Abstract
Background
Post-traumatic stress disorder (PTSD) is highly prevalent in veterans and associated with impairments in family functioning, including parenting. There is a bidirectional relationship between PTSD and familial functioning such that impaired functioning is related to increases in trauma-related symptoms, and vice versa. Despite this known bidirectional association, there is currently no trauma-informed parenting intervention available for veterans within the Department of Veterans Affairs (VA). Strength at Home – Parents (SAHP) is an 8-session telehealth delivered psychotherapy group that aims to improve parenting behaviors and overall parent-child and family functioning among U.S. military veterans with PTSD symptoms. This paper describes the methods of an individually randomized group therapy trial to test the efficacy of SAHP compared to a VA treatment as usual control condition.Methods are reported using SPIRIT guidelines.
Methods
One hundred and ninety veterans with elevated PTSD symptoms and parent-child functioning problems will be randomly assigned to the SAHP intervention or a treatment-as-usual control group. Outcomes are measured at 4 timepoints including baseline. The primary outcome is parenting stress. We will also examine changes in parenting behaviors, whether treatment gains are maintained over time, and will conduct an exploratory analysis to examine results separately by gender. Secondary outcomes include symptoms of PTSD and depression, family functioning, and child psychosocial functioning.
Conclusion
Study findings will determine the efficacy of SAHP, an intervention developed for ease of use and implementation within the VA to improve parenting stress and parenting behaviors in veterans with elevated PTSD symptoms and parenting difficulties.
1. Introduction
A history of lifetime trauma exposure and its related psychological consequences, such as post-traumatic stress disorder (PTSD), are associated with impairments in interpersonal relationship and family functioning in adulthood, including dysfunction in the parent-child domain [1]. For example, increases in trauma symptoms are related to higher reports of stress resulting from parenting and the use of less effective parenting strategies (e.g., inconsistent discipline; [[2], [3], [4], [5]]). There is evidence that these parenting strategies are associated with broad negative consequences for children, ranging from poorer emotion regulation to various psychological disorders [3,6,7]. When parenting difficulties cross over into child maltreatment, they are associated with risk for intimate partner violence (IPV) exposure throughout the child's lifespan [8]. Thus, ineffective parenting may be one pathway through which the intergenerational transmission of trauma symptoms occurs. Further, the association between PTSD and interpersonal functioning appears bidirectional; increased trauma symptoms are related to increased impairment in interpersonal functioning, and, in turn, impairment in interpersonal functioning predicts increased trauma symptoms [9,10]. Therefore, timely intervention to address trauma-related impairments in parenting has the potential to improve mental health of parents, increase the psychological resilience of their children, and decrease rates of IPV.
The interplay between the consequences of trauma exposure and parenting difficulties is particularly relevant among U.S. military veteran populations enrolled in Veterans Administration (VA) care. Post 9/11 Veterans are likely to be living with dependents, with 43.8 % of Veterans in this cohort reporting that they are living with children [11]. In this population, PTSD and IPV are highly prevalent, and parenting-related difficulties associated with PTSD are well-documented [4,12,13]. Apart from a self-paced, online Parenting for Veterans course available at veterantraining.va.gov, there is currently no national resource within the VA for veterans seeking clinician assistance with parenting [14]. Further, the various trauma-focused psychotherapies offered within the VA for PTSD (e.g., Cognitive Processing Therapy; Prolonged Exposure; Eye Movement Desensitization and Reprocessing) do not directly address parenting and family functioning. Recent data suggests that PTSD symptoms often persist following trauma-focused treatment [15], and that improvements in interpersonal functioning associated with decreases in PTSD symptoms are small in nature [10]. Although various parenting programs for service members have been developed in recent years, these programs are offered outside of the VA setting (e.g., on military bases), with a focus on very young children or the specific stressors of deployment [16,17]. Thus, parenting interventions remain largely out of reach for veterans enrolled in VA care who struggle with the negative long-term consequences of trauma and PTSD on the parent-child relationship.
To address this treatment gap, our research team developed Strength at Home – Parents (SAHP), a trauma-informed group psychotherapy to improve parenting for veterans with children ages 3–12.SAHP was developed specifically to address the influence of trauma and PTSD on parenting and parent-child functioning in military and veteran populations while also attending to overall family functioning difficulties and interpersonal conflict that frequently occur with PTSD symptoms. SAHP was adapted from two validated, trauma-informed, cognitive-behavioral, and highly disseminated family functioning and intimate partner violence prevention interventions: Strength at Home - Veterans and Strength at Home - Couples [18,19].
SAHP has three distinct theoretical underpinnings: 1) the Social Information Processing model of trauma and IPV [20], which posits that trauma affects how information from the social environment is processes and used, 2) the Cognitive-Behavioral Interpersonal Theory of PTSD, which posits that behavioral avoidance, maladaptive cognitive processes and emotional disturbances which are present in PTSD negatively impact the parent-child relationships [21,22], and 3) the Family Attachment Network model, which centralizes maladaptive attachment and unclear family roles as mechanisms through which PTSD impacts the family system [23].
SAHP is a manualized treatment that consists of eight group treatment sessions in which veterans are exposed to a variety of psychoeducational and cognitive behavioral skill-based content focused on improving parenting, attachment, emotion regulation, and family functioning behaviors, with an emphasis on the unique impact of trauma exposure and PTSD symptoms on these behaviors. Results from an open trial suggested that SAHP improved parenting behaviors and family functioning, reduced parenting stress, as decreased veteran PTSD and depressive symptoms [24]. Here we outline the protocol for a randomized controlled trial (RCT) comparing an 8-week course of telehealth, therapist delivered, SAHP to the self-paced, online Parenting for Veterans course.
The specific aims of the RCT are twofold: 1) Examine the efficacy of the Strength at Home Parents intervention to improve parenting stress and parenting behaviors in comparison to a treatment as usual control condition. We will also examine whether treatment gains are maintained over time and will conduct an exploratory analysis to examine results separately by gender; 2) Examine the efficacy of SAHP compared with the treatment as usual control on changes in overall family functioning, parenting stress index subscales, and parent and child mental health outcomes from baseline to post-treatment and at the follow-up time-points.
2. Methods
2.1. Design overview
This study is an individually randomized parallel two-group therapy trial. A total of 190 veterans will be randomized to either SAHP intervention or the Parenting for Veterans control group. Both groups will complete one baseline assessment and three follow-up assessments spaced about eight weeks apart (T1 (baseline), T2 (post-treatment), T3 (2-month follow-up), T4 (4-month follow-up)). Over approximately 26 months, we aim to enroll 60 veterans in the control group and 130 veterans to the SAHP treatment group that are stratified by gender. The primary purpose of the trial is to determine whether SAHP results in changes to our primary and secondary outcomes that cannot be better explained by the natural course of parenting behaviors in this population or the standard of care. The control group will receive treatment-as-usual, which consists of a referral to the self-guided, online Parenting for Veterans course available at veterantraining.va.gov. In accordance with purpose-guided trial design [25], comparator guidance, and the stage of testing for this treatment; the control condition is a feasible, relevant, and realistic comparator that approximates real-world conditions, but is not expected to be highly formidable.
2.2. Study setting and population
Recruitment will occur within the VA Veterans Integrated Service Network (VISN) 17, a region that encompasses most VA clinics and hospitals and Texas. Participation will be fully virtual; the treatment will be delivered online through the VA's secure telehealth platform, and all assessments will be completed via Qualtrics, a secure online survey platform.
2.3. Participant inclusion and exclusion criteria
Veterans are included if they meet the following criteria: 1) Current parent or caregiver to a child between the ages of 3 and 12 who resides with the participant or spends at least two days per week with the participant, 2) Elevated PTSD symptoms (PCL scores consistent with at least probable PTSD (PCL-5 >31 [26]), and 3) Parent-child functioning problems (any subscale ≥85th% on the Parenting Stress Index-Short Form [27]). This is intended to be a pragmatic trial with minimal exclusion criteria. The following criteria are intended to identify veterans needing referrals or stabilization prior to enrolling: 1) Untreated and/or poorly managed psychosis or substance dependence as measured by the DSM-5 Cross Cutting Symptom Measure, and 2) Current suicide risk established by the suicide item from the DSM-5 Cross Cutting Symptom Measure and clinician follow-up. Participants who meet criteria for these will need to be engaged in mental health or substance care before they can enroll in the study. Engagement in non-study mental health care is only an inclusion criteria at the eligibility screening and is not continually assessed throughout the study. The following is the only exclusion criterion: 1) Major neurocognitive disorder likely to impact comprehension of material, including severe TBI (as defined as a score on the Ohio State Traumatic Brain Injury Identification Method ≥5 [28]). These exclusions were tested in our pilot trials which identified only one veteran with active, unmanaged substance use disorder.
2.4. Veteran screening and recruitment procedures
All procedures will be completed remotely. Recruitment will rely on a random pull of potential participants’ names and contact information from the VA Corporate data Warehouse records. Veterans included in the data pull must meet three criteria: 1) enrolled in VA VISN 17 healthcare 2) at least two medical record documentations in the past year for the ICD10 code for PTSD and 3) have dependents. No other restrictions apply to the recruitment pull of potential participants. Eligible participants will receive a study invitation letter containing a QR code to a self-screen website. Potential participants who do not respond to the QR code will receive a phone call approximately ten days after the initiation letter was mailed. If they are interested and agree, veterans will complete brief-screening measures, followed by separately conducted informed consent and baseline assessments. Veterans completing the self-screen QR code will receive a phone call within 24 h of survey completion to facilitate next steps. Participants randomized to the SAHP condition will complete the treatment between the baseline and T2 assessment.
2.5. Randomization
Treatment allocation following consent to participate will use a randomly permuted block sequence with blocks of size 4, 6 or 8, in two sequences stratified by gender, with a chain length that is known only to the study statistician. To allow for reduced information in the treatment group owing to within-therapy-group correlation, and to increase the number of veterans who will have access to the new SAHP program, treatment allocation will be 2:1. This is accomplished within the blocks in the following way: an equal number of size 4 and size 8 blocks will be used; the size 4 blocks will be 2:2, while the size 8 blocks will be 6:2, so that the total will be 8:4 (i.e., 2:1). Size 6 blocks will be 4:2.
3. Study conditions
3.1. SAHP
SAHP is a manualized therapy delivered over eight treatment group sessions. Groups will include 5–8 veterans separated by gender. Groups are offered through secure VA telehealth technology. The groups are 2 h long and are facilitated by a licensed mental health provider (psychologist or social worker), or by a clinical psychology intern, psychiatry resident, or post-doctoral level provider under the supervision of a psychologist. SAHP is designed to address cognitive, emotional, and behavioral processes which impair both parent-child functioning and maintain trauma symptomatology. SAHP is explicitly trauma-informed, and content includes psychoeducation about the influence of trauma symptomatology on parent-child interactions, and behavioral exercises that aim to reduce trauma-related affective and behavioral avoidance of family interactions. Further, awareness and regulation of emotional responses for both parent and child are emphasized throughout the treatment. Each session includes didactic content, and in- and between-session behavioral exercises focused on positive parenting behaviors and improved attachment (for a detailed review of session content see Ref. [24]). Study therapists will complete a two-day training followed by co-facilitation of one SAHP treatment group with the treatment developers before providing the treatment on their own. To assess treatment fidelity, 10 % of all sessions will be viewed and live rated for treatment fidelity by a subject matter expert. Veterans randomized to this condition will receive weekly reminders of scheduled group sessions.
3.2. Control condition
The control condition is a VA treatment-as-usual comparison, which is a referral to the self-guided, online Parenting for Veterans course [14], a free online course developed by VA experts for veterans and service members with information and strategies to improve their parenting skills. The course consists of a set of tip sheets and videos covering communication, emotions and behavior, discipline, stress management, and emotional and physical challenges. Veterans in this condition will receive weekly reminders to access the website and they will report on how often they accessed the course, which modules they viewed, and their satisfaction with the modules and the program overall at their follow-up assessment. There are no limits to when and for how long the control group participants can access the self-guided intervention.
3.3. Ancillary and post-trial care
Participants in both conditions will be allowed to start new and continue established non-study treatments during their study participation. No referral or counseling will be withheld during the study. If participants request referrals, or it becomes clear they may benefit from a referral, study staff will facilitate this process.
4. Assessment procedures and study measures
4.1. Participant timeline and plans to promote participant retention
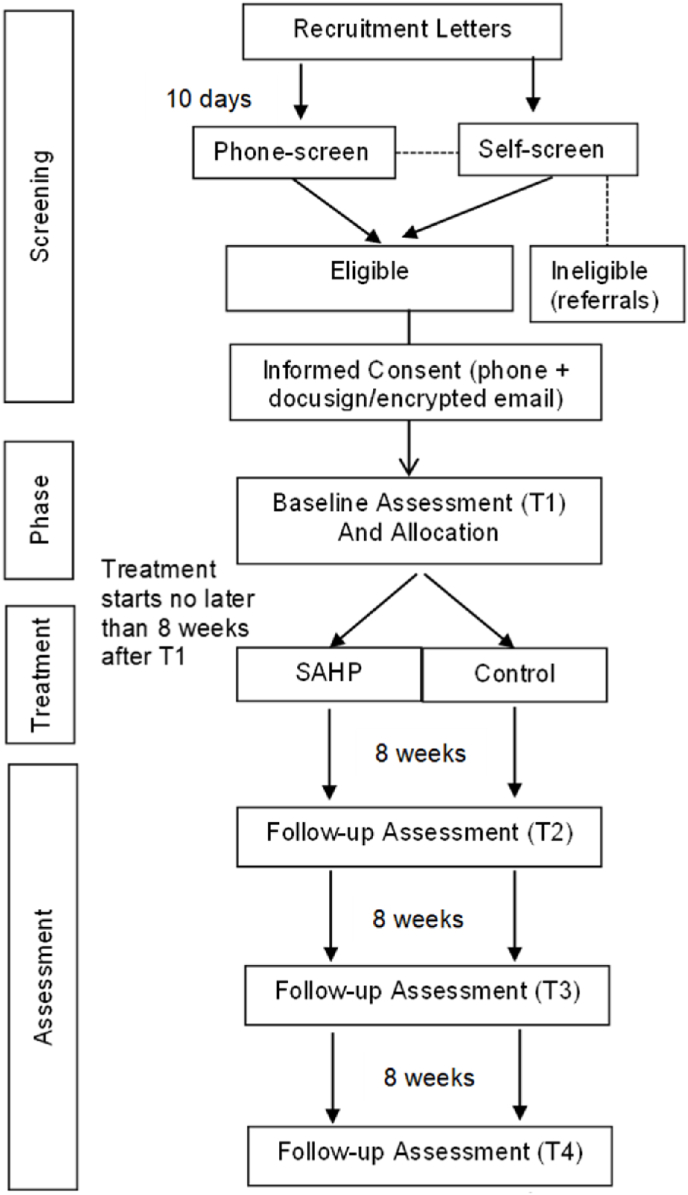
We will leverage text messaging and email to improve retention. Veterans in the control condition will receive weekly reminders to access the Parenting for Veterans website and veterans in the SAHP treatment group will receive appointment reminders before each session. See Fig. 1 for more details on the participant timeline.
Fig. 1.
Study flow.
4.2. Exclusion/stabilization measures
DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure—Adult [29]. The DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure—Adult (DSM-5 CCSM Level 1) is a 23-item self-report measure used to assess mental health domains. Items are scored on a 5-point scale from “none” to “severe” The DSM-5 CCSM Level 1 is used to identify acute need for care related to psychosis, substance use, and suicide risk.
DSM-5 Level 2 Substance Use—Adult [29]. The DSM-5 Level 2 Substance Use—Adult (DSM-5 CCSM Level 2) is a 10-item self-report measure adapted from the NIDA-Modified ASSIST. This measure is used to follow up on positive responses on items 21 or 23 of the DSM-5 Level I Cross-Cutting questionnaire and asks how often during the past 2 weeks the respondent has used the drug. This measure is used to gather more information on substance use to determine the necessity of a clinician follow-up and referral.
Ohio State University Brief ABI Screen [30].The Ohio State University Brief ABI Screen (OSU ABI) is a 3-item structured interview used to screen for the history of Traumatic Brain Injury (TBI) or other Acquired Brain Injury (ABI) exposure.
4.3. Primary outcomes measures
Parenting Stress, Child Stress, and Total Stress. The Parenting Stress Index, 4th edition (PSI [31]) consists of 120 items, with answer options ranging from to “strongly agree” to “strongly disagree” on a 5-point scale. Scores are computed for parent-related stress, child-related stress, and total stress (the combined score of parent and child stress). The short form will be used for eligibility screening, and has 36 identical items forming the same scales. Items on each scale are summed with higher scores indicative of higher stress. Percentiles can be calculated for comparison to standardization samples with the cutoff generally being 84 % and below indicative of typical stress [32]. Any subscale score at or above the 85th percentile will indicate study eligibility.
Parenting behaviors. Parental discipline practices will be measured with the laxness and hostility subscales of the Parenting Scale (PS [33]). Scores are averaged with higher scores reflecting more dysfunctional parenting practices. Items are anchored by one effective and one ineffective discipline strategy rated on a 1 to 7 scale. The laxness scale is comprised of 11 items measuring permissive or inconsistent discipline. Continuous subscale scores are recorded at all timepoints as secondary outcomes. The subscale has adequate internal consistency and reliability and is strongly correlated with observational measures of dysfunctional discipline and child misbehavior [33].
4.4. Secondary outcomes
Family Functioning. The Systematic Clinical Outcome Routine Evaluation (SCORE-15 [34]) Index of Family Functioning and Change is a 15-item scale that assesses self-reported family problems and includes subscales for family strengths, family difficulties, and family communication. Each item is rated on a 5-point scale, and the scores from all individual items are summed into a total family problem score.
PTSD Symptoms. The PTSD Checklist for DSM-5 (PCL-5 [35]) consists of 20 items, with item options ranging from 0 (“not at all”) to 4 (“extremely”). Total scores (range 0–80) are computed, and higher scores are indicative of increased endorsement of trauma symptomatology. A score of 31 or above will indicate eligibility for the study [36].
Depression and suicidal ideation. We will use the QIDS [37]. The QIDS includes 16 items that capture the severity of nine depressive symptoms in the last seven days. Each item is rated on a 4-point scale (0–3); total scores range from 0 to 27, with higher scores indicating more severe depression. This measure is psychometrically strong [37].
Child psychosocial functioning. The Strengths and Difficulties Questionnaire (SDQ [38]) is a parent-report measure of child emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behavior. The SDQ includes 25 questions divided into 5 subscales (emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behavior) with responses “Not True,” “Somewhat True,” or “Certainly True.” Different versions of the questionnaire will be used for 3 years, 4–10 years, and 11–17 years of age. Scores can be used dimensionally and converge with diagnoses, and the measure is sensitive to change (A [39]).
Other covariates, demographic variables: Participants will report their sex, gender identity, race, age, ethnicity, marital status, employment, income, rural/urban locality, years of active military duty, as well as the age, sex and gender of the child of focus.
5. Data analysis
5.1. Power calculation
We consider power for Specific Aim 1 with the primary outcome at only one timepoint (T2) for purposes of designing our study. The foregoing joint model for all timepoints will serve to increase power, so the analysis here is mildly conservative from the perspective of power and sample size. Considering the PSI-Total (PSI-T) score, and preliminary data from our single-arm study, we considered designs with ncont=45, 60, and 75 control subjects; ni=5 subjects per treatment cluster (i.e., therapy group); 90 % power; and treatment effects ranging from to PSI-T points. Sample size was estimated using the R package cluster Power [Kleinman, K. https://cran.r-project.org/web/packages/clusterPower/index.html] with the method described in Ref. [40]. To detect effects as small as PSI-T points, with 60 subjects in the control arm, we estimate needing 26 treatment clusters at 5 subjects/cluster for a total sample size of n = 60 + 5 × 26 = 190.
5.2. Statistical methods for missing data
Data will be analyzed using the intent-to-treat principle, which implies that (a) participants will be assessed according to the treatment condition to which they have been assigned and whether they access that treatment or not; and, (b) so long as a participants completed at least one of T2, T3, or T4 assessments, they will be included in the longitudinal modeling. Because linear mixed effects models use full likelihood inference, analysis naturally accounts for missing data at any of T2, T3, or T4 timepoints under the missing-at-random assumption. For items missing at baseline, we will use single or multiple imputation, depending on how prevalent the missingness is, where single imputation is usually adequate with less than 5 % missingness [41]. For participants that are lost-to-follow up before T2 (i.e., who have no follow up data at all), we will conduct sensitivity analyses after assessing baseline differences between this group and the rest of the study sample.
5.3. Statistical methods for analyzing primary and secondary outcomes
We will develop descriptive analyses of the study sample, presenting data by treatment arm. For formal comparison of outcomes between treatment arms, we will use linear mixed effects models for continuous outcomes [42], as described below, and, for binary or count outcomes, generalized estimating equations with finite sample corrections to correlation parameter estimates and bias-corrected sandwich variance estimates for treatment effects [43]. We will pre-specify (i.e., a priori) baseline variables for which we will adjust, and these will include: the baseline value of the outcome under consideration (e.g., PSI-Total), age, gender, and years of active military duty; such adjustment is not needed for study validity, but can improve statistical efficiency, considerably so when follow-up and baseline values are highly correlated, as is expected to be the case here.
Specific Aim 1. The primary outcome will be Parenting Stress Index - Total (PSI-T) score at T2 (which occurs 8–16 weeks after baseline). For these continuous outcomes, we will analyze the three timepoints (T2, T3, T4) together in a joint linear random (mixed) effects model with: a subject level random intercept and, for those in the SAHP arm, a cluster-level random intercept. We will allow for the random effect and residual variances to differ between arms. For binary outcomes, we will use an analogous approach, separately estimating the within-subject correlation (i.e., across timepoints), and the between-subject within-cluster correlation parameters for the two arms both within- and between-timepoints (T2, T3, T4). By modeling time as a discrete factor and including treatment-by-time effects, these models will directly yield tests and quantification of treatment effects at each follow-up timepoint, while the joint modeling will serve to increase statistical efficiency. The primary outcome from these models will be for PSI-T treatment efficacy at T2.
Secondary outcomes will include the laxness and overreactivity subscales of the Parenting Scale; these will be analyzed in a similar manner to the PSI-T. Additional secondary analyses will test for treatment efficacy at T3 and T4. Should treatment gains be realized at T2, these secondary tests will quantify the degree to which gains are maintained at T3 and T4. Should treatment gains not have been realized at T2, these analyses will test whether such gains are realized at these later time points. Tests for treatment efficacy at each of T3 and T4 can be obtained from the same longitudinal modeling framework as that for T2.
Specific Aim 2. In Aim 2, we will examine the efficacy of SAHP compared with the no-treatment control on changes from baseline in overall family functioning, parenting stress index subscales, and parent and child mental health outcomes. Outcomes for this aim include the Total Family Problems Scale, the two subscales of the PSI, and the Total Difficulties Subscale of the Child Psychosocial Functioning Strengths and Difficulties Questionnaire. For each scale, analyses will proceed as for PSI-T in Aim 1; that is, we will use a mixed effects regression model for the combined outcomes at T2, T3, and T4, where the T2 outcome is considered to be primary. For each outcome model, we will adjust for age, gender, and the value of the measure at baseline (which generally increases power). Because there are four endpoints, we will correct for multiple comparisons using the procedure outlined by Ref. [44]. Follow-up analyses will then examine effects of each of the four measures at T3 and T4 timepoints to quantify the degree to which treatment gains are maintained over time. Further, exploratory analyses in Aim 2 will test for evidence of the effect of the treatment on PTSD symptoms and depression at the primary (T2) or secondary (T3, T4) outcomes.
Additional analyses. Using gender indicator and interactions between gender, treatment condition, and time, we will conduct exploratory analyses testing for evidence of gender differences in the effect of the treatment at the primary (T2) or secondary (T3, T4) outcomes.
6. Discussion
Family functioning has been suggested as an untapped means for improving well-being and underlying psychopathology. This might be especially pertinent for veterans with PTSD, given the high base rate of trauma related symptomatology in this population and the well-documented adverse effects of trauma on family and social functioning [1]. Parenting appears affected in those with PTSD, with reported negative impacts on parenting behaviors, and overall parent-child functioning [[2], [3], [4], [5]]. Despite these known associations, there is currently no validated clinician-delivered parenting intervention available within the VA, thus, parenting interventions remain out of reach for most veterans.
Our team developed and pilot tested SAHP, a trauma-informed parenting intervention for veterans delivered within the VA. Currently available parenting interventions for service members were primarily developed for active duty military families and focus on deployment-related separation and stress [16,17]. SAHP addresses the unmet need for a scalable and accessible parenting intervention developed specifically for veterans with trauma symptoms and related family functioning impairment. A recently completed open trial ([24] demonstrated that SAHP was both satisfactory and reduced parent-child dysfunction, and improved family dynamics and overall veteran mental health.
The study described above will test SAHP against a treatment-as-usual control group, which is a necessary step to establishing the intervention's efficacy. We considered alternative comparators to better test the effect of the intervention against a more “active” treatment; however, we were unable to find a realistic clinician-delivered comparator within the VA system, and, therefore, are referring veterans in the control condition to the Parenting for Veterans course. We believe this research is in line with both congressional mandates for investing in family focused veteran care and a broader shift within the VA towards recovery- and resilience-based alternatives to traditional treatment options. One limitation of the current study is that outcomes are only assessed by veteran self-report. Collateral report by co-parents, other caregivers or teachers would add validity to the measures, and future studies can consider implementing behavioral observations of parent-child functioning. However, there are strict limitations on the study of minors within the VA system and other limitations to the study of non-veterans which makes this logistically challenging.
6.1. Conclusion
PTSD is a signature wound of war for post-9/11 veterans, a young cohort who are likely to be parenting young children. Given the documented negative effects of trauma symptoms on parenting behaviors, parent-child attachment, and child and veteran functioning, interventions specifically addressing these challenges are essential. Results of this trial will advance our understanding of the efficacy of parenting interventions in trauma-exposed veterans, with the overarching goal of paving the way for an integrated and multi-faceted approach to the treatment of PTSD within the VA.
Financial or competing interests
The authors declare no financial or other competing interests.
Study registration
Protocol version
1.
Funding:
This work was supported by a clinical trial award (HT9425-23-1-0869 to Suzannah K. Creech) from the Department of Defense Traumatic Brain Injury and Psychological Health Research Program.
Name and contact information for the trial sponsor
Scientific Officer Nicholas Sanislo nicholas.j.sanislo.ctr@health.mil.
Role of sponsor
The sponsor has no role in the study design or writing of this or any manuscripts. They are not involved in the collection, analysis or interpretation of the data.
CRediT authorship contribution statement
Rahel Pearson: Writing – original draft, Supervision, Project administration, Methodology, Investigation, Conceptualization. Paul J. Rathouz: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Corina Mendoza: Writing – review & editing, Supervision, Project administration, Investigation. Emma Harris: Writing – review & editing, Project administration, Investigation. Allison Metts: Writing – review & editing, Project administration, Investigation. Kathryn Roe: Writing – review & editing, Project administration, Investigation. Justin Benzer: Writing – review & editing, Methodology, Funding acquisition, Conceptualization. Casey Taft: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Suzannah K. Creech: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no financial or other competing interests.
Data availability
No data was used for the research described in the article.
References
- 1.Creech S.K., Misca G. Parenting with PTSD: a review of research on the influence of PTSD on parent-child functioning in military and veteran families. Front. Psychol. 2017;8:1101. doi: 10.3389/fpsyg.2017.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giff S.T., Renshaw K.D., Allen E.S. Post-deployment parenting in military couples: associations with service members' PTSD symptoms. J. Fam. Psychol. 2019;33(2):166. doi: 10.1037/fam0000477. [DOI] [PubMed] [Google Scholar]
- 3.Leen-Feldner E.W., Feldner M.T., Knapp A., Bunaciu L., Blumenthal H., Amstadter A.B. Offspring psychological and biological correlates of parental posttraumatic stress: review of the literature and research agenda. Clin. Psychol. Rev. 2013;33(8):1106–1133. doi: 10.1016/j.cpr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Sherman M.D., Gress Smith J.L., Straits-Troster K., Larsen J.L., Gewirtz A. Veterans' perceptions of the impact of PTSD on their parenting and children. Psychol. Serv. 2016;13(4):401. doi: 10.1037/ser0000101. [DOI] [PubMed] [Google Scholar]
- 5.Tomassetti-Long V.J., Nicholson B.C., Madson M.B., Dahlen E.R. Hardiness, parenting stress, and PTSD symptomatology in US Afghanistan/Iraq era veteran fathers. Psychol. Men Masc. 2015;16(3):239. [Google Scholar]
- 6.Castro-Vale I., Severo M., Carvalho D. Lifetime PTSD is associated with impaired emotion recognition in veterans and their offspring. Psychiatr. Res. 2020;284 doi: 10.1016/j.psychres.2019.112666. [DOI] [PubMed] [Google Scholar]
- 7.Roberts A.L., Galea S., Austin S.B., Cerda M., Wright R.J., Rich-Edwards J.W., Koenen K.C. Posttraumatic stress disorder across two generations: concordance and mechanisms in a population-based sample. Biol. Psychiatr. 2012;72(6):505–511. doi: 10.1016/j.biopsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon K., Hoertel N., Wall M.M., Okuda M., Limosin F., Blanco C. Childhood maltreatment and risk of intimate partner violence: a national study. J. Psychiatr. Res. 2015;69:42–49. doi: 10.1016/j.jpsychires.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lord K.A., Suvak M.K., Holmes S., Shields N., Lane J.E., Sijercic I., Wagner A.C., Stirman S.W., Monson C.M. Bidirectional relationships between posttraumatic stress disorder and social functioning during cognitive processing therapy. Behav. Ther. 2020;51(3):447–460. doi: 10.1016/j.beth.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow B.A., Baker S.N., Leifker F.R., Straud C.L., Rozek D.C., Sippel L.M. The impact of trauma‐focused psychotherapy for posttraumatic stress disorder on interpersonal functioning: a systematic review and meta‐analysis of randomized clinical trials. J. Trauma Stress. 2023 doi: 10.1002/jts.22906. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Veteran Analysis and Statistics Key statistics by veteran status and period of service. KeyStats.pdf (va.gov) 2016 [Google Scholar]
- 12.McGaw V.E., Reupert A.E., Maybery D. Military posttraumatic stress disorder: a qualitative systematic review of the experience of families, parents and children. J. Child Fam. Stud. 2019;28:2942–2952. [Google Scholar]
- 13.Zelkowitz R.L., Archibald E.A., Gradus J.L., Street A.E. Postdeployment mental health concerns and family functioning in veteran men and women. Psychological Trauma: Theory, Research, Practice, and Policy. 2023;15(4):705–714. doi: 10.1037/tra0001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene C., Fenstermacher S., Petty K., Shore P., Murphy P., Lai W., Weingardt K.R. Parenting for veterans [online course] Veterans Health Administration Office of Mental Health and Suicide Prevention. 2013-2022 https://www.veterantraining.va.gov/parenting/index.asp [Google Scholar]
- 15.Larsen S.E., Bellmore A., Gobin R.L., Holens P., Lawrence K.A., Pacella-LaBarbara M.L. An initial review of residual symptoms after empirically supported trauma-focused cognitive behavioral psychological treatment. J. Anxiety Disord. 2019;63:26–35. doi: 10.1016/j.janxdis.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 16.DeVoe E.R., Paris R., Emmert-Aronson B., Ross A., Acker M. A randomized clinical trial of a postdeployment parenting intervention for service members and their families with very young children. Psychological Trauma: Theory, Research, Practice, and Policy. 2017;9(Suppl 1):25–34. doi: 10.1037/tra0000196. [DOI] [PubMed] [Google Scholar]
- 17.Gewirtz A.H., DeGarmo D.S., Zamir O. “After deployment, adaptive parenting tools: 1-year outcomes of an evidence-based parenting program for military families following deployment”: correction. Prev. Sci. 2018;19(4):600–601. doi: 10.1007/s11121-017-0849-2. psyh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creech S.K., Benzer J.K., Bruce L., Taft C.T. Evaluation of the strength at Home group intervention for intimate partner violence in the veterans Affairs health system. JAMA Netw. Open. 2023;6(3) doi: 10.1001/jamanetworkopen.2023.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taft C.T., Creech S.K., Gallagher M.W., Macdonald A., Murphy C.M., Monson C.M. Strength at Home Couples program to prevent military partner violence: a randomized controlled trial. J. Consult. Clin. Psychol. 2016;84(11):935. doi: 10.1037/ccp0000129. [DOI] [PubMed] [Google Scholar]
- 20.Taft C.T., Weatherill R.P., Scott J.P., Thomas S.A., Kang H.K., Eckhardt C.I. Social information processing in anger expression and partner violence in returning US Veterans. J. Trauma Stress. 2015;28(4):314–321. doi: 10.1002/jts.22017. [DOI] [PubMed] [Google Scholar]
- 21.Creech S.K., Trotman A., Michaelson G., Benzer J.K., Copeland L.A. Parenting behaviors and PTSD symptoms predict child psychosocial problems and parenting satisfaction in a sample of US veterans and service members. Military Behavioral Health. 2017;5(4):374–383. [Google Scholar]
- 22.Dekel R., Monson C.M. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress. Violent Behav. 2010;15(4):303–309. doi: 10.1016/j.avb.2010.03.001. [DOI] [Google Scholar]
- 23.Riggs S.A., Riggs D.S. Risk and resilience in military families experiencing deployment: the role of the family attachment network. J. Fam. Psychol. 2011;25(5):5. doi: 10.1037/a0025286. [DOI] [PubMed] [Google Scholar]
- 24.Creech S.K., Pearson R., Saenz J.J., Braciszewski J.M., Riggs S.A., Taft C.T. Trauma-informed parenting intervention for veterans: a preliminary uncontrolled trial of Strength at Home–Parents. J. Fam. Psychol. 2023 doi: 10.1037/fam0001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedland K.E. Purpose-guided trial design in health-related behavioral intervention research. Health Psychol. 2020;39(6):539. doi: 10.1037/hea0000867. [DOI] [PubMed] [Google Scholar]
- 26.Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation: posttraumatic stress disorder checklist for DSM-5. J. Trauma Stress. 2015;28(6):6. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 27.Abidin R.R. Pediatric Psychology Press; 1983. Parenting Stress Index: Manual, Administration Booklet, [and] Research Update. 2915 Idlewood Drive, Charlottesville, VA 22901 ($18. [Google Scholar]
- 28.Corrigan J.D., Bogner J. Initial reliability and validity of the Ohio state university TBI identification method. J. Head Trauma Rehabil. 2007;22(6):318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- 29.Association A.P. American Psychiatric Publishing; Arlington, VA: 2013. DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure—Adult. [Google Scholar]
- 30.McGinley A. DClinPsy, University of Glasgow; 2017. Validating The Brain Injury Screening Index (BISI) and the Ohio State University Traumatic Brain Injury Identification Method (OSU TBI-ID) as Screening Tools for Head Injury in a Scottish Prison Setting: And Clinical Research Portfolio. [DOI] [Google Scholar]
- 31.Abidin R.R. Pediatric Psychology Press, 2915 Idlewood Drive; Charlottesville, VA: 1983. Parenting Stress Index: Manual, Administration Booklet, [and] Research Update. [Google Scholar]
- 32.Abidin R.R. vol. 3. Psychological Assessment Resources; Lutz, FL: 2012. pp. 1–16. (Parenting Stress Index–Fourth Edition (PSI-4)). [Google Scholar]
- 33.Arnold D.S., O'Leary S.G., Wolff L.S., Acker M.M. The Parenting Scale: a measure of dysfunctional parenting in discipline situations. Psychol. Assess. 1993;5(2):2. doi: 10.1037/1040-3590.5.2.137. [DOI] [Google Scholar]
- 34.Stratton P., Bland J., Janes E., Lask J. Developing an indicator of family function and a practicable outcome measure for systemic family and couple therapy: the SCORE: systemic family and couple therapy. J. Fam. Ther. 2010;32(3):232–258. doi: 10.1111/j.1467-6427.2010.00507.x. [DOI] [Google Scholar]
- 35.Weathers F.W., Litz B.T., Keane T.M., Palmieri P., Marx B.P., Schnurr P.P. The PTSD checklist for DSM-5 (PCL-5) 2013. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- 36.Bovin M.J., Marx B.P., Weathers F.W., Gallagher M.W., Rodriguez P., Schnurr P.P., Keane T.M. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychol. Assess. 2016;28(11):1379. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- 37.Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N., Markowitz J.C., Ninan P.T., Kornstein S., Manber R., Thase M.E., Kocsis J.H., Keller M.B. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatr. 2003;54(5):573–583. doi: 10.1016/S0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 38.Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatr. 2001;40(11):1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Goodman A., Goodman R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J. Am. Acad. Child Adolesc. Psychiatr. 2009;48(4):400–403. doi: 10.1097/CHI.0b013e3181985068. [DOI] [PubMed] [Google Scholar]
- 40.Moerbeek M., Wong W.K. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat. Med. 2008;27(15):2850–2864. doi: 10.1002/sim.3115. [DOI] [PubMed] [Google Scholar]
- 41.Rathouz P., Preisser J. Encyclopedia of Health Economics. Elsevier; 2014. Missing data: weighting and imputation. [Google Scholar]
- 42.Laird N.M., Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963. doi: 10.2307/2529876. [DOI] [PubMed] [Google Scholar]
- 43.Preisser J.S., Lu B., Qaqish B.F. Finite sample adjustments in estimating equations and covariance estimators for intracluster correlations. Stat. Med. 2008;27(27):5764–5785. doi: 10.1002/sim.3390. [DOI] [PubMed] [Google Scholar]
- 44.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.