rat primary microglia | N9 cells incubated with LPS and butyrate simultaneously for 22 h, butyrate-pretreated (for 22 h) in rat primary microglia cultures and then incubated with 5 μg/mL LPS | Butyrate (0.6 mM) for N9 cells, butyrate (2.5 mM) for rat primary microglia | ↑ LPS-induced IL-6 level in N9 cells; ↓ IL-6 level in rat primary microglia | Beneficial effect | Huuskonen et al. (2004) |
| – | Fifty-nine participants with insomnia symptoms (≥ 65 years) | Participants were divided into short and normal sleep duration phenotypes for a 2-week period | – | ↑ Short sleep duration in insomnia is associated with an increase in SCFA | Pathological effect | Magzal et al. (2021) |
ASD = autism spectrum disorder; SCFA = short chain fatty acids; SAE = sepsis-associated encephalopathy; IL-1β = interleukin-1β; IL-6 = interleukin-6; LPS = lipopolysaccharide; IFN-γ = interferon-γ; MCP-1 = monocyte chemoattractant protein-1; TNF-α = tumor necrosis factor-α; FTG = fecal transplant gavage. ↑ represents increase. ↓ represents decrease.
Short chain fatty acids indirectly signal to the brain through blood brain barrier (BBB) and intestinal immunity. As the main structural barrier between blood and brain, BBB has extremely low cell paracellular permeability and shields the brain from damaging poisons and pathogens to maintain brain homeostasis (O'Riordan et al., 2022). Barrier integrity could be measured by barrier permeability and the level of TJ proteins. In germ-free mice gavaged with butyrate, there was an increase in the production of TJ proteins and a decrease in BBB (Braniste et al., 2014). In addition, the BBB permeability of germ-free mice with SCFA (acetate, propionate, and butyrate) producing bacteria Clostridium tyrobutyricum and Bacteroides thetaiotaomicron was also reduced (Braniste et al., 2014). Under physiologically normal conditions, brain function is impacted by neuroinflammation, which may be significantly influenced by systemic inflammation. Although immune cell activation and cytokine production have little impact on brain function, systemic infection nevertheless has a considerable impact on the central nervous system, which, in turn, affects cognition and behavior (Cruz-Pereira et al., 2020). It also has been suggested that SCFA may influence immunological cells, which could affect systemic inflammation, peripheral immunity, and ultimately brain function (Dalile et al., 2019). Furthermore, SCFA (acetate, propionate, butyrate, formate, and valerate) individually and collectively lowered the number of cytokines secreted by human THP-1 microglial-like cells, suggesting that SCFA could regulate the function of damaged microglia in Alzheimer's disease (AD) (Wenzel et al., 2020). However, different SCFA had conflicting effects on inflammation. Butyrate reduced the lipopolysaccharide (LPS)-induced inflammation in rat primary microglia, which was observed in colon disease, while in murine proliferating N9 microglial cell line, butyrate exerted a pro-inflammatory effect, and these findings were related to the anti-cancer properties of butyrate (Huuskonen et al., 2004).
It is generally known that SCFA have positive effects on neural diseases including AD, Parkinson's disease, amyotrophic lateral sclerosis (ALS), and mood disorders (Mirzaei et al., 2021; Silva et al., 2020). Short chain fatty acids have been identified as the primary signaling molecules in the “brain-gut axis”, a system of bidirectional communication between the central nervous system and the gut (O'Mahony et al., 2015). Studies showed that ischemic stroke decreased intestinal levels of SCFA. In a cerebral ischemia model (9 weeks), transplanting fecal microbiota rich in SCFA into young rats showed a potential therapeutic effect and supplemental butyrate also had a similar effect (Chen et al., 2019). Literature evidence showed that insufficient SCFA produced by the intestinal microbiota in old mice was ameliorated by fecal transplantation of SCFA, which caused a cognitive impairment in comparison to young mice (Lee et al., 2020b). Additionally, the transplantation of four SCFA-producing bacterial strains alleviated brain inflammation in aged mice (18–20 months) with ischemic stroke (Lee et al., 2020a). The youngsters with autism spectrum disorder (ASD) had lower levels of butyrate and more butyrate markers (Liu et al., 2019b). Short chain fatty acids performed the neuroprotective effect in mice by increasing the TJ protein expression and decreasing the level of cytokines (Liu et al., 2021). However, the patients with impaired sleep continuity had greater concentrations of SCFA, including acetate, propionate, and butyrate (Magzal et al., 2021).
Taken together, the positive regulation of the brain by SCFA via the gut-brain axis (receptors, vagus nerve, and BBB) consists of reduced food intake, incidence of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. However, a short sleep duration may be associated with the harmful effects of SCFA on the brain. It has been reported that SCFA in food are absorbed in the stomach and the small intestine to fulfill their nutritional and physiological roles, but they cannot reach the hindgut under physiological conditions (Braden et al., 1995; Hendry et al., 2010). However, the investigations revealed that oral gavage of SCFA could also play a role in regulating brain function, indicating that the intake of SCFA in food will also have an impact on health. Furthermore, the studies in vivo and in vitro demonstrated that SCFA perform both therapeutic and preventative functions by acting the protective roles via the gut-brain axis whether SCFA are administered before or after modeling.
4.2. SCFA in gut-kidney axis
In this section, the two main forms of renal physiology, acute kidney injury (AKI) and chronic kidney disease (CKD), will be used as examples to highlight the involvement of SCFA in the gut-kidney axis (Table 3). Regarding AKI, butyrate supplementation alleviated renal injury caused by ischemia/reperfusion (I/R) in rats through the upregulated antioxidant and anti-inflammatory activities. Additionally, clinical data revealed that the butyrate level rose coinciding with the recovery of renal function after renal transplantation (Sun et al., 2022). Similarly, acetate and the acetate-producing bacteria also reduced renal I/R injury in C57BL/6 mice (Andrade-Oliveira et al., 2015). By lowering IL-6 level and oxidative stress, two factors involved in the nuclear factor-kappaB (NF-κB) signaling pathway, butyrate was shown to improve kidney function (Machado et al., 2012). In addition, the nephrotoxicity in rats was decreased by butyrate through its antioxidant ability (Sun et al., 2013). Lower levels of butyrate were found in CKD patients compared to healthy controls, which suggests that butyrate supplementation could slow the progression of CKD (Wang et al., 2019). Additionally, propionate partially inhibits renal failure partly through the FFAR2 and FFAR3 signaling pathways (Mikami et al., 2020). It is worth noting that studies in a rat CKD model have shown that high-fiber diets could produce more SCFA and were beneficial for CKD (Kieffer et al., 2016; Vaziri et al., 2014). Short chain fatty acids have also been regarded as potential pharmacological candidates for attenuating CKD progression (Felizardo et al., 2019).
Table 3.
The studies on two-sided role of SCFA in host health via “gut-kidney axis” establishing the connection between gut and kidney.
| Item | Model/subject | Administration route | Dose and duration | Outcomes | Effects type | Reference |
|---|---|---|---|---|---|---|
| Butyrate | Sprague–Dawley rats AKI model induced by renal I/R, saline as control | Tail vein injection 30 min prior to the renal I/R operation | 100 mg/kg, 6, 12, and 24 h | ↑ Function and structure of kidney | Beneficial effect | Sun et al. (2022) |
| – | Thirty-two patients who underwent allograft renal transplantation | – | – | ↑ Fecal butyrate; ↑ renal function after renal transplant recovery | Beneficial effect | Sun et al. (2022) |
| Acetate, Bifidobacterium longumorBifidobacterium adolescentis | C57BL/6 mice AKI model induced by I/R | Bacteria gavage for 10 d before kidney IRI | Bacteria gavage | ↓ Acute kidney injury induced by IR | Beneficial effect | Andrade-Oliveira et al. (2015) |
| SCFA | C57BL/6 mice AKI model induced by I/R, saline as control | 0.5 h before I/R operation | Acetate (200 mg/kg), propionate (200 mg/kg), and butyrate (200 mg/kg) | ↓ Serum level of creatinine and urea; ↑ kidney function after IRI, and acetate performed the best protection. | Beneficial effect | Andrade-Oliveira et al. (2015) |
| Butyrate | Wistar rats AKI model induced by metaglumina diatrizoate sodium, saline as control | Tail vein injection 6 h before modeling | 500 mg/kg, 6 d | ↓ Serum creatinine level and IL-6 level | Beneficial effect | Machado et al. (2012) |
| Butyrate | Sprague–Dawley rats nephrotoxicity model induced by gentamicin | Intraperitoneal injection 30 min prior to gentamicin injection | 50, 100, 200 mg/kg, 8 d | ↑ Activities of superoxide dismutase, catalase; ↓ glutathione and nephrotoxicity | Beneficial effect | Sun et al. (2013) |
| – | One hundred and twenty-seven patients with CKD and 63 healthy 33 controls |
– | – | ↓ Serum SCFA levels in CKD patients, and an inverse correlation between butyrate level and renal function | Beneficial effect | Wang et al. (2019) |
| Butyrate | Sprague–Dawley rats, the 5/6 nephrectomized model | 0.5% butyrate administered by gavage after one week surgery recovery | 313 μL/kg, 8 weeks | ↓ Renal fibrosis and delayed CKD progression | Beneficial effect | Wang et al. (2019) |
| Propionate | C57BL/6 mice, FFA2−/− mice, and FFA3−/− mice CKD model induced by adenine | 0.5% or 1.0% propionate via drinking water for 6 weeks accompanying model construction | 0.5% or 1.0%, 6 weeks | ↓ Kidney damage; ↓serum level of creatinine and blood urea nitrogen | Beneficial effect | Mikami et al. (2020) |
| Diets | Sprague–Dawley rats CKD model induced by adenine | Amylopectin (low-fiber control) and high fermentable fiber for 3 weeks | – | ↑ Kidney function; ↑ gut permeability indexes | Beneficial effect | Kieffer et al. (2016) |
| Diets | Sprague–Dawley rats CKD model induced by adenine | Amylopectin (low-fiber control) and high fermentable fiber for 3 weeks | – | ↓ CKD progression; ↓ oxidative stress and inflammation | Beneficial effect | Vaziri et al. (2014) |
| – | Thirty participants with DKD, 30 normal controls | – | – | ↓ Fecal acetate, propionate, butyrate and total SCFA levels in DKD group | Beneficial effect | Zhong et al. (2021) |
| Diets, SCFA | C57BL/6 mice, GPR43−/− mice, and GPR109A−/− mice diabetes model induced by streptozotocin | High-fiber, normal chow, zero-fiber diets, SCFA in drinking water after modeling | Acetate (100 mM), butyrate (50 mM), and propionate (100 mM) for 3 weeks | ↓ Diabetic nephropathy; ↑ SCFA-producing bacteria; ↑ SCFA production | Beneficial effect | Li et al. (2020) |
| SCFA | Sprague Dawley rats rental stone model induced by ethylene glycol | Acetate, propionate, and butyrate in drinking water | 4 weeks | ↓ Renal crystals | Beneficial effect | Liu et al. (2020) |
| – | Five occasional stones patients, 5 recurrent stones patients, 5 non-kidney stone control | – | – | ↓ SCFA-producing gut bacteria; ↓ metabolic pathways associated with SCFA production in kidney stone patients | Beneficial effect | Liu et al. (2020) |
| SCFA | C57BL/6 mice, GPR43−/− mice, and GPR41−/− mice | Acetate, propionate, and butyrate in drinking water | Acetate (100, 150, and 200 mM), propionate (200 mM), and butyrate (200 mM) for 6 weeks | ↑ Renal hydronephrosis disease; ↑ hydronephrosis and hyperplasia in kidney and ureter tissues | Pathological effect | Park et al. (2016) |
AKI = acute kidney injury; I/R = ischemia/reperfusion; SCFA = short chain fatty acids; IL-6 = interleukin-6; CKD = chronic kidney disease; DKD = diabetic kidney disease. ↑ represents increase. ↓ represents decrease.
Apart from AKI and CKD, diabetic kidney disease (DKD) has gradually grown in popularity as a research topic. A lower SCFA level in the serum and feces of DKD patients was reported (Zhong et al., 2021). Through FFAR2 and GPR109A signaling pathways, a high-fiber diet and SCFA (acetate, butyrate, and propionate) protected diabetic mice from developing diabetic nephropathy. Short chain fatty acids also reduced the expression of pro-inflammatory cytokines in vitro, demonstrating SCFA's ability to prevent diabetic nephropathy (Li et al., 2020). The renal crystals of rats with renal calcium oxalate stones were reduced after administrating with SCFA, and kidney stone patients had lower abundance of SCFA-producing bacteria and the metabolic pathway linked to SCFA production (Liu et al., 2020). A review has proved that SCFA treatment could be a new therapeutic approach for kidney injury induced by a gut-derived inflammatory response (Huang et al., 2017).
However, the literature continues to suggest that SCFA have a certain detrimental impact on kidney function (Table 3). Acetate- or C2-induced renal disease, also known as C2RD disease, is a T cell-mediated renal disease with progressive ureteritis and hydronephrosis. It is caused by the chronic administration of SCFA at levels higher than the physiological level (Park et al., 2016). Additionally, mice fed a high dietary fiber diet had a higher blood level of butyrate and fewer Escherichia species, which made them more susceptible to Escherichia coli and increased Gb3 protein level in the kidney, eventually leading to severe kidney damage (Zumbrun et al., 2014, 2013).
In conclusion, SCFA regulate kidney function by altering receptors and associated signaling pathways in the gut-kidney axis. Short chain fatty acids have a dual effect on kidney function, reducing the severity of AKI, CKD, and DKD (positive regulation) as well as increasing inflammation and bacterial susceptibility in renal hydronephrosis (C2RD disease) (negative regulation). The tail vein injection of SCFA plays a protective effect on renal diseases, which is consistent with the findings that high fiber diets may be beneficial for the kidney via the gut-kidney axis, which could produce more SCFA in the circulatory system. In addition, based on the mode of administration, SCFA have a larger preventive effect on the liver than a therapeutic effect.
4.3. SCFA in gut-liver axis
Nonalcoholic fatty liver disease (NAFLD), including simple steatosis, non-alcoholic hepatitis (NASH) and liver cirrhosis, has become the most common liver disease in the world (Farrell and Larter, 2006; Loomba and Sanyal, 2013). It has become an important cause of chronic liver disease in developed countries, such as Europe and the United States and rich regions of China, which can seriously increase the occurrence of hepatocellular cancer (Behary et al., 2021; Wong et al., 2014). The link between gut bacteria dysbiosis and NASH and NAFLD has been documented (Leung et al., 2016; Wigg et al., 2001). The primary sclerosing cholangitis may potentially be linked to ulcerative colitis via the gut-liver axis (Loftus et al., 2005). Butyrate reverses the development of NASH, which is partly associated with the changes gut microbiome (Ye et al., 2018). Based on the reported studies, we propose that SCFA might contribute to preventing the progression of NAFLD via different mechanisms through the gut-liver axis (Table 4).
Table 4.
The studies on two-sided role of SCFA in host health via “gut-liver axis” establishing the connection between gut and liver.
| Item | Model/subject | Administration route | Dose and duration | Outcomes | Effects type | Reference |
|---|---|---|---|---|---|---|
| Butyrate | C57BL/6J mice NAFLD model |
Gavage | Sodium butyrate (0.12 g/mL), 6 weeks | ↓ NASH development, which may be driven by the protective gut microbiome and metabolome | Beneficial effect | Ye et al. (2018) |
| SCFA | Male C57BL/6J mice, PPAR lox/lox mice, 2 months of age | Dietary intake | Acetate, propionate or butyrate was incorporated into diet at 5% (wt/wt) | ↓ PPARγ expression in the liver; ↓ hepatic triglyceride concentrations | Beneficial effect | den Besten et al. (2015) |
| Butyrate | C57BL/6J mice NAFLD model, liquid as control |
Oral consumption | Sodium butyrate (0.6 g/kg body weight per day), 6 weeks | ↓ Inflammation in the liver; ↓ development of NASH | Beneficial effect | Jin et al. (2015) |
| Acetate | Male wistar rats, water as control | Oral gavage | Sodium acetate (200 mg/kg), 8 weeks | ↓ Xanthine oxidase activity, and performs hepatoprotection | Beneficial effect | Dangana et al. (2020) |
| Acetate | Hyla rabbits, saline as control | Subcutaneous injection | Acetate (2 g/kg body weight per day, one injection each day), 4 days | ↓ Intramuscular triglyceride level; ↑ fatty acid uptake; ↑ fatty acid oxidation | Beneficial effect | Liu et al., 2019b, Liu et al., 2019a |
| SCFA | Thirteen overweight and obese, 20–50 years old Caucasian men | Rectal infusion | SCFA mixtures (acetate, propionate and butyrate), 4 clinical investigation days | ↑ Fat oxidation, energy expenditure and PYY; ↓ lipolysis in overweight/obese men | Beneficial effect | Araujo et al. (2020) |
| Propionate | Human HepG2 hepatocytes | Following propionate treatment of the cells for 24 h | Propionate (0, 0.25, 0.5 mM) for HepG2 cells) | ↓ Gene expression of gluconeogenic enzymes independent of insulin signaling | Beneficial effect | Yoshida et al. (2019) |
| Propionate, butyrate | Duroc × Landrace × Yorkshire pigs (50% male) weaned at day 28 | Dietary intake | Basal diet plus 1 g propionate/kg, or plus 1 g butyrate/kg diet for 14 d | ↑ Serum PYY concentration; ↑ lipid metabolism | Beneficial effect | |
| SCFA | Multi-organ model of UC ex vivo | – | 20 mM of SCFA | ↑ Effector function of activated CD4+ T cells; ↓ acute CD4+ T cell-dependent UC inflammation; ↑ risk of liver injury | Pathological effect | Trapecar et al. (2020) |
| Butyrate | Primiparous purebred female SD rats | Dietary intake | 1% sodium butyrate diet | ↑ Promotes maternal fat mobilization, ↑ fatty acid uptake and lipid accumulation in the liver of offspring | Pathological effect | Zhou et al. (2016) |
NAFLD = nonalcoholic fatty liver disease; NASH = non-alcoholic hepatitis; SCFA = short chain fatty acids; PPAR = peroxisome proliferator-activated receptor; PYY = peptide YY; UC = ulcerative colitis. ↑ represents increase. ↓ represents decrease.
The first is that SCFA affect hepatic steatosis. In mice, feeding a diet containing 5% SCFA (acetate, propionate or butyrate) tripled lipid oxidation, highly elevating the oxidative state leading to decreased liver lipid synthesis in a peroxisome proliferator-activated receptor γ (PPARγ)-dependent manner (den Besten et al., 2015). Gastric administration of butyrate in mice reduced lipid deposition in the liver, markedly reducing inflammation (Jin et al., 2015). Likewise, the supplementation of acetate protected rats from nicotine-induced excess liver lipid by inhibiting xanthine oxidase activity (Dangana et al., 2020). In the liver of rabbits, acetate boosted lipolysis and fatty acid oxidation, preventing lipid accumulation (Liu et al., 2019a). Acetate produced by gut microbiota could inhibit chylomicron secretion, resulting in reduced lipid flow into the circulatory system and alleviated the severity of NAFLD (Araujo et al., 2020). There is evidence that intestinal microbiota and NAFLD is interconnected. Thus, it was found that a reduced abundance of Bacteroidetes (producer of SCFA) was shown in NAFLD adults, compared with healthy controls (Mouzaki et al., 2013). Therefore, both SCFA and high-fiber diets could encourage the proliferation of Bacteroidetes bacteria, relieving liver disease (de Wit et al., 2012; Turnbaugh et al., 2006).
Secondly, another important piece of evidence relates to the ability of SCFA to reduce insulin resistance. Since the liver is the organ responsible for storing and metabolizing glucose, insulin resistance results from the liver's inefficient use of glucose (Zhang et al., 2022a). Short chain fatty acids in the liver could modulate hepatic insulin sensitivity via the AMPK signaling pathway. Additionally, SCFA could bind to GPR41 and GPR43 receptors in hepatocytes thereby suppressing AMPK-dependent gluconeogenesis (den Besten et al., 2015; Tan et al., 2021). Propionate inhibits gluconeogenesis via binding with hepatic GPR43 and activates the AMPK signaling pathway (Yoshida et al., 2019).
Thirdly, SCFA reverse intestinal permeability. According to in vivo findings, a high-fat diet induced hepatic steatosis, which was linked to higher intestinal permeability (Cani et al., 2007). Additionally, the high-fat diet model also revealed a relationship between NAFLD and intestinal permeability; specifically, the severity of steatohepatitis increased when colitis was induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS), as shown by the liver enzyme levels and the NAFLD Activity Score (NAS) (Mao et al., 2015). Given that SCFA can reverse intestinal permeability, it is possible to hypothesize that SCFA can prevent NAFLD by protecting the intestinal barrier.
However, the impact of SCFA on the liver also has two sides. It has been reported that microbiota-derived excessive acetate may encourage liver lipogenesis (Table 4). The excess SCFA produced by the gut microbiota is an extra energy source contributing to liver fat accumulation (Murugesan et al., 2018). Besides acetate, another study showed that higher propionate synthesis boosted hepatic lipogenesis (Gao et al., 2019). Using a multi-organ ex vivo model, it was discovered that SCFA could lead to intestinal barrier breakdown and liver injury in the process of acute T cell-mediated inflammation (Trapecar et al., 2020). Additionally, maternal butyrate supplementation during pregnancy and lactation may cause lipid accumulation in the liver of the offspring (Zhou et al., 2016).
Therefore, SCFA modulate hepatic steatosis, insulin resistance, and intestinal permeability through the gut-liver axis to regulate liver function. The negative effects include increased hepatic lipogenesis and inflammation, whereas favorable effects include halting the progression of NAFLD. Different from the brain and kidney, adding SCFA to the diet while simulating liver disease may have protective effects.
4.4. SCFA in the gut-lung axis
Although the signal of the gut-lung axis has a bi-directional response, the majority of the communication between the two organs occurs from the gut to the lung (Dang and Marsland, 2019). It has been reported that the impairment of lung function may be associated with the deterioration of the diversification of the intestinal microbiota (Chiu et al., 2022). Additionally, the presence of SCFA in the sputum further supported the relationship between the gut and the lung (Ghorbani et al., 2015). The two-sided effects of SCFA in the gut-lung axis are summarized in Table 5.
Table 5.
The studies on two-sided role of SCFA in host health via “gut-lung axis” establishing the connection between gut and lung.
| Item | Model/subject | Administration route | Dose and duration | Outcomes | Effects type | Reference |
|---|---|---|---|---|---|---|
| SCFA | Sputum samples from cystic fibrosis patients, A549, CFBE, corrCFBE or primary human bronchial epithelial cells | Cells incubated for 1 h with various SCFA prior to cytomix stimulation | SCFA: colonic lumen (10-50 mM) cystic fibrosis airways (0.1-5 mM) | ↓ Airway epithelium inflammatory responses, ↑iNOS and Pseudomonas aeruginosa growth | Beneficial effect | Ghorbani et al. (2015) |
| Butyrate | Adult female mice fed a low-fiber diet for 4 weeks then received butyrate | Oral gavage | Sodium butyrate (500 mM) for 2 weeks | Balancing innate and adaptive immunity; ↓ influenza infection; ↓ immune-associated pathology | Beneficial effect | Trompette et al. (2018) |
| SCFA | Differentiating human bronchial epithelial cells (16HBE) | 16HBE cells were stimulated with IL-4, IL-13 or house dust mite, then with SCFA | 10 mM acetate, 0.5 mM propionate or 1 mM butyrate | ↑ Lung immune defense effects; ↑ tight junction proteins expression in the airway epithelial barrier | Beneficial effect | Richards et al. (2021) |
| SCFA | Female C57BL/6 mice cigarette smoking-exposed emphysema model | Mice with standard AIN 76A diet, high-cellulose and high-pectin diet | – | ↓ Emphysema development; ↓ local and systemic inflammation | Beneficial effect | Jang et al. (2021) |
| Butyrate | Sprague–Dawley rat model of hypoxic pH, rat microvascular endothelial cells | SD rat: oral gavage; cells: cells were pretreated with butyrate for either 2 or 24 h, followed by either LPS or TNF-α stimulation |
SD rat: butyrate (220 and 2200 mg/kg intake); cells: butyrate (1 mM) | ↓ Accumulation of CD68+ in the lung alveoli; ↓ CD68+ and CD163+ pulmonary macrophages in lung interstitial | Beneficial effect | Karoor et al. (2021) |
| SCFA | C57BL/6 female mice treated with different fiber content diet | Oral gavage in drinking water before exposed to house dust mite extract | Sodium acetate or sodium propionate (200 mM) for 3 weeks | Shape the immunological environment in the lung; ↓ severity of allergic inflammation | Beneficial effect | Trompette et al. (2014) |
| SCFA | Vancomycin-treated mice, autoclaved water as control | Oral gavage | 40 mM butyrate, 67.5 mM acetate plus 25.9 mM propionate | ↓ Th2 responses to modulate the systemic immune response | Beneficial effect | Cait et al. (2018) |
| SCFA | Primary HLF and ASM cells with TNF-α (1 ng/mL) | Cells stimulated with SCFA for 24 or 96 h, with or without TNF-α for another 12 or 24 h | Acetate (0.5-25 mM), propionate (0.5-25 mM), butyrate (0.01-10 mM) | ↑ TNF-α-induced inflammatory responses; ↑ IL-6 and C-X-C motif chemokine ligand 8 release via activation of FFAR3 and p38 MAPK signaling | Pathological effect | Rufting et al. (2019) |
| SCFA | Peripheral blood mononuclear cells or neutrophils | Cells stimulated with LPS alone or in combination with various SCFA | SCFA (0-20 mM) for 18 h | ↑ Pro-inflammatory milieu in the lower genital tract | Pathological effect | Mirmonsef et al. (2012) |
SCFA = short chain fatty acids; iNOS = inducible nitric oxide synthase; IL-4 = interleukin-4; IL-13 = interleukin-13; LPS = lipopolysaccharide; TNF-α = tumor necrosis factor-α; HLF = human lung fibroblasts; IL-6 = interleukin-6; ASM = airway smooth muscle; FFAR3 = free fatty acid receptor 3; MAPK = mitogen-activated protein kinase kinases; LPS = lipopolysaccharides. ↑ represents increase. ↓ represents decrease.
It has been reported that 500 mM butyrate had an anti-inflammatory effect in the lung and prevented excessive airway infiltration by reducing lung macrophage- and monocyte-produced C-X-C motif chemokine ligand 1 (CXCL1) expression in female mice (Trompette et al., 2018). Besides the lung immune defense effects, SCFA (acetate, propionate, and butyrate) also protect the lung via maintaining the airway epithelial barrier by increasing the TJ protein expression in human bronchial epithelial 16HBE cells (Richards et al., 2021). Chronic obstructive pulmonary disease (COPD) is one of the most widespread respiratory diseases. The role of SCFA in the prevention and treatment of COPD cannot be overlooked (Kotlyarov, 2022). Emphysema is a typical feature of COPD, and experimental animal models have shown a connection between nutritional deficiency and alveolar tissue destruction leading to emphysema (Wright et al., 2008). A high-fiber diet attenuated emphysema-associated pathological changes in mice with cigarette-exposed emphysema, which could partly be explained by increased production of SCFA, including acetate, propionate, and butyrate (Jang et al., 2021). In addition, in a rat hypoxia model, butyrate treatment reduced the accumulation of CD68+ in the lung alveoli as well as CD68+ and CD163+ pulmonary macrophages in lung interstitial microvascular cells (Karoor et al., 2021). Emphysema has been linked to interalveolar septal vascularization, which could be brought on by apoptosis of alveolar epithelial and endothelial cells (Petrache and Petrusca, 2013). Short chain fatty acids may therefore prevent emphysema by protecting endothelial cells and maintaining pulmonary immune homeostasis (Kotlyarov, 2022).
Exacerbations are the other characteristic of COPD. The structural disturbance of the bronchial microbiota is connected to the worsening of COPD (Kotlyarov and Kotlyarova, 2021). The microorganism's colonization in the bronchi is necessary to maintain the immunological tension of the lung. The available data indicate that there are some links between the gut and lung microbiota (Madan et al., 2012). Studies have revealed that diet could alter the microflora in the intestine as well as the microflora in the respiratory tract (Madan et al., 2012; Trompette et al., 2014). Short chain fatty acids have been confirmed to directly affect microorganisms and change their virulence (Machado et al., 2021). High quantities of SCFA dramatically slowed down Pseudomonas aeruginosa development (Ghorbani et al., 2015).
It is worth noting that SCFA have both anti-inflammation and pro-inflammatory functions. Results from both in vivo and in vitro experiments have confirmed that SCFA (acetate, propionate, and butyrate) could inhibit Th2 responses by directly modulating T and dendritic cells, thus ameliorating the gut dysbiosis-driven lung inflammation (Cait et al., 2018). However, the pro-inflammatory effects of SCFA (acetate, propionate, and butyrate) were shown in human primary lung fibroblast cells and airway smooth muscle cells via the p38 MAPK signaling pathway (Rufting et al., 2019). The high concentrations of SCFA in cells increased the secretion of pro-inflammatory cytokines (Mirmonsef et al., 2012).
Therefore, a reduction in the severity and incidence of COPD is a favorable effect of SCFA on lung function, whereas the rise in lung inflammation is a negative effect. These effects are mainly achieved by the ability of SCFA to regulate pulmonary immune homeostasis, airway epithelial barrier and intestinal microbiota.
4.5. SCFA in gut-bone axis
For the body to operate properly overall, bones are essential. Bones are not only essential in supporting the body frame, but also for protecting important organs, acting as a mineral pool of calcium homeostasis, and providing a setting for the development of bone marrow, cytokines and growth factors (Medina-Gomez, 2018). Bone health is significantly impacted by intestinal microbiota and its metabolites. Recent studies have revealed a complex relationship between the gut and bone health (Ahlawat et al., 2021). The effects of SCFA on the gut-bone axis are shown in Table 6.
Table 6.
The studies on two-sided role of SCFA in host health via “gut-bone axis” establishing the connection between gut and bone.
| Item | Model/subject | Administration route | Dose and duration | Outcomes | Effects type | Reference |
|---|---|---|---|---|---|---|
| SCFA | 1-d-old Cherry Valley male ducks, saline as control | 0% RPS diets, SCFA in drinking water | SCFA (67.5 mM acetate, 38.8 mM propionate, 22.8 mM butyrate) for 14 d | ↓ Pro-inflammatory genes expression in both gut and bone marrow; ↓ osteoclastic bone resorption | Beneficial effect | Zhang et al., 2022a, Zhang et al., 2022b |
| SCFA | C57BL/6J female mice, ovariectomized mice, arthritis model | Drinking water | 150 mM acetate, propionate and butyrate for 8 weeks | ↑ Bone mass; ↓ postmenopausal and inflammation-induced bone loss; ↓ osteoclast differentiation and bone resorption | Beneficial effect | Lucas et al. (2018) |
| Butyrate | Rat bone marrow cells and RAW-D cell | RAW-D cells were preincubated with butyrate, then stimulated with TNF-α for 30 min | butyrate (1 mM) for 24 h |
↓ Osteoclast-specific signals; ↓ HDAC activity regulates the process of osteoclastogenesis | Beneficial effect | Rahman et al. (2003) |
| Acetate | Male C57Bl/6 and C57Bl6 GFP Het mice gout model induced by MSU | Mice were treated with acetate, butyrate, and propionate during the 5 d before MSU challenged | 150 mM acetate in drinking water, butyrate (50 mM) or propionate (25 mM) by oral gavage | ↑ Caspase-dependent neutrophil apoptosis, efferocytosis, and ↓ inflammation | Beneficial effect | Vieira et al. (2017) |
| Butyrate | Dietary-obese C57BL/6J mice |
Diet supplementation at 5% (wt/wt) in the high-fat diet | Sodium butyrate at 5 g/kg per day at the normal daily rate of calorie intake | ↓ Diet-induced insulin resistance in mouse; ↑ energy expenditure; ↑ mitochondria function | Beneficial effect | Gao et al. (2009) |
| Butyrate | Five-week-old male C57BL/6J mice | low-fat diet (LFD), high-fat diet (HFD) or HFD supplemented with butyrate | 5% butyrate (wt/wt) for 10 weeks | ↑ Insulin-sensitizing and anti-obesogenic; ↑ muscle mitochondrial function |
Beneficial effect | Henagan et al. (2015) |
| Acetate | Six-week-old C57BL/6 mice | Drinking water | 100, 200 or 300 mM sodium for 3 weeks | ↓ Colitis severity | Beneficial effect | Macia et al. (2015) |
| Butyrate | Four-week-old male db/db and db/m mice | – | 1 g/kg per day at the normal daily rate of calorie intake for 12 weeks | ↓ Muscle atrophy induced by diabetic nephropathy by activating the FFA2 receptor-mediated PI3K/Akt/mTOR pathway | Beneficial effect | Tang et al. (2022) |
SCFA = short chain fatty acids; TNF-α = tumor necrosis factor-α; RPS = raw potato starch; HDAC = histone deacetylases; MSU = monosodium urate; FFA = free fatty acid; PI3K/Akt/mTOR = phosphatidylinositol-3-kinase/Akt/mammalian target of rapamycin. ↑ represents increase. ↓ represents decrease.
The results of intestinal microbiota and microCT analysis showed that the positive effect of raw potato starch on bone mass might be related to the higher proportion of Firmicutes and the production of SCFA in the cecum, which led to the reduction of the expression of the pro-inflammatory genes in the intestine and bone marrow, thereby inhibiting cytokine-mediated osteoclast bone resorption in Cherry Valley male ducks (Zhang et al., 2022a). Studies have shown a connection between bone mineral density and gut microbiota, showing that Crohn's disease and obesity increase the risk of fractures (Villa et al., 2017). Hence it may be postulated that SCFA could protect bone via maintaining bone homeostasis and skeletal muscle function.
The two main components of bone homeostasis, osteoclasts and osteoblasts, act on bone resorption and formation, respectively. Reduced bone density and an imbalance in bone homeostasis are characterized by chronic inflammatory disorders like rheumatoid arthritis, which is due to increased bone resorption brought on by the activation of osteoclasts. A reported study showed that SCFA, especially propionate and butyrate, prevented the development of osteoclast precursor cells in bone marrow by inhibiting the receptor activator of nuclear factor-κB ligand (RANKL) signaling, without affecting osteoblasts (Lucas et al., 2018). In addition, it has been demonstrated that butyrate inhibited histone deacetylase (HDAC) and its downstream genes, hence suppressing the formation of osteoclasts in rat bone marrow cells and RAW-D cells (Rahman et al., 2003).
Systemic autoimmune diseases, including rheumatoid arthritis, osteoarthritis and gout are characterized by the progressive damage of bone and cartilage as well as chronic joint inflammation. Recent studies have demonstrated that gut microbiota and their metabolites are linked to these autoimmune diseases (Abdollahi-Roodsaz et al., 2016; Chang et al., 2001; Kau et al., 2011). The increased fiber intake has been confirmed to have positive effects on alleviating gout development, which could be explained by the production of SCFA (Lyu et al., 2003). Additionally, a study showed that a high-fiber diet and acetate consumption reduced gout-related inflammation induced by monosodium urate crystals. This relationship is related to the acetate's capacity to trigger neutrophil apoptosis (Vieira et al., 2017). The preventive benefits of high fiber diets and SCFA also shown in the experimental mice with arthritis (Lucas et al., 2018).
Regarding the advantageous effects of butyrate administration on skeletal muscle, the study discovered that C57BL/6J mice supplemented with butyrate showed increased gene expression necessary for beneficial mitochondrial adaptation, including peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α, peroxisome proliferators-activated receptors δ (PPARδ), and carnitine palmitoyltransferase (CPT)1b, and also prevented the incomplete oxidation of skeletal muscle caused by high-fat diets (Gao et al., 2009; Henagan et al., 2015). Additionally, butyrate decreased the skeletal muscle atrophy induced by diabetic nephropathy through phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway in mice (Tang et al., 2022). Therefore, by enhancing the skeletal muscle oxidation capacity, butyrate could partially prevent obesity and insulin resistance.
Again SCFA appear to have both beneficial and pathological impacts on bone homeostasis. The results on germ-free mice indicated that insufficient SCFA levels or GPR43 receptor deficiency could lead to defects in inflammasome assembly, which impaired gout inflammation of the knee (Vieira et al., 2015). Similarly, a study demonstrated that the lack of microbiota and SCFA damaged the inflammasome assembly in epithelial cells, impairing their capacity to defend against harmful stimuli (Macia et al., 2015).
In summary, SCFA in the gut-bone axis regulate bone function by modulating intestinal microbiota and bone homeostasis between osteoclasts and osteoblasts. Short chain fatty acids have a dual effect on bone function that includes both a reduction in the severity of rheumatoid arthritis, osteoarthritis, gout, and skeletal muscle atrophy (positive regulation) as well as an increase in gout inflammation (negative regulation). Apart from butyrate, the most studies SCFA, in vivo studies have proved that acetate is beneficial for host bone health with preventive and therapeutic effects.
4.6. SCFA in the gut-cardiovascular axis
There are many risk factors for cardiovascular diseases, including diet. Studies have revealed that intestinal microbiota are an extragenomic contributor to cardiovascular diseases (Tuohy et al., 2014). The majority of risk factors for cardiovascular diseases have the potential to result in microecological disorders linked to intestinal inflammation and the reduction of intestinal barrier integrity, which would increase the number of microorganisms and their metabolites in the circulatory system and speed up the onset of cardiovascular diseases (Battson et al., 2018). Additionally, people with intestinal problems have a higher risk of coronary heart disease even in the absence of usual risk factors (Rogler and Rosano, 2014). Therefore, evidence indicates that there is a bi-directional communication network between the intestine and cardiovascular, namely, the “gut-cardiovascular axis” (Table 7).
Table 7.
The studies on two-sided role of SCFA in host health via “gut-cardiovascular axis” establishing the connection between gut and cardiovascular.
| Item | Model/subject | Administration route | Dose and duration | Outcomes | Effects type | Reference |
|---|---|---|---|---|---|---|
| Free fatty acid | 110 patients with or without heart failure | Metabolomics on blood from artery, coronary sinus, and femoral vein | – | Uptake of acetate was directly proportional to circulating concentrations in both heart | Beneficial effect | Murashige et al. (2020) |
| Butyrate | Cardiac hypertrophy Sprague–Dawley rats, sham operated as control | Hearts perfused | A mix of 0.5 mM butyrate and 0.5 mM 3-hydroxybutyrate | ↑ Oxidization in failing hearts | Beneficial effect | Carley et al. (2021) |
| Acetate | Long Evans rats model of OSA, sham rats receiving PBS as control | Chronic infused acetate into the cecum | 20 μmol/(kg ▪min) and 2 weeks | ↑ Cecal acetate concentrations; ↓ OSA on the microbiota, gut, brain, and blood pressure | Beneficial effect | Ganesh et al. (2018) |
| Acetate | Male C57BL/6 mice hypertensive model | Drinking water | 200 mM magnesium acetate and 3 weeks | Change the gut microbiota; ↓ development of hypertension and heart failure mice | Beneficial effect | Marques et al. (2017) |
| Propionate | Wild-type and Apoe−/− mice | Drinking water | 200 mM | ↓ Cardiac hypertrophy, fibrosis, vascular dysfunction, and hypertension | Beneficial effect | Bartolomaeus et al. (2019) |
| ①SCFA ②Sodium acetate |
①Male and female C57BL/6J mice ②Male C57BL/6J male mice |
①Intraperitoneal injection ②Heart perfused |
①1 g/kg ②1 M |
①↓Mean arterial pressure, HR, and cardiac contractility. ②Acetate ↓ HR after extended exposure |
Beneficial effect | Poll et al. (2021) |
| SCFA | C57BL/6 GPR41 WT male mice and GPR41 KO mice | – | – | ↓ Hypotensive response | Beneficial effect | Natarajan et al. (2016) |
| Butyrate | 14–16 weeks old wistar rats | Administration into the colon | 1.4 (n = 5), 2.8 (n = 5) and 5.6 mmol/kg (n = 5) | ↑ Hypotensive effect, which seems to be mediated by the colon afferent nervous signaling and GPR41/43 receptors | Beneficial effect | Onyszkiewicz et al. (2019) |
| Propionate | ①16 weeks female C57BL/6, Apoe−/− mice ②62 individuals with baseline LDL) cholesterol levels>115 mg/dL |
①Oral gavage ②Oral |
①150 mM calcium propionate and four weeks ②Propionate (500 mg) twice daily for 8 weeks |
①↓ High-fat diet-induced hypercholesterolaemia and atherosclerosis in Apoe−/− mice ②↓ Serum LDL and total cholesterol in hypercholesterolaemic humans |
Beneficial effect | Haghikia et al. (2022) |
| Butyrate | Twenty 6-week-old male apoprotein E deficient (Apoe−/−) mice | Diet | 1% butyrate-supplemented diet (butyrate) for 10 weeks | ↓ Inflammation and activation of NF-κB and atherosclerosis | Beneficial effect | Aguilar et al. (2014) |
SCFA = short chain fatty acids; OSA = obstructive sleep apnea; PBS = phosphate buffer saline; HR = heart rate; GPR = G-protein-coupled receptor; WT = wild type; KO = knock out; LDL = low-density lipoprotein; NF-κB = nuclear factor-kappaB. ↑ represents increase. ↓ represents decrease.
Increasing data indicate that the pathophysiology of heart failure implicates the stomach. The available evidence shows that SCFA are quickly taken and oxidized by the heart to provide energy for the heart and prevent heart failure (Palm et al., 2022). For example, due to factors such as decreased microbiota diversity, fewer butyrate-producing strains, and the fact that SCFA are mainly produced in vivo by microorganisms, patients with heart failure have a limited capacity to create SCFA overall (Jin et al., 2021). Acetate extraction in the heart failure cohort increased by about 20%, according to a study showing that although the contribution of SCFA to cardiac ATP synthesis was relatively low. This result suggested that increasing SCFA levels in circulating system may benefit energy production in heart failure patients (Murashige et al., 2020). Surprisingly, it has been reported that in healthy and failing hearts, ATP synthesis from butyrate was significantly higher than that from ketone bodies, indicating that SCFA are more effective energy generators (Carley et al., 2021).
Hypertension is considered the main risk factor of cardiovascular disease, which is influenced by genetics and environment. The high prevalence rate of hypertension has made it a major global health challenge (Mills et al., 2020). Research has revealed that SCFA produced by dietary fiber under anaerobic fermentation of gut microbiota was involved in the regulation of blood pressure (Forkosh and Ilan, 2019; Yang et al., 2020a). According to a review, blood pressure can be impacted by even a very low concentration of SCFA in the gut (Yang et al., 2020a). Acetate supplementation has been confirmed to have beneficial effects on reducing the development of hypertension in obstructive sleep apnea and a hypertensive mice model (Ganesh et al., 2018; Marques et al., 2017; Poll et al., 2021). Propionate intervention may lower blood pressure by regulating Treg cells to reduce systemic inflammation in mice (Bartolomaeus et al., 2019). The possibility of GPR receptors in vascular tissues as well as olfactory receptor 78 as the mechanism through which propionate reduces blood pressure has been considered (Natarajan et al., 2016). G-protein coupled receptor receptors are thought to be vital effectors in regulating blood pressure induced by SCFA (Miyamoto et al., 2016; Zhang et al., 2022b). Exogenous SCFA were found to lower blood pressure, and GPR41/43 inhibitors could attenuate these effects (Onyszkiewicz et al., 2020). The GPR41/43 receptors and colonic vagus nerve signaling were engaged in butyrate's ability to inhibit the onset of hypertension (Onyszkiewicz et al., 2019). Compared with wild-type rats, salt-sensitive Gper1−/− rats had lower blood pressure and fewer intestinal illnesses, demonstrating that GPER1, one of the GPR receptors, was associated with the regulation of blood pressure (Waghulde et al., 2018). As a bi-directional regulator, hypertension could also disrupt the gut microbiota structure by diminishing its abundance and diversity, thus lowering the concentration of SCFA (Yang et al., 2020a).
Currently, atherosclerosis is considered a chronic inflammatory disease occurring in the large arteries. There is increasing evidence that SCFA may contribute to atherosclerosis. However, different SCFA have various functions in atherosclerosis (Yao et al., 2022). According to the literature, taking a propionate supplement could alleviate atherosclerosis by regulating gut immune system and vascular inflammation (Aguilar et al., 2014; Bartolomaeus et al., 2019; Haghikia et al., 2022). Instead, it is possible to use acetate as the substrate for cholesterol to promote the synthesis of cholesterol (Vourakis et al., 2021).
In summary, the gut-cardiovascular axis is affected by the effects of SCFA on energy production and vascular inflammation, which, in turn, affects cardiovascular function. These outcomes have both beneficial effects (lower incidence of heart failure, hypertension, and atherosclerosis) and pathological effects (induced cholesterol synthesis).
5. Conclusions and perspectives
In conclusion, humans can not only obtain SCFA through microbial fermentation of non-digestible carbohydrates, but also directly through food, especially milk. In addition, the function of the body, including that of the intestine, brain, kidney, liver, lung, and other organs, is significantly influenced by both endogenous synthesis and exogenous supply of SCFA. Through the “gut-organ axes”, SCFA regulate the host's health as a double-edged sword, acting in both positive and negative manner, with the positive effects being more pronounced.
The fundamental processes causing the paradoxical action of SCFA remain poorly understood. Additionally, both their preventive and therapeutic benefits show positive impacts. Several “gut-organ axes” are discussed in the current paper with limitations. For instance, it is not known yet if SCFA affect the “gut-reproductive axis” in any way. What is significantly more crucial is that it is essential to understand how the several “gut-organ axes” that SCFA produce interact with one another. To fully comprehend how SCFA affect organism health and gain new insights into the control and prevention of the onset of diseases, much needs to be done in the future. Additionally, as for the ruminants, an increasing body of evidence suggests that the supplementation of butyrate enhances the function of the rumen, however, the current knowledge regarding the communication between the intestine and other organs via SCFA still awaits elucidation.
Author contributions
Yanan Gao: Investigation, Resources, Writing-original draft; Qianqian Yao: Writing-review & editing; Lu Meng: Writing-review & editing; Jiaqi Wang: Conceptualization, Funding acquisition; Nan Zheng: Conceptualization, Funding acquisition.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by the National Key R&D Program of China (2022YFD1600104), the earmarked fund for CARS (CARS-36), the Agricultural Science and Technology Innovation Program (ASTIP-IAS12).
Footnotes
Peer review under the responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdollahi-Roodsaz S., Abramson S.B., Scher J.U. The metabolic role of the gut microbiota in health and rheumatic disease: mechanisms and interventions. Nat Rev Rheumatol. 2016;12:446–455. doi: 10.1038/nrrheum.2016.68. [DOI] [PubMed] [Google Scholar]
- Aguilar E.C., Leonel A.J., Teixeira L.G., Silva A.R., Silva J.F., Pelaez J.M., Capettini L.S., Lemos V.S., Santos R.A., Alvarez-Leite J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing nfkappab activation. Nutr Metabol Cardiovasc Dis. 2014;24:606–613. doi: 10.1016/j.numecd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Ahlawat S., Asha Sharma KK. Gut-organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2021;72:636–668. doi: 10.1111/lam.13333. [DOI] [PubMed] [Google Scholar]
- Alexander C., Swanson K.S., Fahey G.C., Garleb K.A. Perspective: physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv Nutr. 2019;10:576–589. doi: 10.1093/advances/nmz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Oliveira V., Amano M.T., Correa-Costa M., Castoldi A., Felizardo R.J.F., De Almeida D.C., Bassi E.J., Moraes-Vieira P.M., Hiyane M.I., Rodas A.C.D., Peron J.P.S., Aguiar C.F., Reis M.A., Ribeiro W.R., Valduga C.J., Curi R., Vinolo MaR., Ferreira C.M., Camara N.O.S. Gut bacteria products prevent aki induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26:1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo J.R., Tazi A., Burlen-Defranoux O., Vichier-Guerre S., Nigro G., Licandro H., Demignot S., Sansonetti P.J. Fermentation products of commensal bacteria alter enterocyte lipid metabolism. Cell Host Microbe. 2020;27:358–375. doi: 10.1016/j.chom.2020.01.028. [DOI] [PubMed] [Google Scholar]
- Bai Z.G., Zhang Z.T., Ye Y.J., Wang S. Sodium butyrate induces differentiation of gastric cancer cells to intestinal cells via the pten/phosphoinositide 3-kinase pathway. Cell Biol Int. 2010;34:1141–1145. doi: 10.1042/CBI20090481. [DOI] [PubMed] [Google Scholar]
- Bartolomaeus H., Balogh A., Yakoub M., Homann S., Marko L., Hoges S., Tsvetkov D., Krannich A., Wundersitz S., Avery E.G., Haase N., Kraker K., Hering L., Maase M., Kusche-Vihrog K., Grandoch M., Fielitz J., Kempa S., Gollasch M., Zhumadilov Z., Kozhakhmetov S., Kushugulova A., Eckardt K.U., Dechend R., Rump L.C., Forslund S.K., Muller D.N., Stegbauer J., Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battson M.L., Lee D.M., Weir T.L., Gentile C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. JNB (J Nutr Biochem) 2018;56:1–15. doi: 10.1016/j.jnutbio.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Behary J., Amorim N., Jiang X.T., Raposo A., Gong L., Mcgovern E., Ibrahim R., Chu F., Stephens C., Jebeili H., Fragomeli V., Koay Y.C., Jackson M., O'sullivan J., Weltman M., Mccaughan G., El-Omar E., Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:14. doi: 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2016;89:388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- Braden B., Adams S., Duan L.P., Orth K.H., Maul F.D., Lembcke B., Hor G., Caspary W.F. The c-13 acetate breath test accurately reflects gastric-emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–1055. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., Gulyas B., Halldin C., Hultenby K., Nilsson H., Hebert H., Volpe B.T., Diamond B., Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:11. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning J., Chapp A., Kaurala G.A., Wang R.J., Techtmann S., Chen Q.H. Gut microbiota and short chain fatty acids: influence on the autonomic nervous system. Neurosci Bull. 2020;36:91–95. doi: 10.1007/s12264-019-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger-Van Paassen N., Vincent A., Puiman P.J., Van Der Sluis M., Bouma J., Boehm G., Van Goudoever J.B., Van Seuningen I., Renes I.B. Regulation of the intestinal mucin muc 2 expression by short chain fatty acids: implications for epithelial protection. Faseb J. 2009;23:1. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- Cait A., Hughes M.R., Antignano F., Cait J., Dimitriu P.A., Maas K.R., Reynolds L.A., Hacker L., Mohr J., Finlay B.B., Zaph C., Mcnagny K.M., Mohn W.W. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11:785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Carley A.N., Maurya S.K., Fasano M., Wang Y., Selzman C.H., Drakos S.G., Lewandowski E.D. Short-chain fatty acids outpace ketone oxidation in the failing heart. Circulation. 2021;143:1797–1808. doi: 10.1161/CIRCULATIONAHA.120.052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavini S., Iraira S., Siurana A., Foskolos A., Ferret A., Calsamiglia S. Effect of sodium butyrate administered in the concentrate on rumen development and productive performance of lambs in intensive production system during the suckling and the fattening periods. Small Rumin Res. 2015;123:212–217. [Google Scholar]
- Chang H.Y., Pan W.H., Yeh W.T., Tsai K.S. Hyperuricemia and gout in taiwan: results from the nutritional and health survey in taiwan (1993-96) J Rheumatol. 2001;28:1640–1646. [PubMed] [Google Scholar]
- Chen G.X., Ran X., Li B., Li Y.H., He D.W., Huang B.X., Fu S.P., Liu J.X., Wang W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a tnbs-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.Z., Xu Y., Wu P., Zhou H., Lasanajak Y., Fang Y.Y., Tang L., Ye L., Li X., Cai Z., Zhao J. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:12. doi: 10.1016/j.phrs.2019.104403. [DOI] [PubMed] [Google Scholar]
- Chen Y.J., Zhou X.H., Han B., Li S.M., Xu T., Yi H.X., Liu P., Zhang L.W., Li Y.Y., Jiang S.L., Pan J.C., Ma C.H., Wang B.C. Composition analysis of fatty acids and stereo-distribution of triglycerides in human milk from three regions of China. Food Res Int. 2020;133:8. doi: 10.1016/j.foodres.2020.109196. [DOI] [PubMed] [Google Scholar]
- Chiu Y.C., Lee S.W., Liu C.W., Lan T.Y., Wu L.S.H. Relationship between gut microbiota and lung function decline in patients with chronic obstructive pulmonary disease: a 1-year follow-up study. Respir Res. 2022;23:11. doi: 10.1186/s12931-022-01928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong J., Zhou P., Zhang R.Y. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients. 2022;14:15. doi: 10.3390/nu14091977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Pereira J.S., Rea K., Nolan Y.M., O'leary O.F., Dinan T.G., Cryan J.F. In: Fiske S.T., editor. vol. 71. Annual Reviews; Palo Alto: 2020. Depression's unholy trinity: dysregulated stress, immunity, and the microbiome; pp. 49–78. (Annual review of psychology). [DOI] [PubMed] [Google Scholar]
- Dai X.Y., Yuan T.L., Zhang X.H., Zhou Q., Bi H.Y., Yu R.Q., Wei W., Wang X.G. Short-chain fatty acid (scfa) and medium-chain fatty acid (mcfa) concentrations in human milk consumed by infants born at different gestational ages and the variations in concentration during lactation stages. Food Funct. 2020;11:1869–1880. doi: 10.1039/c9fo02595b. [DOI] [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Dang A.T., Marsland B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- Dangana E.O., Omolekulo T.E., Areola E.D., Olaniyi K.S., Soladoye A.O., Olatunji L.A. Sodium acetate protects against nicotine-induced excess hepatic lipid in male rats by suppressing xanthine oxidase activity. Chem Biol Interact. 2020;316:10. doi: 10.1016/j.cbi.2019.108929. [DOI] [PubMed] [Google Scholar]
- De Vos W.M., Tilg H., Van Hul M., Cani P.D. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit N., Derrien M., Bosch-Vermeulen H., Oosterink E., Keshtkar S., Duval C., De Vogel-Van Den Bosch J., Kleerebezem M., Muller M., Van Der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- Den Besten G., Bleeker A., Gerding A., Van Eunen K., Havinga R., Van Dijk T.H., Oosterveer M.H., Jonker J.W., Groen A.K., Reijngoud D.J., Bakker B.M. Short-chain fatty acids protect against high-fat diet-induced obesity via a ppargamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- Den Besten G., Van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. JLR (J Lipid Res) 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Jiao A.R., Yu B., Mao X.B., Chen D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes and Nutrition. 2019;14:16. doi: 10.1186/s12263-019-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme a (coa): acetate-coa transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamin E.E., Masclee A.A., Dekker J., Pieters H.J., Jonkers D.M. Short-chain fatty acids activate amp-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in caco-2 cell monolayers. J Nutr. 2013;143:1872–1881. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- Fachi J.L., Felipe J.D., Pral L.P., Da Silva B.K., Correa R.O., De Andrade M.C.P., Da Fonseca D.M., Basso P.J., Camara N.O.S., Souza E., Martins F.D., Guima S.E.S., Thomas A.M., Setubal J.C., Magalhaes Y.T., Forti F.L., Candreva T., Rodrigues H.G., De Jesus M.B., Consonni S.R., Farias A.D., Varga-Weisz P., Vinolo MaR. Butyrate protects mice from clostridium difficile-induced colitis through an hif-1-dependent mechanism. Cell Rep. 2019;27:750–761. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Felizardo R.J.F., Watanabe I.K.M., Dardi P., Rossoni L.V., NOS Camara. The interplay among gut microbiota, hypertension and kidney diseases: the role of short-chain fatty acids. Pharmacol Res. 2019;141:366–377. doi: 10.1016/j.phrs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Forkosh E., Ilan Y. The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart. 2019;6:6. doi: 10.1136/openhrt-2018-000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroutan A., Guo A.C., Vazquez-Fresno R., Lipfert M., Zhang L., Zheng J.M., Badran H., Budinski Z., Mandal R., Ametaj B.N., Wishart D.S. Chemical composition of commercial cow's milk. J Agric Food Chem. 2019;67:4897–4914. doi: 10.1021/acs.jafc.9b00204. [DOI] [PubMed] [Google Scholar]
- Foster J.A., Neufeld KaM. Gut-brain: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., Anastasovska J., Ghourab S., Hankir M., Zhang S., Carling D., Swann J.R., Gibson G., Viardot A., Morrison D., Thomas E.L., Bell J.D. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:11. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel G., Aschenbach J.R., Muller F. Transfer of energy substrates across the ruminal epithelium: implications and limitations. Anim Health Res Rev. 2002;3:15–30. doi: 10.1079/ahrr200237. [DOI] [PubMed] [Google Scholar]
- Ganesh B.P., Nelson J.W., Eskew J.R., Ganesan A., Ajami N.J., Petrosino J.F., Bryan R.M., Durgan D.J. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72:1141–1150. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Wang C., Yu L., Sheng T., Wu Z., Wang X., Zhang D., Lin Y., Gong Y. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6769789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.A., Meng L., Liu H.M., Wang J.Q., Zheng N. The compromised intestinal barrier induced by mycotoxins. Toxins. 2020;12:42. doi: 10.3390/toxins12100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.G., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J.P. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudier E., Jarry A., Blottiere H.M., De Coppet P., Buisine M.P., Aubert J.P., Laboisse C., Cherbut C., Hoebler C. Butyrate specifically modulates muc gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- Gaudier E., Rival M., Buisine M.P., Robineau I., Hoebler C. Butyrate enemas upregulate muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res. 2009;58:111–119. doi: 10.33549/physiolres.931271. [DOI] [PubMed] [Google Scholar]
- Ghorbani P., Santhakumar P., Hu Q.D., Djiadeu P., Wolever T.M.S., Palaniyar N., Grasemann H. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J. 2015;46:1033–1045. doi: 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- Górka P., Kowalski Z.M., Pietrzak P., Kotunia A., Kiljanczyk R., Flaga J., Holst J.J., Guilloteau P., Zabielski R. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J Physiol Pharmacol. 2009;60:47–53. [PubMed] [Google Scholar]
- Górka P., Kowalski Z.M., Pietrzak P., Kotunia A., Jagusiak W., Holst J.J., Guilloteau P., Zabielski R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J Dairy Sci. 2011;94:5578–5588. doi: 10.3168/jds.2011-4166. [DOI] [PubMed] [Google Scholar]
- Górka P., Kowalski Z.M., Pietrzak P., Kotunia A., Jagusiak W., Zabielski R. Is rumen development in newborn calves affected by different liquid feeds and small intestine development? J Dairy Sci. 2011;94:3002–3013. doi: 10.3168/jds.2010-3499. [DOI] [PubMed] [Google Scholar]
- Goswami C., Iwasaki Y., Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent. JNB (J Nutr Biochem) 2018;57:130–135. doi: 10.1016/j.jnutbio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Haghikia A., Zimmermann F., Schumann P., Jasina A., Roessler J., Schmidt D., Heinze P., Kaisler J., Nageswaran V., Aigner A., Ceglarek U., Cineus R., Hegazy A.N., Van Der Vorst E.P.C., Doring Y., Strauch C.M., Nemet I., Tremaroli V., Dwibedi C., Krankel N., Leistner D.M., Heimesaat M.M., Bereswill S., Rauch G., Seeland U., Soehnlein O., Muller D.N., Gold R., Backhed F., Hazen S.L., Haghikia A., Landmesser U. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. 2022;43:518–533. doi: 10.1093/eurheartj/ehab644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henagan T.M., Stefanska B., Fang Z.D., Navard A.M., Ye J.P., Lenard N.R., Devarshi P.P. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br J Pharmacol. 2015;172:2782–2798. doi: 10.1111/bph.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry P.O., Van Dam R.M., Bukkems S., Mckeown D.W., Parks R.W., Preston T., Dejong C.H.C., Garden O.J., Fearon K.C.H., Grp E. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198–1206. doi: 10.1002/bjs.7120. [DOI] [PubMed] [Google Scholar]
- Hiltz R.L., Laarman A.H. Effect of butyrate on passive transfer of immunity in dairy calves. J Dairy Sci. 2019;102:4190–4197. doi: 10.3168/jds.2018-15555. [DOI] [PubMed] [Google Scholar]
- Hoyles L., Snelling T., Umlai U.K., Nicholson J.K., Carding S.R., Glen R.C., Mcarthur S. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. 2018;6:13. doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Guo H.L., Deng X., Zhu T.T., Xiong J.F., Xu Y.H., Xu Y. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp Clin Endocrinol Diabetes. 2017;125:98–105. doi: 10.1055/s-0042-121493. [DOI] [PubMed] [Google Scholar]
- Huang X.Z., Li Z.R., Zhu L.B., Huang H.Y., Hou L.L., Lin J. Inhibition of p38 mitogen-activated protein kinase attenuates butyrate-induced intestinal barrier impairment in a caco-2 cell monolayer model. J Pediatr Gastroenterol Nutr. 2014;59:264–269. doi: 10.1097/MPG.0000000000000369. [DOI] [PubMed] [Google Scholar]
- Hungate R.E. Elsevier; Amsterdam, The Netherlands: 1966. The rumen and its microbes. [Google Scholar]
- Huuskonen J., Suuronen T., Nuutinen T., Kyrylenko S., Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141:874–880. doi: 10.1038/sj.bjp.0705682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraporda C., Errea A., Romanin D.E., Cayet D., Pereyra E., Pignataro O., Sirard J.C., Garrote G.L., Abraham A.G., Rumbo M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220:1161–1169. doi: 10.1016/j.imbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Jang Y.O., Kim O.H., Kim S.J., Lee S.H., Yun S., Lim S.E., Yoo H.J., Shin Y., Lee S.W. High-fiber diets attenuate emphysema development via modulation of gut microbiota and metabolism. Sci Rep. 2021;11:11. doi: 10.1038/s41598-021-86404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiminez J.A., Uwiera T.C., Abbott D.W., Uwiera R.R.E., Inglis G.D. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with citrobacter rodentium. mSphere. 2017;2:21. doi: 10.1128/mSphere.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C.J., Sellmann C., Engstler A.J., Ziegenhardt D., Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (nash) Br J Nutr. 2015;114:1745–1755. doi: 10.1017/S0007114515003621. [DOI] [PubMed] [Google Scholar]
- Jin L., Shi X.M., Yang J., Zhao Y.Y., Xue L.X., Xu L., Cai J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein & Cell. 2021;12:346–359. doi: 10.1007/s13238-020-00785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoor V., Strassheim D., Sullivan T., Verin A., Umapathy N.S., Dempsey E.C., Frank D.N., Stenmark K.R., Gerasimovskaya E. The short-chain fatty acid butyrate attenuates pulmonary vascular remodeling and inflammation in hypoxia-induced pulmonary hypertension. Int J Mol Sci. 2021;22:19. doi: 10.3390/ijms22189916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Sato K., Chida H., Roh S.G., Ohwada S., Sato S., Guilloteau P., Katoh K. Effects of Na-butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J Endocrinol. 2011;211:241–248. doi: 10.1530/JOE-11-0299. [DOI] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer D.A., Piccolo B.D., Vaziri N.D., Liu S.M., Lau W.L., Khazaeli M., Nazertehrani S., Moore M.E., Marco M.L., Martin R.J., Adams S.H. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Ren Physiol. 2016;310:F857–F871. doi: 10.1152/ajprenal.00513.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kotlyarov S. Role of short-chain fatty acids produced by gut microbiota in innate lung immunity and pathogenesis of the heterogeneous course of chronic obstructive pulmonary disease. Int J Mol Sci. 2022;23:23. doi: 10.3390/ijms23094768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyarov S., Kotlyarova A. Molecular mechanisms of lipid metabolism disorders in infectious exacerbations of chronic obstructive pulmonary disease. Int J Mol Sci. 2021;22:25. doi: 10.3390/ijms22147634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzinski J., Zitnan R., Albrecht E., Viergutz T., Schweigel-Röntgen M. Modulation of v H+-ATPase is part of the functional adaptation of sheep rumen epithelium to high-energy diet. Am J Physiol Regul Integr Comp Physiol. 2012;303:R909–R920. doi: 10.1152/ajpregu.00597.2011. 2012. [DOI] [PubMed] [Google Scholar]
- Lee C., Kim B.G., Kim J.H., Chun J., Im J.P., Kim J.S. Sodium butyrate inhibits the nf-kappa b signaling pathway and histone deacetylation, and attenuates experimental colitis in an il-10 independent manner. Int Immunopharm. 2017;51:47–56. doi: 10.1016/j.intimp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Lee J., D'aigle J., Atadja L., Quaicoe V., Honarpisheh P., Ganesh B.P., Hassan A., Graf J., Petrosino J., Putluri N., Zhu L., Durgan D.J., Bryan R.M., Mccullough L.D., Venna V.R. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453–465. doi: 10.1161/CIRCRESAHA.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Venna V.R., Durgan D.J., Shi H.N., Hudobenko J., Putluri N., Petrosino J., Mccullough L.D., Bryan R.M. Young versus aged microbiota transplants to germ-free mice: increased short-chain fatty acids and improved cognitive performance. Gut Microb. 2020;12:14. doi: 10.1080/19490976.2020.1814107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in nafld. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- Li Y.J., Chen X.C., Kwan T.K., Loh Y.W., Singer J., Liu Y.Z., Ma J., Tan J., Macia L., Mackay C.R., Chadban S.J., Wu H.L. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid?Mediated activation of g protein?Coupled receptors gpr43 and gpr109a. J Am Soc Nephrol. 2020;31:1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yi C.X., Katiraei S., Kooijman S., Zhou E.C., Chung C.K., Gao Y.Q., Van Den Heuvel J.K., Meijer O.C., Berbee J.F.P., Heijink M., Giera M., Van Dijk K.W., Groen A.K., Rensen P.C.N., Wang Y.A. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang J., He T., Becker S., Zhang G.L., Li D.F., Ma X. Butyrate: a double-edged sword for health? Adv Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.M., Jin Y.J., Ye Y.L., Tang Y.H., Dai S.S., Li M.F., Zhao G.J., Hong G.L., Lu Z.Q. The neuroprotective effect of short chain fatty acids against sepsis-associated encephalopathy in mice. Front Immunol. 2021;12:13. doi: 10.3389/fimmu.2021.626894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Fu C.Y., Li F.C. Acetate affects the process of lipid metabolism in rabbit liver, skeletal muscle and adipose tissue. Animals. 2019;9:12. doi: 10.3390/ani9100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.M., Li E.Y., Sun Z.Y., Fu D.J., Duan G.Q., Jiang M.M., Yu Y., Mei L., Yang P.C., Tang Y.C., Zheng P.Y. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9:9. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jin X., Hong H.G., Xiang L.Y., Jiang Q.Y., Ma Y.C., Chen Z.D., Cheng L., Jian Z.Y., Wei Z.T., Ai J.Z., Qi S.Q., Sun Q., Li H., Li Y., Wang K.J. The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. Faseb J. 2020;34:11200–11214. doi: 10.1096/fj.202000786R. [DOI] [PubMed] [Google Scholar]
- Loftus E.V., Harewood G.C., Loftus C.G., Tremaine W.J., Harmsen W.S., Zinsmeister A.R., Jewell D.A., Sandborn W.J. Psc-ibd: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Sanyal A.J. The global nafld epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- Lucas S., Omata Y., Hofmann J., Bottcher M., Iljazovic A., Sarter K., Albrecht O., Schulz O., Krishnacoumar B., Kronke G., Herrmann M., Mougiakakos D., Strowig T., Schett G., Zaiss M.M. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9:10. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luise D., Motta V., Salvarani C., Chiappelli M., Fusco L., Bertocchi M., Mazzoni M., Maiorano G., Costa L.N., Van Milgen J., Bosi P., Trevisi P. Long-term administration of formic acid to weaners: influence on intestinal microbiota, immunity parameters and growth performance. Anim Feed Sci Technol. 2017;232:160–168. [Google Scholar]
- Lyu L.C., Hsu C.Y., Yeh C.Y., Lee M.S., Huang S.H., Chen C.L. A case-control study of the association of diet and obesity with gout in taiwan. Am J Clin Nutr. 2003;78:690–701. doi: 10.1093/ajcn/78.4.690. [DOI] [PubMed] [Google Scholar]
- Ma J.Y., Piao X.S., Mahfuz S., Long S.F., Wang J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Animal Nutrition. 2022;9:159–174. doi: 10.1016/j.aninu.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Fan P.X., Li L.S., Qiao S.Y., Zhang G.L., Li D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci. 2012;90:266–268. doi: 10.2527/jas.50965. [DOI] [PubMed] [Google Scholar]
- Machado M.G., Sencio V., Trottein F. Short-chain fatty acids as a potential treatment for infections: a closer look at the lungs. Infect Immun. 2021;89:14. doi: 10.1128/IAI.00188-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado R.A., Constantino L.D., Tomasi C.D., Rojas H.A., Vuolo F.S., Vitto M.F., Cesconetto P.A., De Souza C.T., Ritter C., Dal-Pizzol F. Sodium butyrate decreases the activation of nf-kappa b reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:3136–3140. doi: 10.1093/ndt/gfr807. [DOI] [PubMed] [Google Scholar]
- Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S., Maruya M., Mckenzie C.I., Hijikata A., Wong C., Binge L., Thorburn A.N., Chevalier N., Ang C., Marino E., Robert R., Offermanns S., Teixeira M.M., Moore R.J., Flavell R.A., Fagarasan S., Mackay C.R. Metabolite-sensing receptors gpr43 and gpr109a facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:15. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- Madan J.C., Koestler D.C., Stanton B.A., Davidson L., Moulton L.A., Housman M.L., Moore J.H., Guill M.F., Morrison H.G., Sogin M.L., Hampton T.H., Karagas M.R., Palumbo P.E., Foster J.A., Hibberd P.L., O'toole G.A. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio. 2012;3:10. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzal F., Even C., Haimov I., Agmon M., Asraf K., Shochat T., Tamir S. Associations between fecal short-chain fatty acids and sleep continuity in older adults with insomnia symptoms. Sci Rep. 2021;11:8. doi: 10.1038/s41598-021-83389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi M., Gui H., Yao L., Aschenbach J.R., Gäbel G., Shen Z. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J Dairy Sci. 2013;96:7603–7616. doi: 10.3168/jds.2013-6700. [DOI] [PubMed] [Google Scholar]
- Mao J.W., Tang H.Y., Zhao T., Tan X.Y., Bi J., Wang B.Y., Wang Y.D. Intestinal mucosal barrier dysfunction participates in the progress of nonalcoholic fatty liver disease. Int J Clin Exp Pathol. 2015;8:3648–3658. [PMC free article] [PubMed] [Google Scholar]
- Marques F.Z., Nelson E., Chu P.Y., Horlock D., Fiedler A., Ziemann M., Tan J.K., Kuruppu S., Rajapakse N.W., El-Osta A., Mackay C.R., Kaye D.M. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- Mcnabney S.M., Henagan T.M. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. 2017;9:28. doi: 10.3390/nu9121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C. Bone and the gut microbiome: a new dimension. Journal of Laboratory and Precision Medicine. 2018;3:96. [Google Scholar]
- Mentschel J., Leiser R., Mülling C., Pfarrer C., Claus R. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch Anim Nutr. 2001;55:85–102. doi: 10.1080/17450390109386185. [DOI] [PubMed] [Google Scholar]
- Mikami D., Kobayashi M., Uwada J., Yazawa T., Kamiyama K., Nishimori K., Nishikawa Y., Nishikawa S., Yokoi S., Kimura H., Kimura I., Taniguchi T., Iwano M. Short-chain fatty acid mitigates adenine-induced chronic kidney disease via ffa2 and ffa3 pathways. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:8. doi: 10.1016/j.bbalip.2020.158666. [DOI] [PubMed] [Google Scholar]
- Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmonsef P., Zariffard M.R., Gilbert D., Makinde H., Landay A.L., Spear G.T. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol. 2012;67:391–400. doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei R., Bouzari B., Hosseini-Fard S.R., Mazaheri M., Ahmadyousefi Y., Abdi M., Jalalifar S., Karimitabar Z., Teimoori A., Keyvani H., Zamani F., Yousefimashouf R., Karampoor S. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed Pharmacother. 2021;139:23. doi: 10.1016/j.biopha.2021.111661. [DOI] [PubMed] [Google Scholar]
- Miyamoto J., Kasubuchi M., Nakajima A., Irie J., Itoh H., Kimura I. The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 2016;25:379–383. doi: 10.1097/MNH.0000000000000246. [DOI] [PubMed] [Google Scholar]
- Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzaki M., Comelli E.M., Arendt B.M., Bonengel J., Fung S.K., Fischer S.E., Mcgilvray I.D., Allard J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- Murashige D., Jang C., Neinast M., Edwards J.J., Cowan A., Hyman M.C., Rabinowitz J.D., Frankel D.S., Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368. doi: 10.1126/science.abc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S., Nirmalkar K., Hoyo-Vadillo C., Garcia-Espitia M., Ramirez-Sanchez D., Garcia-Mena J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur J Clin Microbiol Infect Dis. 2018;37:621–625. doi: 10.1007/s10096-017-3143-0. [DOI] [PubMed] [Google Scholar]
- Naeem A., Drackley J.K., Stamey J., Loor J.J. Role of metabolic and cellular proliferation genes in ruminal development in response to enhanced plane of nutrition in neonatal Holstein calves. J Dairy Sci. 2012;95:1807–1820. doi: 10.3168/jds.2011-4709. [DOI] [PubMed] [Google Scholar]
- Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N.A., Berkowitz D.E., Pluznick J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial g protein-coupled receptor 41. Physiol Genom. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G.R., Chang P.V. Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol Sci. 2019;40:430–445. doi: 10.1016/j.tips.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwińska B., Hanczakowska E., Arciszewski M.B., Klebaniuk R. Review: exogenous butyrate: implications for the functional development of ruminal epithelium and calf performance. Animal. 2017;11:1522–1530. doi: 10.1017/S1751731117000167. [DOI] [PubMed] [Google Scholar]
- O'hara E., Kelly A., Mccabe M.S., Kenny D.A., Guan L., Waters S.M. Effect of a butyrate-fortified milk replacer on gastrointestinal microbiota and products of fermentation in artificially reared dairy calves at weaning. Sci Rep. 2018;8:11. doi: 10.1038/s41598-018-33122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Hume I., Torell D. Development of microbial activity in the alimentary tract of lambs. J Anim Sci. 1972;35:450–459. doi: 10.2527/jas1972.352450x. [DOI] [PubMed] [Google Scholar]
- O'mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- O'riordan K.J., Collins M.K., Moloney G.M., Knox E.G., Aburto M.R., Fulling C., Morley S.J., Clarke G., Schellekens H., Cryan J.F. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. 2022;546:18. doi: 10.1016/j.mce.2022.111572. [DOI] [PubMed] [Google Scholar]
- Onyszkiewicz M., Gawrys-Kopczynska M., Konopelski P., Aleksandrowicz M., Sawicka A., Kozniewska E., Samborowska E., Ufnal M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and gpr41/43 receptors. Pflueg Arch Eur J Physiol. 2019;471:1441–1453. doi: 10.1007/s00424-019-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszkiewicz M., Gawrys-Kopczynska M., Salagaj M., Aleksandrowicz M., Sawicka A., Kozniewska E., Samborowska E., Ufnal M. Valeric acid lowers arterial blood pressure in rats. Eur J Pharmacol. 2020;877:10. doi: 10.1016/j.ejphar.2020.173086. [DOI] [PubMed] [Google Scholar]
- Palm C.L., Nijholt K.T., Bakker B.M., Westenbrink B.D. Short-chain fatty acids in the metabolism of heart failure - rethinking the fat stigma. Frontiers in Cardiovascular Medicine. 2022;9:9. doi: 10.3389/fcvm.2022.915102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Goergen C.J., Hogenesch H., Kim C.H. Chronically elevated levels of short-chain fatty acids induce t cell-mediated ureteritis and hydronephrosis. J Immunol. 2016;196:2388–2400. doi: 10.4049/jimmunol.1502046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak M., Mandal G.P., Patra A.K., Samanta I., Pradhan S., Haldar S. Effects of dietary supplementation of cinnamaldehyde and formic acid on growth performance, intestinal microbiota and immune response in broiler chickens. Anim Prod Sci. 2017;57:821–827. [Google Scholar]
- Pestana J.M., Gennari A., Monteiro B.W., Lehn D.N., Souza C.F.V. Effects of pasteurization and ultra-high temperature processes on proximate composition and fatty acid profile in bovine milk. Am J Food Technol. 2015;10:265–272. [Google Scholar]
- Petrache I., Petrusca D.N. The involvement of sphingolipids in chronic obstructive pulmonary diseases. Handb Exp Pharmacol. 2013:247–264. doi: 10.1007/978-3-7091-1511-4_12. [DOI] [PubMed] [Google Scholar]
- Poll B.G., Xu J.J., Jun S., Sanchez J., Zaidman N.A., He X.J., Lester L., Berkowitz D.E., Paolocci N., Gao W.D., Pluznick J.L. Acetate, a short-chain fatty acid, acutely lowers heart rate and cardiac contractility along with blood pressure. J Pharmacol Exp Therapeut. 2021;377:39–50. doi: 10.1124/jpet.120.000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice P.M., Schoemaker M.H., Vervoort J., Hettinga K., Lambers T.T., Van Tol EaF., Acerini C.L., Olga L., Petry C.J., Hughes I.A., Koulman A., Ong K.K., Dunger D.B. Human milk short-chain fatty acid composition is associated with adiposity outcomes in infants. J Nutr. 2019;149:716–722. doi: 10.1093/jn/nxy320. [DOI] [PubMed] [Google Scholar]
- Ragaa N.M., Korany R.M.S., Mohamed F.F. Effect of thyme and/or formic acid dietary supplementation on broiler performance and immunity. Agriculture and Agricultural Science Procedia. 2016;10:270–279. [Google Scholar]
- Ragsdale S.W., Pierce E. Acetogenesis and the wood-ljungdahl pathway of co2 fixation. Biochim Biophys Acta Protein Proteonomics. 2008;1784:1873–1898. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., Kukita A., Kukita T., Shobuike T., Nakamura T., Kohashi O. Two histone deacetylase inhibitors, trichostatin a and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood. 2003;101:3451–3459. doi: 10.1182/blood-2002-08-2622. [DOI] [PubMed] [Google Scholar]
- Richards L.B., Li M., Folkerts G., Henricks PaJ., Garssen J., Van Esch B. Butyrate and propionate restore the cytokine and house dust mite compromised barrier function of human bronchial airway epithelial cells. Int J Mol Sci. 2021;22:16. doi: 10.3390/ijms22010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler G., Rosano G. The heart and the gut. Eur Heart J. 2014;35:426–430. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- Rufting S., Xenaki D., Malouf M., Horvat J.C., Wood L.G., Hansbro P.M., Oliver B.G. Short-chain fatty acids increase tnf alpha-induced inflammation in primary human lung mesenchymal cells through the activation of p38 mapk. Am J Physiol Lung Cell Mol Physiol. 2019;316:L157–L174. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- Saleri R., Borghetti P., Ravanetti F., Cavalli V., Ferrari L., De Angelis E., Andrani M., Martelli P. Effects of different short-chain fatty acids (scfa) on gene expression of proteins involved in barrier function in ipec-j2. Porcine Health Management. 2022;8:13. doi: 10.1186/s40813-022-00264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K.P., Martin J.C., Campbell G., Mayer C.D., Flint H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium "roseburia inulinivorans". J Bacteriol. 2006;188:4340–4349. doi: 10.1128/JB.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:14. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H.D., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., Lee J.R., Offermanns S., Ganapathy V. Activation of gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson L.F., Gay M.C.L., Koleva P.T., Eggesb M., Johnson C.C., Wegienka G., Du Toit E., Shimojo N., Munblit D., Campbell D.E., Prescott S.L., Geddes D.T., Kozyrskyj A.L. Human milk from atopic mothers has lower levels of short chain fatty acids. Front Immunol. 2020;11:9. doi: 10.3389/fimmu.2020.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.M., Wu W., Chen L., Yang W.J., Huang X.S., Ma C.Y., Chen F.D., Xiao Y., Zhao Y., Ma C.Y., Yao S.X., Carpio V.H., Dann S.M., Zhao Q.H., Liu Z.J., Cong Y.Z. Microbiota-derived short-chain fatty acids promote th1 cell il-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:15. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.M., Wu W., Liu Z.J., Cong Y.Z. Microbiota metabolite short chain fatty acids, gpcr, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.F., Zhang B.M., Hong X., Zhang X.H., Kong X.B. Histone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in rats. Eur J Pharmacol. 2013;707:147–154. doi: 10.1016/j.ejphar.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Sun Y.Y., Zhou C.X., Chen Y.M., He X.Z., Gao F., Xue D. Quantitative increase in short-chain fatty acids, especially butyrate protects kidney from ischemia/reperfusion injury. J Invest Med. 2022;70:29–35. doi: 10.1136/jim-2020-001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P., Fu H.Y., Ma X. Design, optimization, and nanotechnology of antimicrobial peptides: from exploration to applications. Nano Today. 2021;39:41. [Google Scholar]
- Tang G., Du Y., Guan H.C., Jia J.S., Zhu N., Shi Y.P., Rong S., Yuan W.J. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and ffa2-mediated pi3k/akt/mtor signals. Br J Pharmacol. 2022;179:159–178. doi: 10.1111/bph.15693. [DOI] [PubMed] [Google Scholar]
- Teng F., Wang P., Yang L., Ma Y., Day L. Quantification of fatty acids in human, cow, buffalo, goat, yak, and camel milk using an improved one-step gc-fid method. Food Anal Methods. 2017;10:2881–2891. [Google Scholar]
- Trapecar M., Communal C., Velazquez J., Maass C.A., Huang Y.J., Schneider K., Wright C.W., Butty V., Eng G., Yilmaz O., Trumper D., Griffith L.G. Gut-liver physiomimetics reveal paradoxical modulation of ibd-related inflammation by short-chain fatty acids. Cell Systems. 2020;10:223–239. doi: 10.1016/j.cels.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J., Ubags N., Fajas L., Nicod L.P., Marsland B.J. Dietary fiber confers protection against flu by shaping ly6c(-) patrolling monocyte hematopoiesis and cd8(+) t cell metabolism. Immunity. 2018;48:992–1005. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L.P., Harris N.L., Marsland B.J. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- Tuohy K.M., Fava F., Viola R. 'The way to a man's heart is through his gut microbiota' - dietary pro- and prebiotics for the management of cardiovascular risk. Proc Nutr Soc. 2014;73:172–185. doi: 10.1017/S0029665113003911. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vaziri N.D., Liu S.M., Lau W.L., Khazaeli M., Nazertehrani S., Farzaneh S.H., Kieffer D.A., Adams S.H., Martin R.J. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One. 2014;9:15. doi: 10.1371/journal.pone.0114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas D.P., De La Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (scfas)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:1. doi: 10.3389/fimmu.2019.00277. 10, 277, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira A.T., Galvao I., Macia L.M., Sernaglia E.M., Vinolo MaR., Garcia C.C., Tavares L.P., Amaral F.A., Sousa L.P., Martins F.S., Mackay C.R., Teixeira M.M. Dietary fiber and the short-chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J Leukoc Biol. 2017;101:275–284. doi: 10.1189/jlb.3A1015-453RRR. [DOI] [PubMed] [Google Scholar]
- Vieira A.T., Macia L., Galvao I., Martins F.S., Canesso M.C.C., Amaral F.A., Garcia C.C., Maslowski K.M., De Leon E., Shim D., Nicoli J.R., Harper J.L., Teixeira M.M., Mackay C.R. A role for gut microbiota and the metabolite-sensing receptor gpr43 in a murine model of gout. Arthritis Rheumatol. 2015;67:1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- Villa C.R., Ward W.E., Comelli E.M. Gut microbiota- bone axis. Crit Rev Food Sci Nutr. 2017;57:1664–1672. doi: 10.1080/10408398.2015.1010034. [DOI] [PubMed] [Google Scholar]
- Vourakis M., Mayer G., Rousseau G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int J Mol Sci. 2021;22:20. doi: 10.3390/ijms22158074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghulde H., Cheng X., Galla S., Mell B., Cai J.W., Pruett-Miller S.M., Vazquez G., Patterson A., Kumar M.V., Joe B. Attenuation of microbiotal dysbiosis and hypertension in a crispr/cas9 gene ablation rat model of gper1. Hypertension. 2018;72:1125–1132. doi: 10.1161/HYPERTENSIONAHA.118.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Ross R.P., Shanahan F., O'mahony L., O'mahony C., Coakley M., Hart O., Lawlor P., Quigley E.M., Kiely B., Fitzgerald G.F., Stanton C. Metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am J Clin Nutr. 2009;89:1393–1401. doi: 10.3945/ajcn.2008.27023. [DOI] [PubMed] [Google Scholar]
- Wang D., Day E.A., Townsend L.K., Djordjevic D., Jorgensen S.B., Steinberg G.R. Gdf15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17:592–607. doi: 10.1038/s41574-021-00529-7. [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Lv D., Jiang S.H., Jiang J.P., Liang M., Hou F.F., Chen Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci. 2019;133:1857–1870. doi: 10.1042/CS20190171. [DOI] [PubMed] [Google Scholar]
- Welch C.B., Ryman V.E., Pringle T.D., Lourenco J.M. Utilizing the gastrointestinal microbiota to modulate cattle health through the microbiome-gut-organ axes. Microorganisms. 2022;10:21. doi: 10.3390/microorganisms10071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel T.J., Gates E.J., Ranger A.L., Klegeris A. Short-chain fatty acids (scfas) alone or in combination regulate select immune functions of microglia-like cells. Mol Cell Neurosci. 2020;105:10. doi: 10.1016/j.mcn.2020.103493. [DOI] [PubMed] [Google Scholar]
- Wigg A.J., Roberts-Thomson I.C., Dymock R.B., Mccarthy P.J., Grose R.H., Cummins A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.J., Cheung R., Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the u. S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- Wright J.L., Cosio M., Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1–L15. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Sun W., Ding L.K., Yan M., Sun C., Zhang M., Li S.Y., Qian X., Ma J., Wu L. Three important short-chain fatty acids (scfas) attenuate the inflammatory response induced by 5-fu and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022;23:13. doi: 10.1186/s12865-022-00495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.M., Chen X., Yu S.Q., Su Y., Zhu W.Y. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PLoS One. 2016;11:20. doi: 10.1371/journal.pone.0162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Chen H.W., Gao Y.H., An N., Li X.Y., Pan X.D., Yang X.Y., Tian L., Sun J.H., Xiong X.J., Xing Y.W. Gut microbiota-derived short-chain fatty acids and hypertension: mechanism and treatment. Biomed Pharmacother. 2020;130:15. doi: 10.1016/j.biopha.2020.110503. [DOI] [PubMed] [Google Scholar]
- Yang W.J., Yu T.M., Huang X.S., Bilotta A.J., Xu L.Q., Lu Y., Sun J.R., Pan F., Zhou J., Zhang W.B., Yao S.X., Maynard C.L., Singh N., Dann S.M., Liu Z.J., Cong Y.Z. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell il-22 production and gut immunity. Nat Commun. 2020;11:18. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Chen Y., Xu M. The critical role of short-chain fatty acids in health and disease: a subtle focus on cardiovascular disease-nlrp3 inflammasome-angiogenesis axis. Clin Immunol. 2022;238:11. doi: 10.1016/j.clim.2022.109013. [DOI] [PubMed] [Google Scholar]
- Ye J.Z., Lv L.X., Wu W.R., Li Y.T., Shi D., Fang D.Q., Guo F.F., Jiang H.Y., Yan R., Ye W.C., Li L.J. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front Microbiol. 2018;9:16. doi: 10.3389/fmicb.2018.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Ishii M., Akagawa M. Propionate suppresses hepatic gluconeogenesis via gpr43/ampk signaling pathway. Arch Biochem Biophys. 2019;672:13. doi: 10.1016/j.abb.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Zeng H.W., Umar S., Rust B., Lazarova D., Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. 2019;20:19. doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Qin S.M., Zhu Y., Zhang X.L., Du P.F., Huang Y.Q., Michiels J., Zeng Q.F., Chen W. Dietary resistant starch from potato regulates bone mass by modulating gut microbiota and concomitant short-chain fatty acids production in meat ducks. Front Nutr. 2022;9:16. doi: 10.3389/fnut.2022.860086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ko C.Y., Zeng Y.M. Immunoregulatory effect of short-chain fatty acids from gut microbiota on obstructive sleep apnea-associated hypertension. Nat Sci Sleep. 2022;14:393–405. doi: 10.2147/NSS.S354742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Jiang F., Zhuang H., Chu Y., Zhang F., Wang C. Microrna mir-124-3p suppresses proliferation and epithelial-mesenchymal transition of hepatocellular carcinoma via arrdc1 (arrestin domain containing 1) Bioengineered. 2022;13:8255–8265. doi: 10.1080/21655979.2022.2051686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C.Y., Dai Z.W., Chai L.X., Wu L.P., Li J.H., Guo W.Y., Zhang J., Zhang Q., Xue C.P., Lin H.X., Luo Q., Cai K.D. The change of gut microbiota-derived short-chain fatty acids in diabetic kidney disease. J Clin Lab Anal. 2021;35:11. doi: 10.1002/jcla.24062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Sun J., Ge L.P., Liu Z.H., Chen H., Yu B., Chen D.W. Exogenous infusion of short-chain fatty acids can improve intestinal functions independently of the gut microbiota. J Anim Sci. 2020;98:10. doi: 10.1093/jas/skaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.B., Gao S.X., Chen J.L., Zhao R.Q., Yang X.J. Maternal sodium butyrate supplement elevates the lipolysis in adipose tissue and leads to lipid accumulation in offspring liver of weaning-age rats. Lipids Health Dis. 2016;15:8. doi: 10.1186/s12944-016-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrun S.D., Melton-Celsa A.R., O'brien A.D. When a healthy diet turns deadly. Gut Microb. 2014;5:40–43. doi: 10.4161/gmic.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrun S.D., Melton-Celsa A.R., Smith M.A., Gilbreath J.J., Merrell D.S., O'brien A.D. Dietary choice affects shiga toxin-producing escherichia coli (stec) o157:H7 colonization and disease. Proc Natl Acad Sci USA. 2013;110:E2126–E2133. doi: 10.1073/pnas.1222014110. [DOI] [PMC free article] [PubMed] [Google Scholar]